Abstract
Host antimicrobial mechanisms reduce iron availability to pathogens. Iron proteins influencing the innate immune response include hepcidin, lactoferrin, siderocalin, haptoglobin, hemopexin, Nramp1, ferroportin and the transferrin receptor. Numerous global health threats are influenced by iron status and provide examples of our growing understanding of the connections between infection and iron metabolism.
Keywords: Innate immunity, Inflammation, Hepcidin
1. Introduction
The innate immune response serves as the first line of host defense against pathogens that have breached natural mechanical barriers such as the skin, mucus, and gastric acid. Cells of the innate immune response include monocytes, macrophages, neutrophils, and dendritic cells, which react rapidly to control pathogen growth and promote inflammation and development of adaptive immunity [1]. Initial identification of foreign invaders is governed by the interaction of pattern-recognition receptors (PRRs) with pathogen-specific motifs (e.g., peptidoglycan, lipopolysaccharide, flagellin, and viral dsRNA) known as pathogen-associated molecular patterns (PAMPs) or products of host cell damage (e.g., extracellular ATP) known as danger-associated molecular patterns (DAMPs) [1]. PRRs are separated into two categories, sensor and phagocytic based on the activation of pro-inflammatory signaling cascades or pathogen phagocytosis, respectively [2]. These innate pathways induce various antimicrobial mechanisms, including depletion of iron available to pathogens at the systemic and cellular levels. This review particularly focuses on the role of iron metabolism in the innate immune response. The first part summarizes the metabolism and proteins regulating iron status that affect innate immunity, and the second focuses on key global infectious diseases (tuberculosis, HIV, malaria, and trypanosomatid diseases) that are influenced by iron status.
2. Systemic Iron Metabolism
Iron’s chemistry cycles between ferrous (Fe2+) and ferric (Fe3+) states to support the redox potential critical for oxygen and energy metabolism. Iron that is associated with heme represents the largest pool of in our body and is required for oxygen transport in hemoglobin, oxygen storage in myoglobin, and electron transport for cytochrome function in aerobic respiration. The second largest pool of iron is in the nonheme form stored in ferritin. When needed, iron can be released from ferritin to fulfill demands for oxygen transport and energy metabolism. This mechanism limits excess free iron that would otherwise generate reactive oxygen species. Circulating transferrin, which delivers iron to cells through receptor-mediated uptake, also provides a second storage pool that is more dynamic in nature than ferritin storage by distributing iron from storage sites (e.g., liver) to peripheral tissues (e.g., muscle, bone marrow, etc.) [3].
Iron homeostasis must balance the needs for iron while limiting toxicity associated with the metal’s absorption, utilization, and storage. Our body has developed a high degree of iron conservation such that iron is not eliminated from the body; rather the amount of iron present in the system is regulated at its point of entry from dietary iron. Uptake of iron into the intestinal epithelium involves the apical membrane transporter Divalent Metal Transporter-1 (DMT1). Export across enterocytes into circulation involves a second membrane transporter called ferroportin (Fpn). When iron is depleted from the body due to blood loss, for example, absorption from the diet is increased primarily through regulation of the transport activity of these two factors and their associated oxidoreductases which help to transport the appropriate ferrous or ferric form of iron. Iron entering the system binds to transferrin and is delivered by portal circulation to the liver. While any excess non-heme iron can be stored in liver ferritin, the immediate metabolic pool circulates bound to transferrin. Transferrin is bound by cell surface receptors and is internalized by receptor-mediated endocytosis to acidic compartments wherein iron is released. This mechanism delivers iron to peripheral tissues for metabolic purposes [4].
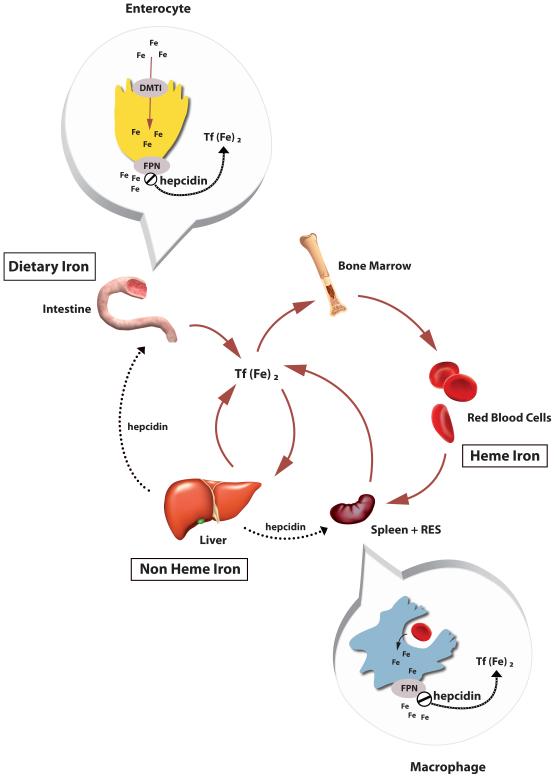
The dominant site for iron utilization is the bone marrow for developing red blood cell hemoglobinization. The body has a substantial reservoir of iron in erythrocytes and 20 to 25 mg of iron is turned each day due to erythrophagocytosis of senescent red blood cells by macrophages in spleen and reticuloendothelial system (RES). This released iron can be recycled and either utilized or stored as needed; thus uptake of dietary iron is quite limited with only 1-2 mg/day required. There is a complex sensing mechanism that responds to the saturation of iron-binding by transferrin and other regulatory cues – including infection and inflammation – that help to tightly control iron metabolism to limit excess yet prevent deficiency. Hepcidin, the key iron regulatory hormone, along with other iron proteins involved in transport and regulation, underpin iron’s key role in innate immunity to modulate the body’s iron balance and in the battle against infection [5]. Figure 1 summarizes the relationships between different iron pools, regulation of homeostasis and the transporters involved.
Figure 1. Iron Homeostasis and Hepcidin Regulation.
Dietary iron is taken up across intestinal enterocyte by a transport network that involves the apical importer DMT1 (Divalent Metal Transporter 1) and the basolateral exporter Fpn (Ferroportin). Iron circulates bound to Transferrin (Tf) and is delivered to cells by endocytosis of Tf receptors. Excess iron delivered to the liver is stored in ferritin (non-heme iron) while the majority of iron is utilization to manufacture hemoglobin (heme iron). As sensescent red cells are destroyed by macrophages in spleen and reticuloendothelial system, iron is recycle via Fpn. Entry of iron into circulation is controlled by hepcidin which is a regulatory hormone secreted by the liver, and also as part of the innate immune response. Hepcidin binds to Fpn, causing its internalization and ultimate degradation. Part of liver iron homeostasis detecting changes in iron status involves pathways sensing the saturation of circulating Tf and state of iron overload.
2. Iron proteins influencing disease
2.1 Hepcidin
Hepcidin is not only a central player in the integration of iron metabolism but it also provides a critical connection with the immune response to infection and inflammation. Upon activation of sensor PPRs, secreted IL-6 induces hepcidin expression and release from the liver [6]. Its mechanism of action is to bind directly to the iron efflux protein ferroportin. This ligand/receptor coupling triggers two immediate consequences. First, hepcidin’s interactions with ferroportin induces the internalization and lysosomal degradation of the transporter [7]; thus, iron egress from duodenal enterocytes and macrophages is diminished, resulting in hypoferremia [6]. This systemic iron retention serves to deplete circulating iron that would otherwise be available to extracellular pathogens. Second, the protein kinase Jak2 becomes activated resulting in phosphorylation of not only ferroportin, but also the transcription factor Stat3. Hepcidin therefore can also induce transcriptional changes to modulate downstream acute cytokine-induced inflammatory responses [8].
Disruption of the hepcidin/ferroportin axis is known to impact susceptibility to various bacterial and viral pathogens. Hereditary diseases of iron overload, or Types IIII hemochromatoses (Table 1), are characterized by low levels of hepcidin, over-expression of ferroportin, macrophage iron depletion, and hyperferremia [9]. Type IV, or ferroportin disease, has some overlapping features because it results from mutations in the ferroportin transport protein. It has been postulated that increased plasma iron is responsible for the association of hemochromatoses with Vibrio vulnificus, Yersinia enterocolitica, and Escherichia coli infections [10]; however, hemochromatoses have also been implicated in resistance to infection, particularly by intracellular pathogens. For example, Mycobacterium tuberculosis growth and iron acquisition was significantly impaired in iron-depleted macrophages isolated from patients with HFE-associated hemochromatosis [11]. Similarly, other models of ferroportin over-expression have exhibited impaired growth of intracellular Salmonella enterica, Chlamydia trachomatis, and Legionella pneumophila that could be reversed with the addition of hepcidin [12;13]. Thus, it is apparent that pathophysiological disruption of hepcidin’s regulation of ferroportin has direct impact on nutritional iron available to extracellular and intracellular pathogens.
Table 1. Hereditary Hemochromatoses: Iron-loading Diseases with Altered Susceptibility to Infections.
| TYPE | NAME | MUTATION/LOCUS | CLINICAL SIGNS |
|---|---|---|---|
| I | HFE-associated Hemochromatosis | HFE/6p21.3 | Elevated transferrin saturation; high serum ferritin; liver iron loading |
|
IIA
IIB |
Juvenile Hemochromatosis |
HJV/1q21 HEPC/19q13 |
Severe iron loading with early age of onset |
| III | TfR2-associated hemochromatosis |
hTFR2/7q22 | High iron, normal transferrin saturation and serum ferritin |
| IV | Ferroportin Disease |
FPN/2q32 | High transferrin saturation and serum ferritin, relatively normal iron stores |
2.2 Lactoferrin
Lactoferrin, a potent chelator of ferric iron, is also involved in modulation of extracellular iron availability. Constitutively high levels of lactoferrin are found in most surface secretions, including tears, saliva, bile, and breast milk [14]. Pro-inflammatory cytokines such as TNF-α also induce the release of lactoferrin from neutrophilic granules at the site of infection [14;15]. Accordingly, the addition of exogenous lactoferrin has been shown to reduce pulmonary M. tuberculosis burden in a mouse model of iron overload [16]. The capacity of lactoferrin to sequester iron also has been implicated in the ability of patients with cystic fibrosis to control opportunistic Pseudomonas aeruginosa infection and biofilm formation [17;18]. Despite this direct link between exogenous lactoferrin and inhibition of bacterial growth, lactoferrin knockout mice do not display enhanced susceptibility to M. tuberculosis [19], P. aeruginosa[20], or Staphylococcus aureus [20]. However, neutrophils isolated from these mice exhibit a stimuli-specific defect in the oxidative burst [20]. This suggests that the exact role of lactoferrin in innate immunity has yet to be completely elucidated.
2.3 Siderocalin
Siderocalin, which is also known as lipocalin-2 or neutrophil-gelatinase-associated lipocalin (Ngal), is produced by neutrophil granules and in epithelial cells in response to the pro-inflammatory cytokine IL-1β [21]. Numerous pathogenic microorganisms secrete small organic siderophores to scavenge for iron. Siderocalin subverts this iron acquisition system through binding catecholate-type and salicylate-derived iron-laden siderophores such as enterobactin secreted by E. coli and mycobactin secreted by M. tuberculosis, respectively [22;23]. Consequently, the addition of recombinant siderocalin to culture media dramatically reduces growth of both by E. coli and M. tuberculosis [22;24;25]. Siderocalin knock-out mice exhibit increased susceptibility and mortality in response to E. coli and M. tuberculosis infections [24;26]. A recent study involving contacts of patients with active pulmonary tuberculosis revealed an inverse relationship between serum levels of siderocalin and infection [25]. Additionally, neutrophil deficient HIV-infected patients demonstrate a significant reduction in serum siderocalin when compared to healthy individuals that can be reversed with highly active anti-retroviral therapy [27] further implicating this protein in human disease outcomes. Endogenous ligands for siderocalin have been identified to include urinary catechol [28] and its relative 2,5-dihydroxybenzoic acid [29]. Thus, siderocalin also has an emerging role to sequester host systemic free iron within these complexes, thereby limiting its availability to pathogens.
2.4 Heme iron factors (hemoglobin, haptoglobin, hemopexin)
The vast majority of iron in the body serves as a co-factor bound to heme in hemoglobin. When released by cellular lysis, hemoglobin and heme are bound by haptoglobin and hemopexin, respectively. Both of these complexes can be taken up and cleared by macrophages, but they may also serve as iron sources for infectious agents such as Staphylococcus [19], Leishmania [30], Trypanosomes [31], and Plasmodium falciparum [32], the causative agent of malaria. In malaria and other infections, the host enzyme heme oxygenase-1 is induced to limit iron availability and to provide other anti-inflammatory functions [33;34]. Because heme present in red blood cells represents a rich iron source, many pathogens trigger hemophagocytic macrophages to engulf erythrocytes, providing a survival niche for growth (e.g., Influenza, Mycobacteria, and Salmonella) [35].
2.5 Non-heme iron factors (TfRs, ferroportin and Nramp1)
Activation of phagocytes via PRRs and the Th1 cell-derived cytokine interferon-γ results in decreased expression of transferrin receptors and enhanced expression of the natural resistance associated macrophage protein-1 (Nramp1) and ferroportin [36-39]. The transferrin receptor is the major pathway for delivery of iron to peripheral tissues through endocytosis of its ligand transferrin, which binds iron and circulates in plasma. Down-regulation of the transferrin receptor limits the cell’s ability to acquire transferrin-bound iron and concomitantly reduces the endosomal pool of iron accessed by intracellular pathogens such as L. pneumophila, M. tuberculosis, and Mycobacterium avium to restrict their growth [36;37;40;41]. Nramp1 was identified in mice with a mutation that conferred susceptibility to infections [42], and loss of Nramp1 function in mice is associated with reduced inflammatory response to infection [43]. Certain mutations in the human gene also influence the response to infection and inflammation [44]. Nramp1 is a pH-dependent divalent metal transporter that localizes to phagosomal compartments and reduces intraphagosomal iron [45] and macrophage recycling of erythrophagocytosed iron is defective in Nramp1 knockout mice [46]. Functional Nramp1 is known to confer resistance to intraphagosomal parasites including Mycobacterium bovis BCG, Leishmania donovanii, and Salmonella typhimurium, whereas parasites that escape to the cytosol such as Listeria monocytogenes are unaffected by Nramp1 status [45;47]. Recent work has also demonstrated that the mobilization of iron by Nramp1 suppresses the expression of the inhibitory cytokine IL-10 and enhances macrophage production of iNOS further promoting microbial growth restriction [39;48;49]. Finally, the regulation of ferroportin expression at the cellular level opposes the systemic regulation mediated by hepcidin described above. Challenge with S. typhimurium or M. tuberculosis up-regulates ferroportin expression and reduces intracellular iron in isolated murine macrophages [48;50]. This is presumably a pathogen-specific localized response to limit intracellular iron but it is presently unclear as to how these opposing mechanisms are balanced in vivo.
3. Global diseases influenced by iron status
3.1 Tuberculosis
M. tuberculosis is a leading cause of infectious disease morbidity and mortality with approximately 8 million new cases and 2 million deaths reported annually [51]. M. tuberculosis is an intracellular pathogen that requires iron for growth and virulence. To ensure the metabolic demand for iron is met, M. tuberculosis has evolved numerous mechanisms to sequester host cell iron. Upon infection of alveolar macrophages, M. tuberculosis establishes a unique phagosomal compartment that retains the capacity to fuse with early endosomes but fails to fuse with lysosomes [52;53]. This allows for access to transferrin-bound iron, while preventing the bactericidal effects of low pH and lysosomal degradative enzymes [52;53]. Additionally, M. tuberculosis siderophore production is markedly increased in response to iron deprivation in culture and in activated macrophages [54]. These siderophores tightly bind available iron and return it to the M. tuberculosis via receptor-mediated internalization [55]. Mutant strains of M. tuberculosis with a targeted deletion of the siderophore biosynthetic machinery are unable to grow in iron-depleted media. Furthermore, these strains exhibit attenuated growth inside THP-1 monocytes, demonstrating the importance of iron acquisition for virulence [56].
Both in vitro and in vivo models consistently have demonstrated that the addition of exogenous iron enhances mycobacterial growth [57-60]. Disruptions in iron metabolism have also been shown to impact mycobacterial infection. β2-microglobulin knockout mice that exhibit tissue iron overload demonstrated enhanced susceptibility to M. tuberculosis infection that could be reversed with iron chelation using lactoferrin [16]. HFE knockout mice with a similar iron phenotype also exhibit enhanced susceptibility to mycobacterial infection [61]. These data appear to contradict the studies mentioned above showing iron-depleted macrophages from HFE patients are resistant to M. tuberculosis growth; however, it is becoming more apparent that there is a complex relationship between iron status and macrophage effector function. Recent work has indicated that HFE knockout mice have a defect in the production of the pro-inflammatory cytokines TNF-α and IL-6 when challenged with Salmonella [62]. The reduction in these cytokines was associated with decreased enterocolitis and enhanced bacterial growth [62]. Additionally, the iron transporters Nramp1 and ferroportin have been shown to influence cytokine production and NO production in macrophages [49;62-65] . Therefore, in vivo, it is important to consider both the nutritional iron stores as well as immune cell function when evaluating disease outcomes.
Clinical studies also have characterized a strong association between iron status and tuberculosis. An early study conducted with iron-deficient Somali nomads demonstrated an increase in infections, including tuberculosis, upon oral iron repletion [66]. Subsequent studies in Africa have confirmed this link. Tuberculosis also has been associated with increased dietary iron [67]. Similar to experimental murine models, tissue iron overload in some African populations (also known as African iron overload) resulting from increased dietary intake of iron or non-HFE hemochromatosis is positively associated with morbidity and mortality from pulmonary tuberculosis [67-70]. Finally, the efficacy of commonly used antimycobacterial drugs is compromised in mice with iron overload [59]. As the threat of drug resistant M. tuberculosis continues to increase, unraveling the relationship between host iron status and tuberculosis susceptibility may reveal new opportunities for therapeutic approaches or interventions.
3.2 Human immunodeficiency virus type 1 (HIV-1)
Approximately 30 million people worldwide are living with chronic HIV-1 infection. Progression of HIV-1 infection into acquired immunodeficiency syndrome (AIDS) accounts for nearly 2 million deaths annually [71]. Since the discovery of the HIV-1 retrovirus in the early 1980s, elucidation of the molecular mechanisms governing the viral lifecycle has demonstrated a requirement for host cell iron during replication.
Transcription of proviral DNA is initiated by the binding of host nuclear factor (NF)-kB to the NF-kB response element located in the transcriptional enhancer region of the HIV-1 5′-long terminal repeat (LTR) [72]. NF-kB is held latent in the cytoplasm through interaction with I-kB. Phosphorylation of I-kB by I-kB kinase targets it for degradation resulting in the translocation of active NF-kB into the nucleus. Treatment of hepatic macrophages with exogenous iron results in the activation of I-kB kinase and, subsequently, upregulation NF-kB-mediated transcription [73]. Accordingly, treatment of persistently infected monocytes with the iron chelator deferoxamine limits HIV-1 reactivation by decreasing NF-kB activity [74]. These and other findings suggest that high iron status may negatively affect infection.
Elongation and translation of HIV-1 transcripts requires virally-encoded transcriptional activator (Tat) and regulator of viral gene expression (Rev) proteins, respectively. Treatment of T-cells with the iron chelator ICL670 reduces Tat and RNA polymerase II phosphorylation through disruption of iron-dependent kinases, resulting in a significant reduction in Tat-mediated transcription [75]. Recent work also has demonstrated that ferroportin over-expression inhibits HIV-1 Tat-mediated proviral transcription from genomic DNA through reduction of intracellular iron [76]. Similarly, iron chelation with ciclopirox and deferiprone prevents maturation of Rev cofactors and subsequently inhibits HIV-1 replication [77].
Similar to bacterial pathogens, HIV-1 has evolved a mechanism to support host cell iron retention. The HIV-1 negative effector (Nef) protein is a virulence factor required for progression to AIDS. Nef has numerous functions including shielding infected cells from immune detection by down-regulating cell surface expression of major histocompatability (MHC) I molecules [72]. Nef also blocks the transport of HFE to the cell surface of cultured THP-1 monocytes. Functional HFE has been shown to increase [78;79] and decrease [80;81] intracellular iron stores in a cell type-dependent manner. Disruption of HFE trafficking by Nef results in iron retention in THP-1 cells as indicated by increased ferritin expression [82]. This increase in intracellular iron was associated with an increase in HIV-1 gag expression, suggesting a beneficial role for the Nef/HFE interaction in viral replication [82]. Additionally, human macrophages from patients with HFE-hemochromatosis were resistant to Nef-mediated iron accumulation [82].
In vitro experimental evidence clearly defines the role of iron in HIV-1 replication. Clinical evidence also suggests an important relationship between host iron status and HIV-1 progression. Anemia has long been described as a sequela of HIV-1 infection that negatively impacts survival [83;84]; however, several recent studies have demonstrated that elevated iron is positively associated with viral load [85] and mortality [86;87]. The fact that genetic polymorphisms in two separate iron regulatory genes (Nramp1 and haptoglobin) are also strong predictors of mortality provides additional support for the role of iron metabolism in HIV-1 infection [88]. In the context of HIV/AIDS, disruption of iron balance in either direction appears to be detrimental, which undoubtedly complicates current therapeutic interventions aimed at correcting anemia. Furthermore, the hypermutability of HIV-1 has contributed to drug resistance and has complicated vaccine efforts. In vitro chelation data suggest that new therapeutic avenues that target iron-dependent host factors may hold promise for combating these issues.
3.3 Malaria
Despite successful control efforts in many sub-tropical countries, P. falciparum still causes upwards of 200 million infections, and nearly 1 million deaths annually, a significant portion of which are children [89]. This vector-borne eukaryotic parasite displays a complex multistage life-cycle involving host hepatocytes and erythrocytes. Iron acquisition by replicating P. falciparum trophozoites involves the proteolysis of hemoglobin within red blood cells that eventually lyse. Activation of the immune system results in further destruction of parasitized and uninfected red blood cells, leading to severe anemia [90]. Recent evidence suggests that this anemia may be exacerbated by IL6-mediated release of hepcidin which reduces circulating iron that would otherwise be available for erythropoiesis [91;92].
Because severe malarial anemia is associated with increased morbidity and mortality, iron supplementation was considered a logical intervention. However, early clinical and experimental studies aimed at characterizing the effect of host iron status on malaria outcomes reported conflicting results. The aforementioned study with Somali nomads found that iron repletion was associated with an increase in P. falciparum parasitemia [66]. A subsequent study in Papua New Guinea demonstrated that infants receiving parenteral iron-dextran had significantly higher malaria positivity rates at 4 and 10 months after injection, when compared to untreated infants [93]. Experimental infection of anemic mice also suggested that iron-deficiency was protective against Plasmodium chabaudi [94]. Iron chelation has been found to improve coma in children with cerebral malaria [95]. Ensuing studies contradicted these results. For example, oral iron supplements improved anemia without any significant impact on susceptibility to malaria in Tanzanian infants [96]. Similarly, no adverse consequences were observed when iron was given to Gambian children with acute malaria [97]. Finally, iron supplementation or deficiency had no impact on Plasmodium bergei infection in rats [98].
More recent results obtained from a powerful study in Pemba, on over 24,000 children, demonstrated that iron supplementation of iron-replete subjects greatly increased risk of malaria-related and non-malarial adverse events, including death [99]. However, subjects that were iron deficient at the beginning of the trial were protected from adverse events [99]. This provides some clarification to the conflicting reports in that baseline iron status rather than supplementation alone significantly contributes to malarial outcomes. On account of this, the investigators in the Pemba trial suggested a re-evaluation of providing blanket iron supplementation to children in areas with high rates of malaria. It is clear that increased awareness of the role that iron sequestration plays during the immune response could greatly impact the burden of this disease.
3.4 Trypanosomatid diseases
Vector-borne trypanosomatid diseases including human African sleeping sickness, Chagas disease, and leishmaniasis are responsible for nearly 30 million infections and 150,000 deaths per year [100]. Iron is essential for trypanosomatid growth and its acquisition is species and lifecycle-dependent [101].
Trypanosoma brucei gambiense and rhodesiense are the causative agents of African trypanosomiasis, with T. b. gambiense accounting for approximately 90% of all cases of sleeping sickness [100]. These parasites display an exclusively extracellular lifecycle, replicating in blood and tissue fluids. T. brucei obtains iron through receptor-mediated endocytosis of host transferrin [101;102]. The T. brucei transferrin receptor is a GPI-anchored heterodimer encoded by expression site-associated genes (ESAG) 6 and 7 [102]. ESAG-6 and ESAG-7 are cotranscribed with the genes encoding the variant surface glycoproteins (VSG). There are approximately 20 VSG expression sites that contain functional ESAG-6 and ESAG-7 genes. T. brucei evades immune detection through the ability to alter cell surface expression of VSG by switching which VSG gene is active. This also allows for expression of variant transferrin receptors [102]. Because these variants have different affinities for transferrin, it has been postulated that expression site switching allows for adaptation to different mammalian host transferrin and thus expansion of the host range [102]. Chelation studies have also demonstrated the importance of iron in T. brucei growth. In vitro treatment of the bloodstream form of T. brucei with lipophilic chelators results in growth inhibition which is most likely due to reduced assimilation of iron into newly synthesized proteins [103;104].
Chagas disease results from infection with Trypanosoma cruzi. This disease is also known as American trypanosomiasis as it is endemic in Central and South America [100]. T. cruzi replicates primarily within the cytoplasm of smooth, cardiac, and skeletal muscle cells [105]. Unlike T. brucei, little is known about the mechanisms of iron acquisition by T. cruzi. In vitro studies have demonstrated transferrin uptake via receptor-mediated endocytosis [106]. This may be a mechanism for iron acquisition in the bloodstream; however, the mechanism(s) governing cytoplasmic iron uptake remain unknown. Despite this, a relationship between host iron status and Chagas disease outcomes clearly exists. Early evidence from a murine model of T. cruzi infection demonstrated an increase in parasitemia and mortality associated with elevated iron stores that could be reversed with chelation [107]. Subsequent cell culture and animal models have confirmed this relationship [108-110]. Furthermore, a recent study has demonstrated enhanced efficacy of benznidazole (a drug of choice for the treatment of Chagas disease) in the presence of the iron chelator desferroxiamine [111].
Leishmaniasis can present as a localized cutaneous infection or a systemic visceral infection. Visceral leishmaniasis is the most deadly form and can be caused by L. donovani, Leishmania infantum, and Leishmania chagasi, depending on geographic location [100]. Leishmania spp. are intracellular parasites that reside in macrophage phagolysosomes. Infection of murine macrophages with L. donovani decreases the cytoplasmic labile iron pool, resulting in enhanced stability of transferrin receptor mRNA and subsequent upregulation of the receptor [112]. This allows for more iron to be delivered to the phagolysosome. Iron released from transferrin in this compartment can be imported via the plasma membrane localized Leishmania iron transporter-1 (LIT-1). This member of the ZIP family of cation transporters is required for replication within the parasitophorous vacuole [113]. However, LIT-1 null mutants can persist within the skin of mice, suggesting an alternate source of iron can be utilized [113]. Recent work has demonstrated that a 46 kDa protein located in the flagellar pocket serves as a hemoglobin receptor [114;115]. Therefore, these parasites are capable of acquiring both non-heme and heme iron to meet their metabolic demands. Manipulation of host iron status through the use of chelators or supplemental iron has produce conflicting outcomes. For example, treatment of mice with desferroxamine for 2 weeks resulted in reduced L. chagasi burden in both the liver and spleen [116]. In contrast, parenteral iron supplementation protected mice against Leishmania major, however this outcome appears to be the result of an enhanced immune response as the oxidative burst was enhanced in the iron treated animals [117].
Despite the obvious relationship between iron and trypanosomatids, there is a dearth of relevant clinical information regarding host iron status and African sleeping sickness, Chagas disease, or leishmaniasis. In fact, these diseases are among the most neglected diseases in the world [100]. As such, there are no vaccines and treatment relies solely on antiquated, and often toxic, chemotherapy [100]. The aforementioned advances in understanding trypanosomatid iron metabolism may provide insight into much needed new therapeutics.
4. Conclusion
Iron is an essential nutrient at the forefront of the battle between the human host and infectious microbes. Research efforts over the last 30 years have significantly advanced our understanding of iron acquisition in pathogens as well as host mechanisms aimed at sequestering iron from these invaders. Presently, there are no effective vaccines for tuberculosis, HIV-1, malaria, or trypanosomatid diseases. Additionally, drug resistance has been described in all of these microbes. As the utility of current chemotherapeutics declines, these diseases will continue to cause significant morbidity and mortality, especially among the impoverished. Therefore, it is imperative to consider new therapeutic interventions such as targeting host or pathogen iron metabolism in order to effectively combat these as well as other microbial killers.
TABLE 2. Key Iron Proteins Involved in Immune Response.
| Protein | Role in Iron Metabolism | Influence on Infection |
|---|---|---|
| Hepcidin | Ligand for iron export protein ferroportin; reduced expression disrupts ferroportin regulation in hemochromatosis |
Regulates efflux of iron from enterocytes and macrophages to promote hypoferremia; activates JAK2 to modulate cytokine response |
| Ferroportin | Membrane exporter of iron; overexpression leads to hyperferremia in hemochromatosis |
Hemochromatosis associated with infections (Vibrio, Yersinia, E. coli) but resistance to intracellular pathogen (Mycobacteria, Salmonella, Legionella) |
| HFE | MHC Class-I like molecule associated with TfRs |
Mutations in HFE are associated with low hepcidin production; most common genetic origin of hemochromatoses |
| Transferrin receptors (TfR1 and TfR2) |
Deliver iron bound to transferrin to cells; sensors of iron status through HFE regulation (primarily in liver) |
Down-regulation during infection reduces endosomal iron to restrict pathogen growth (Legionella, Mycobacteria) |
| Lactoferrin | Present in secretions to bind iron |
Released from neutrophils at infection sites to sequester iron |
| Siderocalin (lipocalin-2 or Ngal) |
Binds catecholate and salicylate type siderophores |
Released from neutrophils and epithelia to sequester iron to limit infections |
| Nramp1 | Divalent metal transporter localized to macrophage phagosomal membrane |
Confers resistance to intraphagosomal pathogens (Mycobacteria, Leishmania, Salmonella) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Eisenbarth SC, Flavell RA. Innate instruction of adaptive immunity revisited: the inflammasome. EMBO Mol. Med. 2009;1:92–98. doi: 10.1002/emmm.200900014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit Rev. Immunol. 2010;30:463–487. doi: 10.1615/critrevimmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Theil EC. Iron homeostasis and nutritional iron deficiency. J.Nutr. 2011;141:724S–728S. doi: 10.3945/jn.110.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem. J. 2011;434:365–381. doi: 10.1042/BJ20101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [8].De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, Daynes RA, Kushner JP, Li D, Ward DM, Kaplan J. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J. Clin. Invest. 2010;120:2395–2405. doi: 10.1172/JCI42011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pietrangelo A. Hereditary hemochromatosis. Biochim. Biophys. Acta. 2006;1763:700–710. doi: 10.1016/j.bbamcr.2006.05.013. [DOI] [PubMed] [Google Scholar]
- [10].Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int. J. Infect. Dis. 2007;11:482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- [11].Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J. Leukoc. Biol. 2007;81:195–204. doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- [12].Chlosta S, Fishman DS, Harrington L, Johnson EE, Knutson MD, Wessling-Resnick M, Cherayil BJ. The iron efflux protein ferroportin regulates the intracellular growth of Salmonella enterica. Infect. Immun. 2006;74:3065–3067. doi: 10.1128/IAI.74.5.3065-3067.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paradkar PN, De Domenico I, Durchfort N, Zohn I, Kaplan J, Ward DM. Iron depletion limits intracellular bacterial growth in macrophages. Blood. 2008;112:866–874. doi: 10.1182/blood-2007-12-126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ward PP, Uribe-Luna S, Conneely OM. Lactoferrin and host defense. Biochem. Cell Biol. 2002;80:95–102. doi: 10.1139/o01-214. [DOI] [PubMed] [Google Scholar]
- [15].Afeltra A, Caccavo D, Ferri GM, Addessi MA, De Rosa FG, Amoroso A, Bonomo L. Expression of lactoferrin on human granulocytes: analysis with polyclonal and monoclonal antibodies. Clin. Exp. Immunol. 1997;109:279–285. doi: 10.1046/j.1365-2249.1997.4351333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 2002;196:1507–1513. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Britigan BE, Hayek MB, Doebbeling BN, Fick RB., Jr. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect. Immun. 1993;61:5049–5055. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–555. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- [19].Schaible UE, Kaufmann SH. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- [20].Ward PP, Mendoza-Meneses M, Park PW, Conneely OM. Stimulus-dependent impairment of the neutrophil oxidative burst response in lactoferrin-deficient mice. Am. J. Pathol. 2008;172:1019–1029. doi: 10.2353/ajpath.2008.061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Borregaard N, Cowland JB. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals. 2006;19:211–215. doi: 10.1007/s10534-005-3251-7. [DOI] [PubMed] [Google Scholar]
- [22].Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- [23].Holmes MA, Paulsene W, Jide X, Ratledge C, Strong RK. Siderocalin (Lcn 2) also binds carboxymycobactins, potentially defending against mycobacterial infections through iron sequestration. Structure. 2005;13:29–41. doi: 10.1016/j.str.2004.10.009. [DOI] [PubMed] [Google Scholar]
- [24].Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- [25].Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Invest. 2007;117:1988–1994. doi: 10.1172/JCI31097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Saiga H, Nishimura J, Kuwata H, Okuyama M, Matsumoto S, Sato S, Matsumoto M, Akira S, Yoshikai Y, Honda K, Yamamoto M, Takeda K. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J. Immunol. 2008;181:8521–8527. doi: 10.4049/jimmunol.181.12.8521. [DOI] [PubMed] [Google Scholar]
- [27].Landro L, Damas JK, Flo TH, Heggelund L, Ueland T, Tjonnfjord GE, Espevik T, Aukrust P, Froland SS. Decreased serum lipocalin-2 levels in human immunodeficiency virus-infected patients: increase during highly active anti-retroviral therapy. Clin.Exp.Immunol. 2008;152:57–63. doi: 10.1111/j.1365-2249.2008.03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, Viltard M, Williams D, Paragas N, Leete T, Kulkarni R, Li X, Lee B, Kalandadze A, Ratner AJ, Pizarro JC, Schmidt-Ott KM, Landry DW, Raymond KN, Strong RK, Barasch J. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat. Chem. Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Devireddy LR, Hart DO, Goetz DH, Green MR. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sengupta S, Tripathi J, Tandon R, Raje M, Roy RP, Basu SK, Mukhopadhyay A. Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J. Biol. Chem. 1999;274:2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- [31].Stijlemans B, Vankrunkelsven A, Brys L, Magez S, De Baetselier P. Role of iron homeostasis in trypanosomiasis-associated anemia. Immunobiology. 2008;213:823–835. doi: 10.1016/j.imbio.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [32].Francis SE, Sullivan DJ, Jr., Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu. Rev. Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- [33].Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- [34].Epiphanio S, Mikolajczak SA, Goncalves LA, Pamplona A, Portugal S, Albuquerque S, Goldberg M, Rebelo S, Anderson DG, Akinc A, Vornlocher HP, Kappe SH, Soares MP, Mota MM. Heme oxygenase-1 is an anti-inflammatory host factor that promotes murine plasmodium liver infection. Cell Host Microbe. 2008;3:331–338. doi: 10.1016/j.chom.2008.04.003. [DOI] [PubMed] [Google Scholar]
- [35].Silva-Herzog E, Detweiler CS. Intracellular microbes and haemophagocytosis. Cell Microbiol. 2008;10:2151–2158. doi: 10.1111/j.1462-5822.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Byrd TF, Horwitz MA. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Invest. 1989;83:1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Byrd TF, Horwitz MA. Regulation of transferrin receptor expression and ferritin content in human mononuclear phagocytes. Coordinate upregulation by iron transferrin and downregulation by interferon gamma. J. Clin. Invest. 1993;91:969–976. doi: 10.1172/JCI116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].ter-Koltunoff M, Goren S, Nousbeck J, Feng CG, Sher A, Ozato K, Azriel A, Levi BZ. Innate immunity to intraphagosomal pathogens is mediated by interferon regulatory factor 8 (IRF-8) that stimulates the expression of macrophage-specific Nramp1 through antagonizing repression by c-Myc. J. Biol. Chem. 2008;283:2724–2733. doi: 10.1074/jbc.M707704200. [DOI] [PubMed] [Google Scholar]
- [39].Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- [40].Clemens DL, Horwitz MA. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, Brech A, Sato S, Husebye H, Cangelosi GA, Akira S, Strong RK, Espevik T, Flo TH. Intracellular Mycobacterium avium intersect transferrin in the Rab11(+) recycling endocytic pathway and avoid lipocalin 2 trafficking to the lysosomal pathway. J. Infect. Dis. 2010;201:783–792. doi: 10.1086/650493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vidal SM, Malo D, Vogan K, Skamene E, Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- [43].Valdez Y, Grassl GA, Guttman JA, Coburn B, Gros P, Vallance BA, Finlay BB. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell Microbiol. 2009;11:351–362. doi: 10.1111/j.1462-5822.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- [44].Blackwell JM, Goswami T, Evans CA, Sibthorpe D, Papo N, White JK, Searle S, Miller EN, Peacock CS, Mohammed H, Ibrahim M. SLC11A1 (formerly NRAMP1) and disease resistance. Cell Microbiol. 2001;3:773–784. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- [46].Soe-Lin S, Apte SS, Andriopoulos B, Jr., Andrews MC, Schranzhofer M, Kahawita T, Garcia-Santos D, Ponka P. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Forbes JR, Gros P. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 2001;9:397–403. doi: 10.1016/s0966-842x(01)02098-4. [DOI] [PubMed] [Google Scholar]
- [48].Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- [49].Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11:1365–1381. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Van Zandt KE, Sow FB, Florence WC, Zwilling BS, Satoskar AR, Schlesinger LS, Lafuse WP. The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J. Leukoc. Biol. 2008;84:689–700. doi: 10.1189/jlb.1107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr. Opin. Immunol. 2008;20:371–376. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- [52].Sturgill-Koszycki S, Schaible UE, Russell DG. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- [53].Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL, Fok AK, Allen RD, Gluck SL, Heuser J, Russell DG. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- [54].Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J. Exp. Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- [56].De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE., III The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cronje L, Edmondson N, Eisenach KD, Bornman L. Iron and iron chelating agents modulate Mycobacterium tuberculosis growth and monocyte-macrophage viability and effector functions. FEMS Immunol. Med. Microbiol. 2005;45:103–112. doi: 10.1016/j.femsim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [58].Lounis N, Truffot-Pernot C, Grosset J, Gordeuk VR, Boelaert JR. Iron and Mycobacterium tuberculosis infection. J. Clin. Virol. 2001;20:123–126. doi: 10.1016/s1386-6532(00)00136-0. [DOI] [PubMed] [Google Scholar]
- [59].Lounis N, Maslo C, Truffot-Pernot C, Grosset J, Boelaert RJ. Impact of iron loading on the activity of isoniazid or ethambutol in the treatment of murine tuberculosis. Int. J. Tuberc. Lung Dis. 2003;7:575–579. [PubMed] [Google Scholar]
- [60].Raghu B, Sarma GR, Venkatesan P. Effect of iron on the growth and siderophore production of mycobacteria. Biochem. Mol. Biol. Int. 1993;31:341–348. [PubMed] [Google Scholar]
- [61].Gomes-Pereira S, Rodrigues PN, Appelberg R, Gomes MS. Increased susceptibility to Mycobacterium avium in hemochromatosis protein HFE-deficient mice. Infect. Immun. 2008;76:4713–4719. doi: 10.1128/IAI.00612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang L, Johnson EE, Shi HN, Walker WA, Wessling-Resnick M, Cherayil BJ. Attenuated inflammatory responses in hemochromatosis reveal a role for iron in the regulation of macrophage cytokine translation. J. Immunol. 2008;181:2723–2731. doi: 10.4049/jimmunol.181.4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- [64].Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur. J. Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- [65].Johnson EE, Sandgren A, Cherayil BJ, Murray M, Wessling-Resnick M. Role of ferroportin in macrophage-mediated immunity. Infect. Immun. 2010;78:5099–5106. doi: 10.1128/IAI.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Murray MJ, Murray AB, Murray MB, Murray CJ. The adverse effect of iron repletion on the course of certain infections. Br. Med. J. 1978;2:1113–1115. doi: 10.1136/bmj.2.6145.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gangaidzo IT, Moyo VM, Mvundura E, Aggrey G, Murphree NL, Khumalo H, Saungweme T, Kasvosve I, Gomo ZA, Rouault T, Boelaert JR, Gordeuk VR. Association of pulmonary tuberculosis with increased dietary iron. J. Infect. Dis. 2001;184:936–939. doi: 10.1086/323203. [DOI] [PubMed] [Google Scholar]
- [68].Moyo VM, Gangaidzo IT, Gordeuk VR, Kiire CF, Macphail AP. Tuberculosis and iron overload in Africa: a review. Cent. Afr. J. Med. 1997;43:334–339. [PubMed] [Google Scholar]
- [69].Gordeuk VR, McLaren CE, Macphail AP, Deichsel G, Bothwell TH. Associations of iron overload in Africa with hepatocellular carcinoma and tuberculosis: Strachan’s 1929 thesis revisited. Blood. 1996;87:3470–3476. [PubMed] [Google Scholar]
- [70].Gordeuk V, Mukiibi J, Hasstedt SJ, Samowitz W, Edwards CQ, West G, Ndambire S, Emmanual J, Nkanza N, Chapanduka Z, Randall M, Boone P, Romano P, Martell RW, Yamashita Y, Effler P, Brittenham G. Iron overload in Africa. Interaction between a gene and dietary iron content. N. Engl. J. Med. 1992;326:95–100. doi: 10.1056/NEJM199201093260204. [DOI] [PubMed] [Google Scholar]
- [71].Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N. Engl. J. Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Greene WC, Peterlin BM. Charting HIV’s remarkable voyage through the cell: Basic science as a passport to future therapy. Nat. Med. 2002;8:673–680. doi: 10.1038/nm0702-673. [DOI] [PubMed] [Google Scholar]
- [73].Chen L, Xiong S, She H, Lin SW, Wang J, Tsukamoto H. Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IkappaB kinase in hepatic macrophages. J. Biol. Chem. 2007;282:5582–5588. doi: 10.1074/jbc.M609273200. [DOI] [PubMed] [Google Scholar]
- [74].Sappey C, Boelaert JR, Legrand-Poels S, Forceille C, Favier A, Piette J. Iron chelation decreases NF-kappa B and HIV type 1 activation due to oxidative stress. AIDS Res. Hum. Retroviruses. 1995;11:1049–1061. doi: 10.1089/aid.1995.11.1049. [DOI] [PubMed] [Google Scholar]
- [75].Debebe Z, Ammosova T, Jerebtsova M, Kurantsin-Mills J, Niu X, Charles S, Richardson DR, Ray PE, Gordeuk VR, Nekhai S. Iron chelators ICL670 and 311 inhibit HIV-1 transcription. Virology. 2007;367:324–333. doi: 10.1016/j.virol.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xu M, Kashanchi F, Foster A, Rotimi J, Turner W, Gordeuk VR, Nekhai S. Hepcidin induces HIV-1 transcription inhibited by ferroportin. Retrovirology. 2010;7:104. doi: 10.1186/1742-4690-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hoque M, Hanauske-Abel HM, Palumbo P, Saxena D, D’Alliessi GD, Park MH, Pe’ery T, Mathews MB. Inhibition of HIV-1 gene expression by Ciclopirox and Deferiprone, drugs that prevent hypusination of eukaryotic initiation factor 5A. Retrovirology. 2009;6:90. doi: 10.1186/1742-4690-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Davies PS, Enns CA. Expression of the hereditary hemochromatosis protein HFE increases ferritin levels by inhibiting iron export in HT29 cells. J. Biol. Chem. 2004;279:25085–25092. doi: 10.1074/jbc.M400537200. [DOI] [PubMed] [Google Scholar]
- [79].Drakesmith H, Sweetland E, Schimanski L, Edwards J, Cowley D, Ashraf M, Bastin J, Townsend AR. The hemochromatosis protein HFE inhibits iron export from macrophages. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15602–15607. doi: 10.1073/pnas.242614699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gross CN, Irrinki A, Feder JN, Enns CA. Co-trafficking of HFE, a nonclassical major histocompatibility complex class I protein, with the transferrin receptor implies a role in intracellular iron regulation. J. Biol. Chem. 1998;273:22068–22074. doi: 10.1074/jbc.273.34.22068. [DOI] [PubMed] [Google Scholar]
- [82].Drakesmith H, Chen N, Ledermann H, Screaton G, Townsend A, Xu XN. HIV-1 Nef down-regulates the hemochromatosis protein HFE, manipulating cellular iron homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11017–11022. doi: 10.1073/pnas.0504823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lundgren JD, Mocroft A. Anemia and survival in human immunodeficiency virus. Clin. Infect. Dis. 2003;37(Suppl 4):S297–S303. doi: 10.1086/376909. [DOI] [PubMed] [Google Scholar]
- [84].Moore RD. Human immunodeficiency virus infection, anemia, and survival. Clin. Infect. Dis. 1999;29:44–49. doi: 10.1086/520178. [DOI] [PubMed] [Google Scholar]
- [85].Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Madsen PH, Michaelsen KF. Iron, haptoglobin phenotype, and HIV-1 viral load: a cross-sectional study among pregnant Zimbabwean women. J. Acquir. Immune Defic. Syndr. 2003;33:74–81. doi: 10.1097/00126334-200305010-00011. [DOI] [PubMed] [Google Scholar]
- [86].Gordeuk VR, Onojobi G, Schneider MF, Dawkins FW, Delapenha R, Voloshin Y, von, V W, Bacon M, Minkoff H, Levine A, Cohen M, Greenblatt RM. The association of serum ferritin and transferrin receptor concentrations with mortality in women with human immunodeficiency virus infection. Haematologica. 2006;91:739–743. [PubMed] [Google Scholar]
- [87].McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J. Acquir. Immune. Defic. Syndr. 2007;46:498–507. doi: 10.1097/qai.0b013e31815b2d4b. [DOI] [PubMed] [Google Scholar]
- [88].McDermid JM, van der Loeff MF, Jaye A, Hennig BJ, Bates C, Todd J, Sirugo G, Hill AV, Whittle HC, Prentice AM. Mortality in HIV infection is independently predicted by host iron status and SLC11A1 and HP genotypes, with new evidence of a gene-nutrient interaction. Am. J. Clin. Nutr. 2009;90:225–233. doi: 10.3945/ajcn.2009.27709. [DOI] [PubMed] [Google Scholar]
- [89].Hafalla JC, Silvie O, Matuschewski K. Cell biology and immunology of malaria. Immunol. Rev. 2011;240:297–316. doi: 10.1111/j.1600-065X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- [90].Nweneka CV, Doherty CP, Cox S, Prentice A. Iron delocalisation in the pathogenesis of malarial anaemia. Trans. R. Soc. Trop. Med. Hyg. 2010;104:175–184. doi: 10.1016/j.trstmh.2009.08.007. [DOI] [PubMed] [Google Scholar]
- [91].de Mast Q, van Dongen-Lases EC, Swinkels DW, Nieman AE, Roestenberg M, Druilhe P, Arens TA, Luty AJ, Hermsen CC, Sauerwein RW, van der Ven AJ. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br. J. Haematol. 2009;145:657–664. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- [92].de Mast Q, Nadjm B, Reyburn H, Kemna EH, Amos B, Laarakkers CM, Silalye S, Verhoef H, Sauerwein RW, Swinkels DW, van der Ven AJ. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J. Infect. Dis. 2009;199:253–262. doi: 10.1086/595790. [DOI] [PubMed] [Google Scholar]
- [93].Oppenheimer SJ, Gibson FD, Macfarlane SB, Moody JB, Harrison C, Spencer A, Bunari O. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 1986;80:603–612. doi: 10.1016/0035-9203(86)90154-9. [DOI] [PubMed] [Google Scholar]
- [94].Harvey PW, Bell RG, Nesheim MC. Iron deficiency protects inbred mice against infection with Plasmodium chabaudi. Infect. Immun. 1985;50:932–934. doi: 10.1128/iai.50.3.932-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gordeuk V, Thuma P, Brittenham G, McLaren C, Parry D, Backenstose A, Biemba G, Msiska R, Holmes L, McKinley E, Vargas L, Gilkeson R, Poltera AA. Effect of iron chelation therapy on recovery from deep coma in children with cerebral malaria. N. Engl. J. Med. 1992;327:1473–1477. doi: 10.1056/NEJM199211193272101. [DOI] [PubMed] [Google Scholar]
- [96].Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, Acosta CJ, Schellenberg DM, Galindo CM, Kimario J, Urassa H, Brabin B, Smith TA, Kitua AY, Tanner M, Alonso PL. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- [97].van Hensbroek MB, Morris-Jones S, Meisner S, Jaffar S, Bayo L, Dackour R, Phillips C, Greenwood BM. Iron, but not folic acid, combined with effective antimalarial therapy promotes haematological recovery in African children after acute falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1995;89:672–676. doi: 10.1016/0035-9203(95)90438-7. [DOI] [PubMed] [Google Scholar]
- [98].Cardoso MA, Ferreira MU, Ribeiro GS, Penteado MD, ndrade Junior HF. Dietary iron supplementation does not aggravate experimental malaria in young rats. J. Nutr. 1996;126:467–475. doi: 10.1093/jn/126.2.467. [DOI] [PubMed] [Google Scholar]
- [99].Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, Dhingra U, Kabole I, Deb S, Othman MK, Kabole FM. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367:133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- [100].Nussbaum K, Honek J, Cadmus CM, Efferth T. Trypanosomatid parasites causing neglected diseases. Curr. Med. Chem. 2010;17:1594–1617. doi: 10.2174/092986710790979953. [DOI] [PubMed] [Google Scholar]
- [101].Taylor MC, Kelly JM. Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology. 2010;137:899–917. doi: 10.1017/S0031182009991880. [DOI] [PubMed] [Google Scholar]
- [102].Gerrits H, Mussmann R, Bitter W, Kieft R, Borst P. The physiological significance of transferrin receptor variations in Trypanosoma brucei. Mol. Biochem. Parasitol. 2002;119:237–247. doi: 10.1016/s0166-6851(01)00417-0. [DOI] [PubMed] [Google Scholar]
- [103].Breidbach T, Scory S, Krauth-Siegel RL, Steverding D. Growth inhibition of bloodstream forms of Trypanosoma brucei by the iron chelator deferoxamine. Int. J. Parasitol. 2002;32:473–479. doi: 10.1016/s0020-7519(01)00310-1. [DOI] [PubMed] [Google Scholar]
- [104].Merschjohann K, Steverding D. In vitro growth inhibition of bloodstream forms of Trypanosoma brucei and Trypanosoma congolense by iron chelators. Kinetoplastid. Biol. Dis. 2006;5:3. doi: 10.1186/1475-9292-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, Rodrigues MM, Gazzinelli RT. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert. Rev. Mol. Med. 2010;12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- [106].Lima MF, Villalta F. Trypanosoma cruzi receptors for human transferrin and their role. Mol. Biochem. Parasitol. 1990;38:245–252. doi: 10.1016/0166-6851(90)90027-j. [DOI] [PubMed] [Google Scholar]
- [107].Lalonde RG, Holbein BE. Role of iron in Trypanosoma cruzi infection of mice. J. Clin. Invest. 1984;73:470–476. doi: 10.1172/JCI111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Pedrosa ML, Silva ME, Silva ME, Silva ME, Nicoli JR, Vieira EC. The effect of iron deficiency and iron overload on the evolution of Chagas disease produced by three strains of Trypanosoma cruzi in CFW mice. Comp Biochem. Physiol. A: Physiol . 1990;97:235–243. doi: 10.1016/0300-9629(90)90178-u. [DOI] [PubMed] [Google Scholar]
- [109].Loo VG, Lalonde RG. Role of iron in intracellular growth of Trypanosoma cruzi. Infect. Immun. 1984;45:726–730. doi: 10.1128/iai.45.3.726-730.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Arantes JM, Pedrosa ML, Martins HR, Veloso VM, de LM, Bahia MT, Tafuri WL, Carneiro CM. Trypanosoma cruzi: treatment with the iron chelator desferrioxamine reduces parasitemia and mortality in experimentally infected mice. Exp. Parasitol. 2007;117:43–50. doi: 10.1016/j.exppara.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [111].Francisco AF, de, V A, Arantes JM, Pedrosa ML, Martins HR, Silva M, Veloso VM, de LM, Bahia MT, Tafuri WL, Carneiro CM. Trypanosoma cruzi: effect of benznidazole therapy combined with the iron chelator desferrioxamine in infected mice. Exp. Parasitol. 2008;120:314–319. doi: 10.1016/j.exppara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [112].Das NK, Biswas S, Solanki S, Mukhopadhyay CK. Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cell Microbiol. 2009;11:83–94. doi: 10.1111/j.1462-5822.2008.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J. Exp. Med. 2006;203:2363–2375. doi: 10.1084/jem.20060559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Carvalho S, Cruz T, Santarem N, Castro H, Costa V, Tomas AM. Heme as a source of iron to Leishmania infantum amastigotes. Acta Trop. 2009;109:131–135. doi: 10.1016/j.actatropica.2008.10.007. [DOI] [PubMed] [Google Scholar]
- [115].Krishnamurthy G, Vikram R, Singh SB, Patel N, Agarwal S, Mukhopadhyay G, Basu SK, Mukhopadhyay A. Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J. Biol. Chem. 2005;280:5884–5891. doi: 10.1074/jbc.M411845200. [DOI] [PubMed] [Google Scholar]
- [116].Malafaia G, Marcon LN, Pereira LF, Pedrosa ML, Rezende SA. Leishmania chagasi: effect of the iron deficiency on the infection in BALB/c mice. Exp. Parasitol. 2011;127:719–723. doi: 10.1016/j.exppara.2010.11.010. [DOI] [PubMed] [Google Scholar]
- [117].Bisti S, Konidou G, Boelaert J, Lebastard M, Soteriadou K. The prevention of the growth of Leishmania major progeny in BALB/c iron-loaded mice: a process coupled to increased oxidative burst, the amplitude and duration of which depend on initial parasite developmental stage and dose. Microbes Infect. 2006;8:1464–1472. doi: 10.1016/j.micinf.2006.01.014. [DOI] [PubMed] [Google Scholar]