Abstract
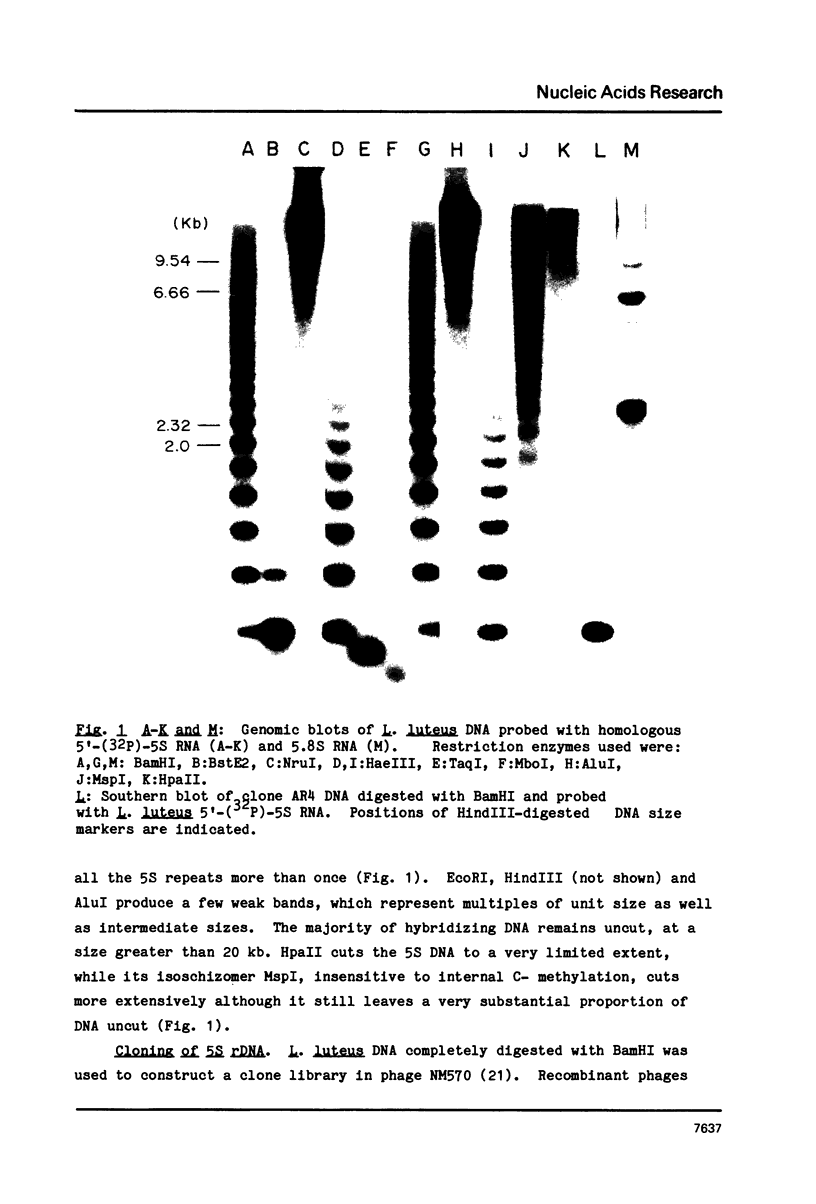
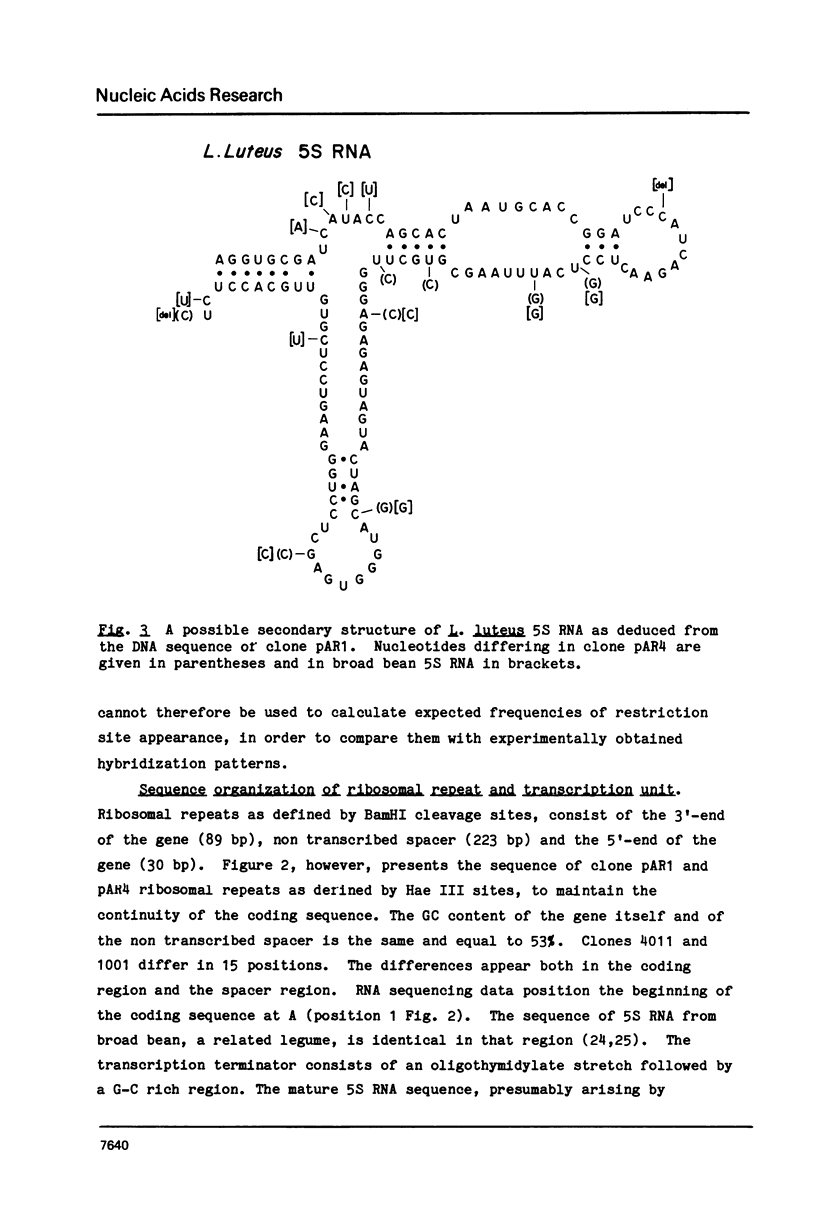
Genomic blots of yellow lupin (Lupinus luteus) DNA digested with restriction nucleases and probed with 32P-labelled Lupinus 5S RNA reveal that 5S DNA is organized as tandemly repeated sequences of one size class, 342 bp. The DNA is extensively methylated. Two cloned BamHI ribosomal repeats were sequenced, revealing sequence divergence within both the coding and spacer regions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barciszewski J., Joachimiak A., Rafalski A., Barciszewska M., Twardowski T., Wiewiórowski M. Conservation of the structures of plant tRNAs and aminoacyl-tRNA synthetases. FEBS Lett. 1979 Jun 1;102(1):194–197. doi: 10.1016/0014-5793(79)80958-8. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Backman K. Plasmids of Escherichia coli as cloning vectors. Methods Enzymol. 1979;68:245–267. doi: 10.1016/0076-6879(79)68018-7. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Castagnoli L., Melton D. A., Cortese R. Promoter of a eukaryotic tRNAPro gene is composed of three noncontiguous regions. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1195–1199. doi: 10.1073/pnas.79.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delihas N., Andersen J., Sprouse H. M., Kashdan M., Dudock B. The nucleotide sequence of spinach cytoplasmic 5 S ribosomal RNA. J Biol Chem. 1981 Jul 25;256(14):7515–7517. [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S RNA sequences and their precursors. Nucleic Acids Res. 1982 Jan 22;10(2):r93–115. doi: 10.1093/nar/10.2.762-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach W. L., Dyer T. A. Sequence organization of the repeating units in the nucleus of wheat which contain 5S rRNA genes. Nucleic Acids Res. 1980 Nov 11;8(21):4851–4865. doi: 10.1093/nar/8.21.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbrough P. B., Ellis T. H., Cullis C. A. Organisation of the 5S RNA genes in flax. Nucleic Acids Res. 1981 Nov 25;9(22):5895–5904. doi: 10.1093/nar/9.22.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbrough P. B., Ellis T. H., Lomonossoff G. P. Sequence variation and methylation of the flax 5S RNA genes. Nucleic Acids Res. 1982 Aug 11;10(15):4501–4514. doi: 10.1093/nar/10.15.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H., Kressman A., Birnstiel M. L. A split promoter for a eucaryotic tRNA gene. Cell. 1981 May;24(2):573–585. doi: 10.1016/0092-8674(81)90348-2. [DOI] [PubMed] [Google Scholar]
- Hori H., Osawa S. Evolutionary change in 5S RNA secondary structure and a phylogenic tree of 54 5S RNA species. Proc Natl Acad Sci U S A. 1979 Jan;76(1):381–385. doi: 10.1073/pnas.76.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein B., Murray K. Phage lambda receptor chromosomes for DNA fragments made with restriction endonuclease I of Bacillus amyloliquefaciens H. J Mol Biol. 1979 Sep 15;133(2):289–294. doi: 10.1016/0022-2836(79)90537-0. [DOI] [PubMed] [Google Scholar]
- Korn L. J. Transcription of Xenopus 5S ribosomal RNA genes. Nature. 1982 Jan 14;295(5845):101–105. doi: 10.1038/295101a0. [DOI] [PubMed] [Google Scholar]
- Mascia P. N., Rubenstein I., Phillips R. L., Wang A. S., Xiang L. Z. Localization of the 5S rRNA genes and evidence for diversity in the 5S rDNA region of maize. Gene. 1981 Oct;15(1):7–20. doi: 10.1016/0378-1119(81)90099-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Corry M. J., Dyer T. A. Nucleotide sequence analysis of the cytoplasmic 5S ribosomal ribonucleic acid from five species of flowering plants. Biochem J. 1973 Dec;135(4):845–851. doi: 10.1042/bj1350845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne P. I., Dyer T. A. Evidence for the nucleotide sequence of 5-S rRNA from the flowering plant Secale cereale (Rye). Eur J Biochem. 1976 Dec;71(1):33–38. doi: 10.1111/j.1432-1033.1976.tb11086.x. [DOI] [PubMed] [Google Scholar]
- Rafalski A. J., Barciszewski J., Gulewicz K., Twardowski T., Keith G. Nucleotide sequence of tRNAPhe from the seeds of lupin (Lupinus luteus). Comparison of the major species with wheat germ tRNAPhe. Acta Biochim Pol. 1977;24(4):301–318. [PubMed] [Google Scholar]
- Roe B. A. Studies on human tRNA. I. The rapid, large scale isolation and partial fractionation of placenta and liver tRNA. Nucleic Acids Res. 1975 Jan;2(1):21–42. doi: 10.1093/nar/2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Dingermann T., Farrell P., Söll D. Internal control regions for transcription of eukaryotic tRNA genes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6657–6661. doi: 10.1073/pnas.78.11.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]