Abstract
Methamphetamine (MA) increases dopamine (DA) levels within the mesolimbic pathway and acetylcholine (ACh), a neurotransmitter known to increase DA cell firing and release and mediate reinforcement, within the ventral tegmental area (VTA). The laterodorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei provide cholinergic input to the VTA; however, the contribution of LDT- and PPT-derived ACh to MA-induced DA and ACh levels and locomotor activation remains unknown. The first experiment examined the role of LDT-derived ACh in MA locomotor activation by reversibly inhibiting these neurons with bilateral intra-LDT microinjections of the M2 receptor agonist oxotremorine (OXO). Male C57BL/6 J mice were given a bilateral 0.1 µl OXO (0, 1, or 10 nM/side) microinjection immediately prior to IP saline or MA (2 mg/kg). The highest OXO concentration significantly inhibited both saline-and MA-primed locomotor activity. In a second set of experiments we characterized the individual contributions of ACh originating in the LDT or pedunculopontine tegmental nucleus (PPT) to MA-induced levels of ACh and DA by administering intra-LDT or PPT OXO and performing in vivo microdialysis in the VTA and NAc. Intra-LDT OXO dose-dependently attenuated the MA-induced increase in ACh within the VTA but had no effect on DA in NAc. Intra-PPT OXO had no effect on ACh or DA levels within the VTA or NAc, respectively. We conclude that LDT, but not PPT, ACh is important in locomotor behavior and the cholinergic, but not dopaminergic, response to systemic MA.
Keywords: In vivo microdialysis, Locomotor activity, Dopamine, Methamphetamine
1. Introduction
The mesopontine tegmentum, which contains the laterodorsal (LDT) and pedunculopontine tegmentum (PPT), is a neurochemically and functionally diverse structure that is important for the interpretation of sensory input and selection of an appropriate motor output [1,2]. The LDT and PPT provide the only known cholinergic projections to midbrain dopamine (DA) neurons in the substantia nigra (SN) and ventral tegmental area (VTA). Specifically, the cholinergic projection from the LDT preferentially targets VTA-DA neurons and GABA neurons within the VTA [3,4], while projections from the PPT primarily target DA neurons within the SN. Previous research has used electrical and pharmacological manipulation of the LDT-to-VTA acetylcholine (ACh) projection to assess its contribution to DA neuron firing and extracellular DA levels in the mesolimbic pathway [5–7]. By measuring the DA oxidation current within the nucleus accumbens (NAc) and DA neuron firing in the VTA, this research suggests LDT ACh can increase DA neuron firing and release through its actions on muscarinic M5-type and nicotinic ACh receptors located on DA neurons in the VTA [for a review see 8]. Furthermore, specific targeting of the PPT [9] or LDT [10] cholinergic projection has implicated mesopontine ACh in exploratory behavior, spontaneous locomotion and the rewarding value of intra-cranial self-stimulation (ICSS) [9]. In addition, application of cholinergic agonists and antagonists in the VTA suggests that nicotinic and muscarinic ACh receptors on DA neurons in the VTA affect ICSS and extracellular DA levels in terminal regions of the mesolimbic pathway, as measured by microdialysis [11–13]. Taken together, these data suggest that mesopontine cholinergic projections to the VTA could be a key contributor to mesolimbic DA responses and DA-mediated reinforcement.
Cholinergic modulation of DA responding is one mechanism drugs of abuse can use to increase DA levels within the mesolimbic pathway [13,14]. Methamphetamine (MA), a highly addictive psychostimulant, is one such drug that induces DA release in terminal regions such as the NAc and at the cell bodies in the VTA [15–18]. In addition, recent evidence suggests that MA also induces a prolonged increase in ACh levels within the VTA [18]; however, it is unclear whether this MA-induced increase in ACh contributes to increased DA levels within NAc or MA-related behaviors, such as locomotor activation. It is well known that acute administration of MA induces robust locomotor activation [17] and a recent study in C57BL/6 J mice found brain MA concentration to be correlated with locomotor activity [19]. Indeed, it has been hypothesized that a common neural substrate may underlie the rewarding and locomotor activating effects of drugs of abuse [20] and recent evidence suggests that mesolimbic DA may mediate the stimulating and rewarding effects of MA [21,22]. Thus, MA-induced locomotor activity is a measurable behavior that may be an analog of the rewarding effects of MA. In addition, the cholinergic projection to the mesolimbic pathway from the PPT and LDT has been implicated in reward-related behavior and locomotor behavior. However, little is known about the role of ACh in MA-related behaviors and neurochemical effects. The present study evaluated the contribution of LDT-derived ACh in MA- (or saline) induced locomotor activity. A separate set of experiments used in vivo microdialysis to clarify whether the source of ACh primarily originated in the LDT and/or the PPT and whether the resulting intra-VTA cholinergic tone contributes to MA-induced increases in NAc DA levels.
In order to test this, we reversibly inhibited cholinergic neurons in the LDT or PPT via a bilateral microinjection of the muscarinic ACh receptor agonist oxotremorine sesquifumarate (OXO). OXO preferentially binds to M2-type ACh receptors, which are inhibitory autoreceptors that activate a TTX-insensitive hyperpolarization thereby inhibiting neuronal activity and terminal ACh release. We hypothesized that reversible inhibition of LDT-, but not PPT-, derived ACh would attenuate MA-induced levels of ACh and DA in the VTA and NAc, respectively. We also hypothesized that inhibition of LDT derived ACh would attenuate MA-induced locomotor behavior.
2. Methods
2.1. Subjects
Male C57BL/6 J mice between 8 and 13 weeks old (Jackson Laboratories, Sacramento, CA) were used in all five experiments. Experiment 1 consisted of 39 experimentally naïve male mice, 19 of which were subsequently used in Experiment 2 after a 3-week wash-out period. An additional 15 experimentally naïve male C57BL/6 J mice were added to finish Experiment 2, for a total of 31 mice used in Experiment 2. Experiments 3, 4 and 5 all consisted of 10, 16 and 13 experimentally naïve male mice, respectively. All animals were maintained in a climate and humidity controlled vivarium on a 12 h:12 h light–dark cycle with lights on at 0600 h. Animals were allowed ad libitum access to food and water while in their home cage. All experiments were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee and carried out in accordance with the guidelines set forth by the National Research Council of the National Academies [23].
2.2. Drugs
MA hydrochloride was provided by the National Institutes on Drug Abuse drug supply program (Research Triangle Park, NC). Mice received an intraperitoneal (IP) injection of either 2 or 3.5 mg/kg MA in the locomotor or microdialysis experiments, respectively. MA doses were selected by their ability to stimulate an intermediate increase in locomotor activation or ACh levels within the VTA while not producing a floor or ceiling effect [18]. MA was dissolved in 0.9% saline and administered in a volume of 0.1 ml/25 g. As the control, IP saline was injected in a volume of 0.1 ml/25 g. Oxotremorine sesquifumurate (Sigma, St. Louis, MO) was dissolved in artificial cereberospinofluid (aCSF) to 1, 5 or 10 nM for bilateral microinfusions of 0.1 µl/side into the LDT or PPT. These concentrations are expressed as the salt and were selected based on competition binding studies in the human and rat pons (OXO for the M2 receptor subtype: Kd = 1.45 nM, Bmax = 14,093 cpm) [24,25] and from previous experiments in the lab administering intra-LDT OXO microinjections in the rat. The aCSF consisted of (in mM): 128 NaCl, 3.9 KCl, 1.2 CaCl2, 1.0 MgCl2, 25 NaHCO3, 0.3 NaH2PO4 pH 7.3.
2.3. Surgery
Mice were placed under isoflurane anesthesia and stereotaxically implanted with bilateral 6.5 mm long, 23-gauge thin wall guide shafts positioned above the LDT (AP: −4.96, ML: ±0.65, DV: −1. 5) or the PPT (AP: −4.72, ML:±1.25, DV: −1.75). In the same surgery, mice were also implanted with a unilateral 5 mm long 21-gauge guide shaft positioned above the VTA (AP: −2.92, ML: ±0.6, DV: −1.3; Experiments 2 and 4) or the NAc (AP: +1.1, ML: ±0.8, DV: −1.5; Experiments 3 and 5). Coordinates were selected from the atlas of Paxinos and Franklin [26]. A stylet was kept in the guide shafts until the time of testing. Subcutaneous saline and an analgesic (carprofen; 0.5 mg/kg) were administered at the beginning of the surgery. Mice were singly housed following surgery to prevent damage to head-mounts and allowed one week to recover prior to experimental testing.
2.4. Microinjection procedure
The procedure for bilateral microinjections was adapted from experiments in our lab using rats and mice [27,28]. Briefly, microinjectors were constructed from fused silica glass tubing (150 µm od × 75 µm id) protruding 0.5 or 2.5 mm from the end of a 28-gauge stainless steel guide to reach the target nuclei of the inferior colliculus (IC) or the LDT, respectively. The shorter microinjector was used in the locomotor experiment in order to target the IC. Bilateral microinjections into the IC were made as an anatomical control to assess the potential effect of OXO diffusing up the injector tract on locomotor activity. Microinjections were delivered over 60 s at a rate of 1.67 nl/s for a total volume of 100 nl per side. Injectors remained in place for 30 s following the injection.
2.5. Apparatus
2.5.1. Locomotor activity
Locomotor activity was assessed in a rectangular (30.5 cm × 15.2 cm) clear Plexiglas chamber with a paper floor housed in a sound attenuating cabinet. An infrared activity camera (Coulbourn Instruments, H24-61) mounted 18 cm above the arena recorded all activity during the trial. Horizontal activity data were recorded in the dark as total distance (cm) moved using Ethovision (Noldus, Tacoma, WA) tracking software.
2.5.2. Microdialysis
Mice were housed in a cylindrical clear polycarbonate chamber (38 cm × 27 cm; Instech Laboratories, Plymouth Meeting, PA) in a sound-attenuated enclosure during in vivo microdialysis. A fiber optic lamp on the lowest setting was positioned 30 cm above the polycarbonate chamber and provided illumination during the experiment. A counter-balanced arm held a single channel swivel extended over the middle of the chamber. A Hamilton syringe (Glenco, 2.5 cc gastight; Sigma, St. Louis, MO) was fitted onto a pump (Razel A99; Braintree Inc, Stamford, CT), which delivered buffered dialysis ringer (142 mM NaCl, 3.9 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 1.35 mM Na2HPO4, 0.3 mM NaH2PO4; pH 7.3) via PE20 tubing through the swivel to the microdialysis probe at a rate of 1 µl/min. In Experiments 1 and 3 the acetylcholinesterase inhibitor neostigmine (0.3 µM neostigmine·Br) was added to the dialysis ringer to delay the breakdown of ACh before it could diffuse across the dialysis membrane and be collected.
2.6. Behavioral procedure
Locomotor activity and measurements of ACh and DA were performed in separate experiments because previous experiments in our lab have found that timed collection of dialysis samples can alter an animal’s behavior. In addition, we have noted that ACh levels within the VTA are extremely sensitive to an animal’s wakefulness and activity level. Therefore, since measuring ACh and DA could affect an animal’s activity (which, in turn, would artificially alter transmitter levels) we decided to perform these measures separately.
2.6.1. Locomotor activity
Locomotor activity was tested over 3 consecutive days. Day 1 served as habituation to handling, injection procedures and the testing apparatus. Day 2 established baseline locomotor activity for all animals. On the first 2 days the microinjection and IP injection administered were aCSF and saline, respectively. Day 3 was the test day, in which mice were administered an OXO microinjection (0.1 µl of 0, 1 or 10 nM per side) followed by an IP MA injection (0 or 2 mg/kg).
Each day, animals were brought into the testing room, weighed and their stylets were removed. Mice were then returned to their home cage to acclimate for 45 min prior to testing. After acclimation, each animal was lightly restrained, given a bilateral microinjection immediately followed by an IP injection and went into the locomotor arena. Activity was recorded for 30 min. At the end of the session, the animals were removed from the testing chamber and returned to their home cage.
2.6.2. In vivo microdialysis
Approximately 14 h before testing, a 7.5 mm long microdialysis probe was inserted into the guide shaft that extended 2.5 mm into the VTA or NAc. This allowed time for tissue trauma to dissipate and for the mouse to habituate to the testing chamber. Microdialysis probes were constructed as described previously [18] and had a 0.75 or 1.0 mm long active membrane tip for use in the VTA or NAc, respectively. The microdialysis procedure for measuring DA and ACh was performed as previously described [18], with the exception that neostigmine was not added to the dialysis ringer when collecting dialysates for DA. Neostigmine was removed for the DA analysis since it is only necessary to prevent the breakdown of ACh and may artificially inflate DA levels [29].
Baseline samples were collected every 20 min for 1 h and analyzed for ACh or DA content using high performance liquid chromatography (HPLC) or liquid-chromatography tandem mass spectrometry (LC–MS/MS). Following baseline sampling, each mouse was given a bilateral microinjection into the LDT or PPT, immediately followed by an intraperitoneal (IP) saline injection (0.1 ml per 25 g). Samples were taken every 20 min for 80 min total. Mice were then administered a second bilateral microinjection into the LDT or PPT followed by an IP MA injection (3.5 mg/kg). Samples were taken every 20 min for up to 4 h until ACh or DA levels returned to baseline.
2.7. Experiment 1: Locomotor activity following MA and LDT/IC microinjection
The purpose of this experiment was to assess the role of LDT ACh on MA-induced locomotor activity. Each mouse experienced two bouts of the 3-day locomotor testing procedure, for a total of 6 testing days. During one testing bout the bilateral microinjection was administered into the LDT, while during the other bout a bilateral microinjection was administered into a dorsal control site, the inferior colliculus (IC). Thus, each mouse served as its own control for the dorsal diffusion of the drug. There was a 2 week wash-out period between the testing bouts. Mice were counterbalanced for microinjection site, so that half of the mice received the IC microinjection in the first testing bout. On the test day (day 3) each mouse only received one OXO concentration (0.1 µl of 0, 1, or 10 nM per side) and one MA dose (0 or 2 mg/kg). This dose remained the same for each testing bout.
2.8. Experiment 2: Intra-VTA in vivo microdialysis for ACh following intra-LDT OXO
The goal of this experiment was to determine whether the LDT was the source of the MA-induced increases in intra-VTA ACh. In vivo microdialysis was performed within the VTA and dialysates were collected and assessed for ACh content.
After establishing a stable baseline of ACh levels each mouse received a control injection. This consisted of a bilateral intra-LDT microinjection (0.1 µl per side) of aCSF or OXO (1, 5, or 10 nM) immediately followed by an IP saline injection. The control injection served to assess the effect of intra-LDT OXO on basal VTA ACh levels and the effect of handling and injection on ACh levels. A total of 80 min after the control injection each mouse received a test injection, thus each mouse served as its own control. This consisted of a bilateral intra-LDT microinjection immediately followed by IP MA (3.5 mg/kg). Each mouse only received one dose of OXO and this dose was the same during the control and test injections.
2.9. Experiment 3: Intra-VTA in vivo microdialysis for ACh following intra-PPT OXO
The purpose of this experiment was to determine whether the PPT was the involved in the MA-induced increases in intra-VTA ACh. In vivo microdialysis was performed within the VTA just as in Experiment 2. Each mouse received a control injection after baseline ACh levels were established. The control injection included a bilateral intra-PPT microinjection (0.1 µl per side) of aCSF or OXO (1 or 10 nM) immediately followed by an IP saline injection. A total of 80 min after the control injection each mouse received a test injection, which included a bilateral intra-PPT microinjection immediately followed by IP MA (3.5 mg/kg). Just as in Experiment 2, each mouse only received one dose of OXO and this dose was the same during the control and test injections.
2.10. Experiment 4: Intra-NAc in vivo microdialysis for DA following intra-LDT OXO
This experiment examined whether LDT-derived ACh influenced MA-induced increases in DA levels within the NAc. In vivo microdialysis was performed within the NAc and dialysate samples were collected and analyzed for DA content. Each mouse received a control injection after baseline ACh levels were established, which consisted of a bilateral intra-LDT microinjection (0.1 µl per side) of aCSF or OXO (1 or 10 nM per side) immediately followed by an IP saline injection. Eighty min following the control injection each mouse received a test injection. This consisted of a bilateral intra-LDT microinjection immediately followed by IP MA (3.5 mg/kg). Just as in Experiments 2 and 3, each mouse only received one dose of OXO and this dose was the same during the control and test injections.
2.11. Experiment 5: Intra-NAc in vivo microdialysis for DA following intra-PPT OXO
In this experiment, we examined whether PPT-derived ACh played a role MA-induced increases in DA levels within the NAc. In vivo microdialysis was performed within the NAc just as in Experiment 4. Each mouse received a control injection after baseline ACh levels were established. This control injection included a bilateral intra-PPT microinjection (0.1 µl per side) of aCSF or OXO (1 or 10 nM) immediately followed by an IP saline injection. Each mouse received a test injection 80 min after the control injection. This consisted of a bilateral intra-PPT microinjection immediately followed by IP MA (3.5 mg/kg). Just as in Experiments 2 through 4, each mouse only received one concentration of OXO and this concentration was the same during the control and test injections.
2.12. Sample and standard preparation
DA and ACh samples and their corresponding standard curves that were analyzed using high performance liquid chromatography with electrochemical detection (HPLC) were prepared as described previously [18]. Approximately two-thirds the way through the dialysis experiment, we were not able to obtain immobilized enzyme reactors with suitable sensitivity for the electrochemical detection of ACh using HPLC. As a result, a different analytical method, liquid chromatography–tandem mass spectrometry (LC–MS/MS), was used for the last third of subjects. The total number of samples in each OXO treatment group that were analyzed using LC–MS/MS was: 62 for aCSF, 24 for 1 nM OXO and 69 for 5 nM OXO. The total number of samples per OXO treatment group that were analyzed using HPLC was: 76 for aCSF, 104 for 1 nM OXO, 37 for 5 nM OXO and 128 for 10 nM OXO.
ACh dialysate samples that were analyzed with LC–MS/MS were spiked with 180 µl of an internal standard of deuterated ACh (d4-ACh; CDN Isotopes, Quebec, Canada). The internal standard was prepared in 75% acetonitrile, 25% methanol and 0.2% formic acid (v/v/v) to a final concentration of 0.0001 ng/µl. After addition of the internal standard each sample was centrifuged at 10,000 rpm for 5 min at room temperature through a 22 µm filter (Fisher Scientific, Pittsburgh, PA). A standard curve was prepared using a range of ACh concentrations (Sigma, St. Louis, MO), with each containing 180 µl of d4-ACh. Aliquots of 20 µl were injected onto a poly-hydroxylethyl column (50 × 2.1, 3 µm, 100 Å; Poly LC Inc., Columbia, MD) with guard column for analysis.
2.12.1. Sample analysis
The procedure for samples analyzed for ACh or DA content using HPLC has been described previously [18].
Analysis of ACh content using the LC–MS/MS system consisted of a quadrupole ion trap (4000 Q trap, Applied Biosystems/MDX SCIEX) equipped with electron spray ionization, a Prominence UFLC-XR autosampler, system controller, column oven and UV/VIS detector for liquid chromatography (Shimadzu Corp., Kyoto, Japan). Positive ioninzation was attained using single reaction monitoring (SRM). The transition detected for ACh was m/z = 146/87 and for d4-ACh was m/z = 150/91.1. The ion spray voltage was 3 kV and maintained at 600 °C. nitrogen was used for the curtain gas (50 psi) and for the two ion source gasses (40 and 60 psi). Collision fragmentation was attained using nitrogen. Mobile phase for this system consisted of 0.1% formic acid, 20 mM ammonium formate, acetonitrile and methanol and was maintained at 3 ml/min. ACh and d4-ACh analytes were identified by retention time and their respective fragment m/z. The amount of ACh was quantified by peak area ratio of d4-ACh versus ACh within each sample using Analyst version 1.5 (Applied Biosystems).
Table 1 shows the picograms of ACh per microliter of dialysate at baseline or after saline injection for samples analyzed with the HPLC and LC–MS/MS analytical methods. Analysis with Welch’s t-test (to correct for unequal variance) showed no significant difference in the amount of ACh at baseline (t24 = 0.43, p = 0.668) or after saline injection (t45 = 1.17, p = 0.247) between the two analytical methods. In addition, there was no significant change in the amount of ACh when comparing baseline and post-saline injection using either the HPLC method (t119 = 0.17, p = 0.868) or the LC–MS/MS method (t58 = 0.32, p = 0.753).
Table 1.
Extracellular levels of VTA ACh at baseline and following a control saline injection analyzed with HPLC and LC–MS/MS.
| HPLCa | LC–MS/MSa | |
|---|---|---|
| Baseline | 0.85 ± 0.07 (N = 58) | 0.98 ± 0.29 (N = 23) |
| Post-saline | 0.83 ± 0.07 (N = 63) | 1.09 ± 0.20 (N = 37) |
There was no difference in extracellular ACh between two analytical methods, high performance liquid chromatography (HPLC; left column) or liquid chromatography tandem mass spectrometry (LC–MS/MS; right column) using a Welch’s corrected t-test at baseline (p = 0.668) or post-saline (p = 0.247). There was also no significant change in extracellular ACh levels between baseline and post-saline for the HPLC (p = 0.868) or LC–MS/MS method (p = 0.753).
Data expressed as pg ACh/µl dialysate.
2.13. Histology
Mice were deeply anesthetized before a thionin (300 nl) microinjection was administered into the LDT or PPT at the end of each experiment. Microdialysis probe placement was confirmed by intercalating a thionin pulse into the inlet of the probe that perfused thionin through the VTA. Mice were then decapitated and their brains flash-frozen in isopentane on dry ice. Frozen brains were stored at −80° C until they were sectioned (40 µm) on a Leica cryostat. Data were excluded if the subjects’ microdialysis fiber intruded on the substantia nigra (SN), when targeting the VTA, or if at least half of the fiber intruded on the caudate putamen, when targeting the NAc. Subjects were excluded if one or both injections were located outside of the target region. The number of mice excluded was 14, 23, 6, 14 and 5 for Experiments 1 through 5, respectively. Fig. 1 shows the range of microdialysis probe and microinjection locations (with target areas shaded) overlaid on plates from the atlas of Paxinos and Franklin [26] for mice included in the analyses of each experiment. Microinjection locations from Experiment 1 are shown in panel A. Panels B–E show the microdialysis probe placements (top) and microinjection locations (bottom) for Experiments 2–5, respectively.
Fig. 1.
Microdialysis probe placements in the VTA and NAc and bilateral microinjection locations in the LDT and PPT are shaded and shown overlaid on plates taken from the atlas of Paxinos and Franklin [26]. Successful LDT microinjection targets for Experiment 1 are shown in panel A. Panels B and C show successful intra-VTA microdialysis probe placements for Experiments 2 and 3 (top) with the corresponding LDT (B, bottom) and PPT (C, bottom) microinjection locations. Panels D and E show successful intra-NAc microdialysis probe placements for Experiments 4 and 5 (top) with the corresponding LDT (D, bottom) and PPT (E, bottom) microinjection locations. Probe placements were counterbalanced for side and plates are labeled as mm from bregma. CIC: central nu of inferior colliculus; CPu: caudate putamen; LDT: laterodorsal tegmental nu; LPAG: lateral periaqueductal grey; NAc: nucleus accumbens; PnO: pontine reticular nu (oral part); PPT: pedunculopontine tegmental nu; SNc: substantia nigra, compact; SNr: substantia nigra, reticular; VTA: ventral tegmental area.
2.14. Statistics
Locomotor data were analyzed using a three-way repeated measures ANOVA with Day as the within subjects factor and IP treatment (IP) and Microinjection Pretreatment (PreTx) as the between subjects factors. Greenhouse-Geisser was used to correct for violations to sphericity. Dialysis data were analyzed using 2-way repeated measures ANOVA with microinjection treatment as the between subjects factor (4 levels for Experiment 2, 2 levels for Experiments 3–5) and time as the within subjects factor. For the within subject factor, time was collapsed into 2 time bins, post-saline and post-MA. For Experiments 2, 4 and 5 the post-saline time bin consisted of collapsing the 80 min of data following the saline injection. For Experiment 3 the last 60 min of post-saline injection data were collapsed. These time bins were chosen to create an equal baseline between OXO groups. For Experiments 2 and 3, data in the first 80 min following MA injection were collapsed to create the post-MA time bin. For Experiments 4 and 5, data in the first 40 min following MA injection were collapsed to create the post-MA time bin. Since some subjects had a sample missing or excluded following MA administration, a repeated measures ANOVA would have required eliminating these subjects from analysis. Therefore, we collapsed into one time bin following MA administration in order to include all subjects. Missing samples were due to problems such as the probe coming disconnected during dialysate collection, technical problems with sample injection onto the HPLC column, or HPLC/LC–MS/MS detection problems. A sample was considered below detection limit and excluded from the analysis if (1) the amount of ACh detected fell outside the range of the standard curve, (2) no sample peak was detected by the HPLC or LC–MS/MS, or (3) the signal-to-noise ratio was not at least 2:1. The total number of samples across all microdialysis experiments that were excluded from analysis was 80 out of 760 (10.5%). Post-MA time bins were selected that best represented the peak of the response following MA injection. Significant two-way interactions were followed up with simple main effects analyses and post hoc tests. Effects were considered significant at an alpha of 0.05.
3. Results
3.1. Experiment 1
3.1.1. Locomotor activity following LDT microinjection pretreatment
Data from a total of 39 mice were used in this analysis. A summary of activity (cm ± SEM) on baseline and test day by all treatment groups is presented in Table 2. IP injection of saline or MA resulted in a significant change in locomotor activity on test day compared to baseline (Fig. 2A; F1,34 = 31.7, p < 0.001). IP administration of saline on the test day resulted in significantly lower activity compared to baseline (from 3941 ± 1328 to 2053 ± 412) (F1,18 = 10.0, p = 0.005), while IP injection of 2 mg/kg MA resulted in a significant increase in locomotor activity (from 3759 ± 371 to 8235 ± 1075) (F1,19 = 17.5, p = 0.001). Since these comparisons were collapsed across pretreatment dose the saline effect may be driven by the ability of the high concentration of OXO to inhibit basal locomotor activity (Table 2). There were significant main effects of day (F1,34 = 7.94, p = 0.008) and IP (F1,34 = 23.4, p < 0.001); however, the 3-way interaction between day, IP and preTx was not significant.
Table 2.
Mean distance traveled in 30 min on baseline and test day following a microinjection pretreatment in the LDT or IC and IP injection of saline or MA.
| Microinjection site | IP | Microinjection pretreatment | Baseline activity* | Test day activity*, † | N |
|---|---|---|---|---|---|
| LDT | Saline | aCSF | 2547 (720) | 2968 (1435)a | 5 |
| 1 nM OXO | 3415 (657) | 2336 (1326)a | 6 | ||
| 10 nM OXO | 5207 (569) | 1269 (1148) | 8 | ||
| 2 mg/kg | aCSF | 3535 (720) | 10114 (1453)a | 5 | |
| MA | 1 nM OXO | 3736 (569) | 9890 (1148)a | 8 | |
| 10 nM OXO | 3946 (609) | 5004 (1228) | 7 | ||
| IC | Saline | aCSF | 3920 (713) | 3234 (985) | 7 |
| 1 nM OXO | 3691 (713) | 3662 (985) | 7 | ||
| 10 nM OXO | 5084 (843) | 3647 (1165) | 5 | ||
| 2 mg/kg | aCSF | 3950 (943) | 9149 (1303)b | 4 | |
| MA | 1 nM OXO | 4216 (770) | 9811 (1064)b | 6 | |
| 10 nM OXO | 3800 (713) | 8146 (985)b | 7 |
Data expressed as distance (cm ± SEM).
In mice that received microinjection pretreatment into the LDT, simple main effects analysis of a significant day × IP interaction revealed test activity to be significantly different from baseline in mice that received IP saline (p = 0.001) or MA (p = 0.001).
Simple main effects analysis of a significant day × pretreatment interaction revealed test day activity was significantly increased compared to baseline in mice pretreated with aCSF (p = 0.01) or 1 nM OXO (p < 0.05).
Test day activity was significantly increased in mice that received microinjection pretreatment in the inferior colliculus and IP MA (IC; p < 0.0001).
Fig. 2.
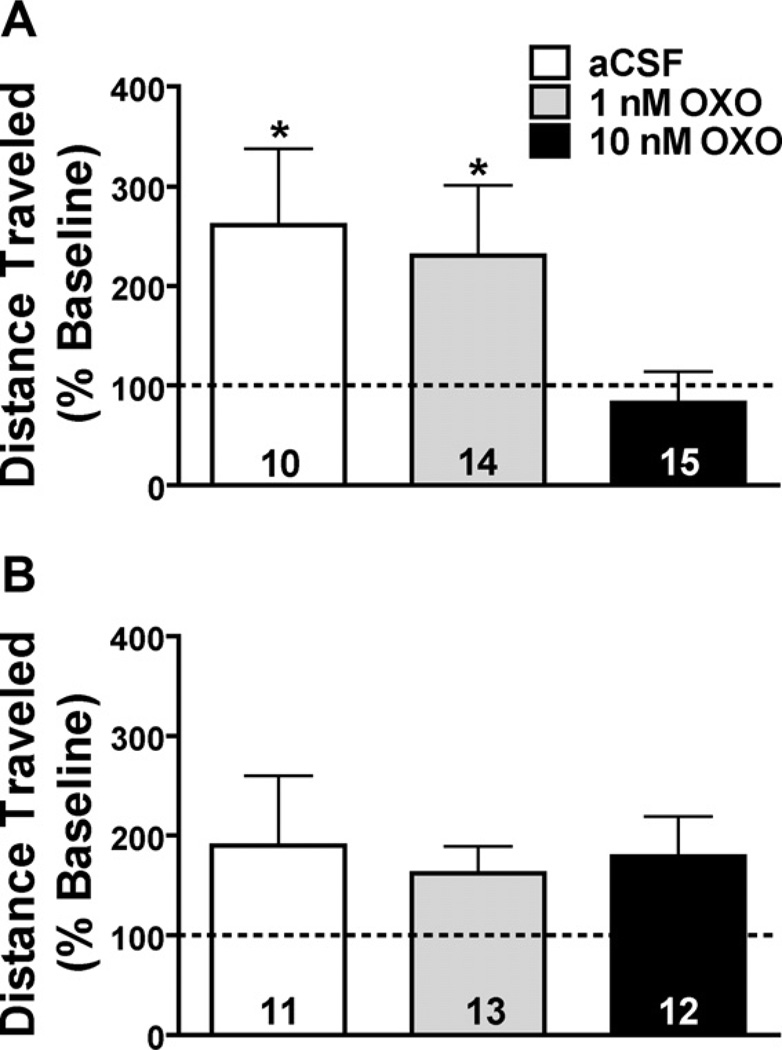
Effect of bilateral OXO microinjections into the LDT (A) or a dorsal control site, the inferior colliculus (IC; B). Mice received IP saline or MA immediately following the microinjection. Data are depicted as a percent of baseline (represented by the dotted line) and collapsed on IP treatment with OXO group sample sizes on their respective bar. (A) Mice pretreated with a bilateral microinjection into the LDT of aCSF (white bar) or 1 nM OXO (grey bar) immediately followed by IP saline or MA (2 mg/kg) showed a significant increase in locomotor activity on test day. Pretreatment with 10 nM OXO (black bar) had no significant effect on locomotor activity. (B) Bilateral OXO pretreatment into the IC had no effect on test day locomotor activity. *p < 0.05 vs. baseline activity.
Administration of OXO into the LDT differentially affected test day locomotor activity compared to baseline (Fig. 2A; F2,34 = 8.17, p = 0.001). Simple main effects analysis of this interaction revealed that pretreatment with intra-LDT aCSF (F1,9 = 8.51, p = 0.017) or 1 nM OXO (F1,13 = 6.45, p = 0.025) resulted in significantly higher activity on test day, regardless of IP drug administered. However, animals pretreated with 10 nM OXO did not show an increase in activity on test day. These data suggest that 10 nM OXO inhibited activity, while 1 nM and aCSF did not, regardless of IP drug administered.
3.1.2. Locomotor activity following IC microinjection pretreatment
Data from a total of 36 mice were used for this analysis. Table 2 lists the mean activity by all treatment groups on baseline and test day (cm ± SEM). IP administration of saline or MA resulted in difference in activity on test day compared to baseline (F1,30 = 55.9, p < 0.001). Further exploration of the Day × IP interaction revealed that 2 mg/kg MA significantly increased locomotor activity on test day irrespective of intra-IC pretreatment (F1,16 = 52.4, p < 0.001; Fig. 2B). In addition, there was a trend for saline-treated mice to have higher locomotor activity on baseline day (F1,18 = 3.96, p = 0.062). There was also a significant main effect of Day (F1,30 = 31.5, p < 0.001) and IP (F1,30 = 15.4, p < 0.001), but no other effects or interactions were significant. Importantly, none of the data analyses suggested there was an effect of OXO administered into this dorsal control site.
3.2. Experiment 2
3.2.1. Intra-VTA in vivo microdialysis for ACh following intra-LDT OXO
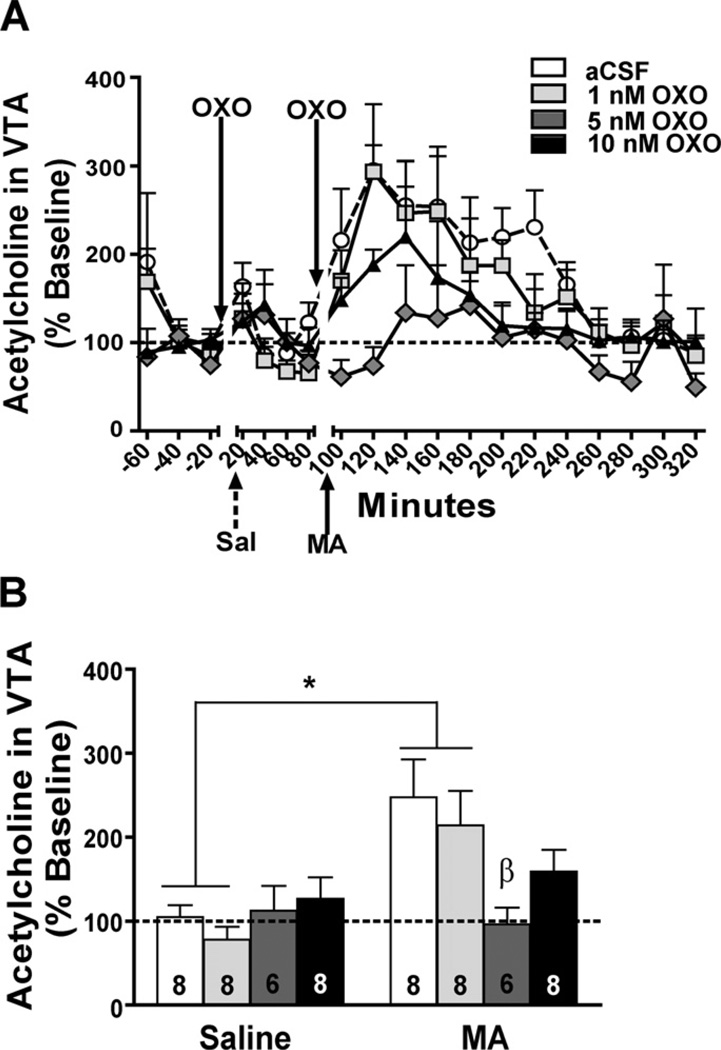
Data from a total of 30 mice were used in these analyses. Bilateral OXO microinjections in the LDT dose-dependently attenuated intra-VTA ACh levels following IP MA, but not IP saline (Time ×OXO: F0 = 3.44, p = 0.031). Fig. 3A shows the time course of ACh responding in relation to OXO microinjections and IP (saline and MA) injections. The lack of an OXO effect on extracellular ACh in the VTA after saline injection may have been due to a floor effect. Simple main effect analyses showed that pretreatment with aCSF (F1,7 = 10.12, p = 0.015) or 1 nM OXO (F1,7 = 10.8, p = 0.013) significantly increased extracellular ACh in the 40 min post-MA injection compared to post-saline injection (Fig. 3B). Pretreatment with 5 or 10 nM OXO inhibited the MA-induced increase in extracellular ACh; however, no OXO concentration significantly affected ACh levels after saline injection. Following IP MA, the aCSF-pretreated mice had significantly higher levels of ACh compared to 5 nM OXO-pretreated mice (p = 0.019).
Fig. 3.
Effect of bilateral OXO microinjections into the LDT on ACh levels in the VTA. Data are depicted as a percent of baseline (represented by the dotted line). (A) The ACh time course in response to aCSF (white circles), 1 nM (light grey squares), 5 nM (dark grey diamonds), or 10 nM (black triangles) OXO microinjection and IP saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) ACh samples were collapsed for each mouse after saline injection (Saline) and in the first 80 min after MA injection (3.5 mg/kg; MA) for analysis. There was a significant increase in ACh levels in mice pretreated with aCSF (white bar) or 1 nM OXO (light grey bar) following IP MA compared to saline. Compared to aCSF, microinjection pretreatment with 5 nM OXO significantly decreased MA-induced ACh levels. Treatment group sample sizes are shown on their respective bar. *p < 0.05 MA vs. saline; β p < 0.05 vs. aCSF MA group.
3.3. Experiment 3
3.3.1. Intra-VTA in vivo microdialysis for ACh following intra-PPT OXO
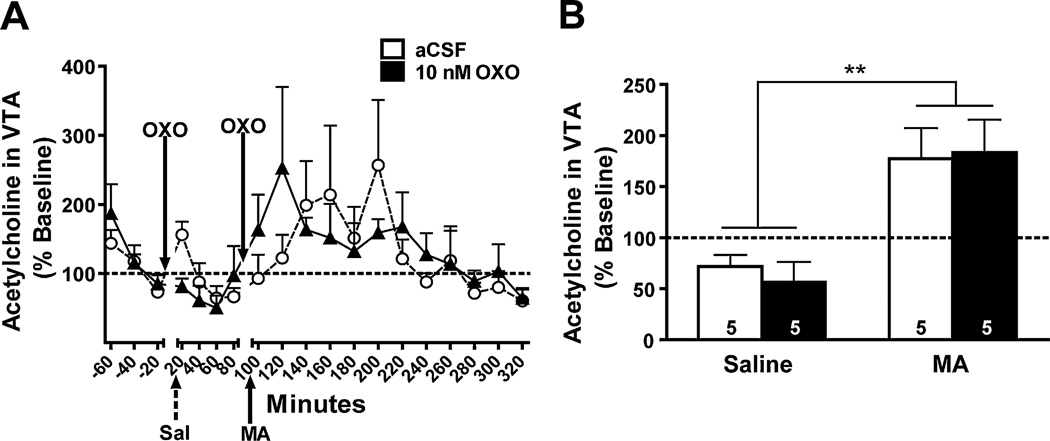
Data from a total of 10 mice were used in these analyses. A main effect of time suggested that MA induced an increase in extracellular ACh regardless of intra-PPT pretreatment (F1,8 = 27.8, p = 0.001; Fig. 4A). Microinjections of 10 nM OXO into the PPT had no effect on extracellular levels of ACh after saline or MA injection (Fig. 4B). When analyzing the data it appeared that the 10 nM OXO-treated group showed a shift in the peak ACh response compared to aCSF-treated animals. Therefore, we performed a repeated measures ANOVA comparing the collapsed control time bin with the individual post-MA 20-min time bins, which required the removal of 3 subjects due to missing samples (1, aCSF and 2, 10 nM). This analysis showed no significant main effects of time or treatment group and no significant interaction (data not shown).
Fig. 4.
Effect of bilateral OXO microinjections into the PPT on ACh levels in the VTA. Data are expressed as a percent of baseline (represented by the dotted line). (A) The time course of ACh in response to intra-PPT OXO (10 nM; solid triangles) or aCSF (open circles) microinjection and IP injection of saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) ACh samples were collapsed for each mouse after saline injection (Saline) and in the first 80 min after MA injection (3.5 mg/kg; MA) in each OXO treatment group for analysis. There was a significant increase in ACh levels following IP MA in both 10 nM OXO- (solid bar) and aCSF-pretreated (open bar) mice. Treatment group sample sizes are shown on their respective bar. **p = 0.001 vs. IP saline.
3.4. Experiment 4
3.4.1. Intra-NAc in vivo microdialysis for DA following intra-LDT OXO
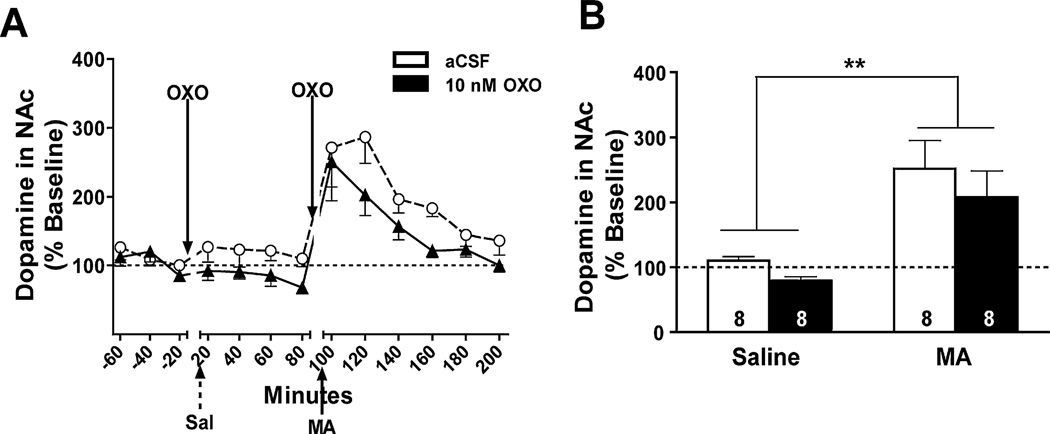
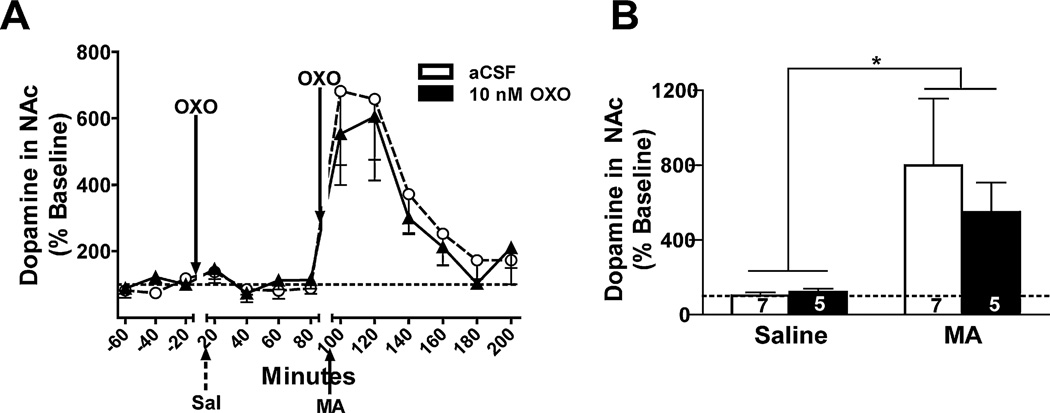
Data from a total of 16 mice were used in the DA analyses. The time course of the DA and DA metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), response is shown in Figs. 5A and 6A, respectively. A main effect of time showed a significant increase in NAc DA levels in the first 40 min after MA injection compared to after saline injection (F1,14 = 18.5, p = 0.001; Fig. 5B). No other significant effects or interactions were detected.
Fig. 5.
Effect of bilateral OXO microinjections into the LDT on DA levels in the NAc. Data are expressed as a percent of baseline (represented by the dotted line). (A) The time course of DA levels in response to intra-LDT OXO (10 nM; solid triangles) or aCSF (open circles) microinjection and IP injection of saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) DA samples were collapsed for each mouse after saline injection (Saline) and in the first 40 min after MA injection (3.5 mg/kg; MA) in each OXO treatment group for analysis. Microinjection of 10 nM OXO (solid bar) into the LDT had no effect on the MA-induced increase in DA levels. Treatment group sample sizes are shown on their respective bar. **p = 0.001 vs. IP saline.
Fig. 6.
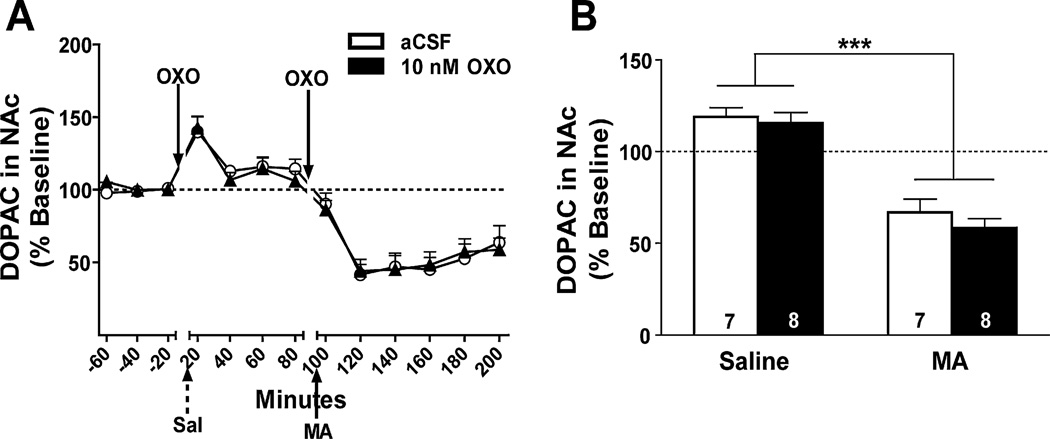
Effect of bilateral OXO microinjections into the LDT on levels of the DA metabolite, DOPAC, in the NAc. Data are expressed as a percent of baseline (represented by the dotted line). (A) The time course of DOPAC levels in response to intra-LDT OXO (solid triangles) or aCSF (open circles) microinjection and IP injections of saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) Data were collapsed after saline injection (Saline) and the first 40 min after MA injection (MA) in each OXO treatment group for analysis. There was a significant decrease in DOPAC levels after IP MA in both 10 nM OXO- (solid bar) and aCSF-pretreated (open bar) mice. Treatment group sample sizes are shown on their respective bar. ***p < 0.0001 vs. IP saline.
As a measure of DA metabolism, levels DOPAC were measured post-saline injection and compared to the first 40 min post-MA injection (Fig. 6A). Data from a total of 15 mice were used in the DOPAC analyses. A main effect of time showed a decrease in extracellular DOPAC levels after MA injection compared to after saline injection (F1, 13 = 90.7, p < 0.0001). This sustained decrease in DOPAC levels is most likely due to a MA-induced reversal of the DA transporter (DAT), which would inhibit DA re-uptake and metabolism into DOPAC. No other effects or interactions were significant.
3.5. Experiment 5
3.5.1. Intra-NAc in vivo microdialysis for DA following intra-PPT OXO
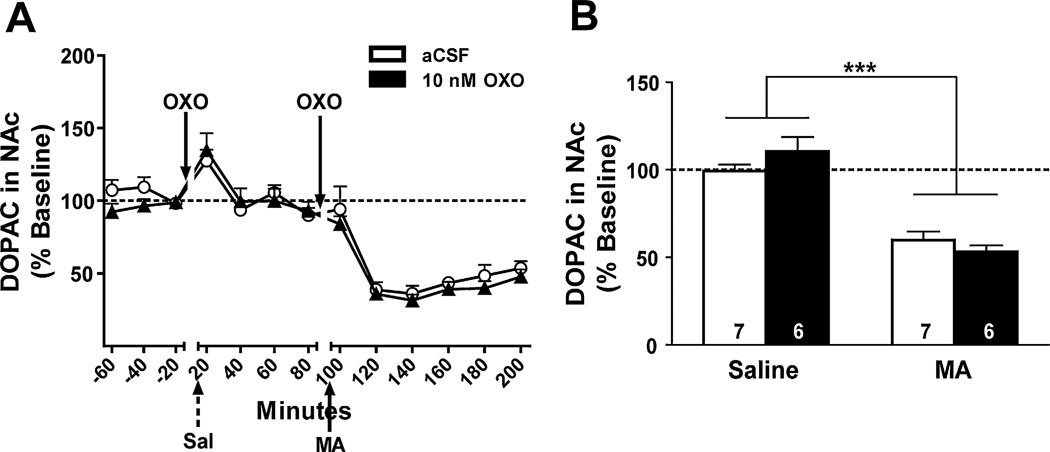
Data from 12 mice were used in the DA analyses. The time course of the DA and DOPAC response is shown in Figs. 7A and 8A, respectively. DA levels increased significantly after the MA injection compared to after the saline injection (F1,10 = 6.1, p = 0.034), and this effect was irrespective of intra-PPT OXO pretreatment (Fig. 7B). Similar to when OXO was microinjected into the LDT, NAc levels of the DA metabolite, DOPAC, in the first 40 min after MA injection were significantly lower than after the saline injection (F1,11 = 79.5, p < 0.0001, Fig. 8B). No other effects or interactions were significant.
Fig. 7.
Effect of bilateral OXO microinjections into the PPT on DA levels in the NAc. Data are expressed as a percent of baseline (represented by the dotted line). (A) The time course of DA levels in response to OXO (solid triangles) or aCSF (open circles) and IP saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) Data were collapsed after saline injection (Saline) and the first 40 min after MA injection (MA) in each OXO treatment group for analysis. There was a significant increase in DA levels following IP MA compared to saline in both 10 nM OXO- (solid bar) and aCSF-pretreated (open bar) mice. Treatment group sample sizes are shown on their respective bar. *p < 0.05 vs. IP saline.
Fig. 8.
Effect of bilateral OXO microinjections into the PPT on levels of the DA metabolite, DOPAC, in the NAc. Data are expressed as a percent of baseline (represented by the dotted line). (A) The time course of DOPAC levels in response to OXO (black triangles) or aCSF (white circles) and IP saline (Sal; broken arrow) or MA (3.5 mg/kg; solid arrow) is shown in 20-min bins. (B) Data were collapsed after saline injection (Saline) and the first 40 min after MA injection (MA) in each OXO treatment group for analysis. There was a significant decrease in DOPAC levels following IP MA compared to saline in 10 nM OXO- (black bars) and aCSF-pretreated (white bars) mice. ***p < 0.0001 vs. IP saline.
4. Discussion
Acetylcholine has been implicated in mediating DA neuron burst firing, locomotor activity and reward processing within the DA mesolimbic circuit. The experiments presented in this paper sought to examine the origin of the cholinergic tone within the VTA, namely the LDT and PPT within the mesopontine region, and determine their contribution to MA-induced changes in locomotor activity and ACh and DA within the mesolimbic circuit. The results support the proposal that while MA activates LDT cholinergic neurons that project to the VTA, leading to increased ACh levels in the VTA, this is not critical for MA-induced increases in NAc DA levels. In addition, PPT cholinergic neurons do not seem to be recruited for basal or MA-induced increases in ACh levels within the VTA. These data also suggest that LDT cholinergic neurons are involved in locomotor activity after saline or MA injection.
4.1. Basal versus methamphetamine-induced acetylcholine responses
While intra-LDT OXO dose-dependently attenuated the MA-induced increase in intra-VTA ACh, basal levels of ACh were unaffected. Previous studies applying the mixed nicotinic/muscarinic ACh agonist carbachol to the mesopontine tegmentum suggest there may be separate groups or sub-types of cholinergic neurons within the LDT and PPT [30,31]. These groups may be differentially responsive to MA and OXO or have a different expression of the M2 ACh receptor, resulting in differential effects on basal and MA-induced levels of ACh.
Investigations of the mesopontine cholinergic contribution to the ponto-geniculo-occipital waves associated with rapid eye movement (REM) sleep have revealed two electrophysiologically different sub-types of cholinergic neurons within the LDT and PPT. Low-threshold bursting (LTB) neurons are suspected to maintain high levels of ACh in terminal regions through the ability to produce burst firing while non-LTB neurons, which are incapable of burst-firing, possess a potassium channel-mediated after-hyperpolarization (AHP) and are thought to maintain tonic levels of ACh in terminal regions [32,33]. In cats, cholinergic agonists applied to the mesopontine tegmentum decreased burst firing-induced ACh levels in the lateral geniculate nucleus, likely through an action on M2 autoreceptors [30]. These sub-types of cholinergic neurons within the LDT and PPT are heterogeneously distributed among other types of cells (principally GABAergic and glutamatergic neurons), making them difficult to target individually. It is not clear whether non-LTB cholinergic neurons possess the inhibitory M2 autoreceptor. One speculation is that cholinergic neurons on which MA and OXO act possess the inhibitory M2-type ACh receptor, while cholinergic neurons responsible for maintaining basal tone in terminal regions do not express these receptors (at least to the same degree or sensitivity). This is, of course, only one hypothesis for the method of action of these drugs and future studies are needed to clarify whether these sub-types of cholinergic neurons differentially express M2 (or other) ACh receptors.
4.2. Mesopontine acetylcholine in methamphetamine-induced locomotor activity and dopamine levels
These experiments are the first to show that MA specifically activates LDT, but not PPT, ACh neurons and that inhibition of the LDT-to-VTA cholinergic projection is related to a non-selective impairment of locomotor activation (MA-induced and spontaneous). Furthermore, these data show that neither inhibition of LDT- nor PPT-derived cholinergic tone in the VTA affected MA-induced increases in DA levels within the NAc.
Although previous studies clearly support the role of mesopontine ACh in the modulation of DA burst firing and release [7,34,35], the current findings are not necessarily incompatible with the literature. MA is capable of inducing DA release in the absence of neuronal stimulation, thus cholinergic enhancement of DA activity may be insignificant compared to MA’s affects at DA terminals. Several mechanisms have been reported to underlie the ability of amphetamine compounds to induce stimulation independent DA release. These include, but are not limited to, the weak base hypothesis and actions at the vesicular monoamine transporter (VMAT) and DAT [for an in depth review the reader is referred to 36]. The MA-induced increase in ACh, which can increase DA neuron excitability [37,38], is likely masked by the action of MA on DA terminals. The current data also suggest that mesopontine-derived cholinergic tone within the VTA is not necessary for basal DA levels in the NAc. Interestingly, the MA-induced increase in extracellular DA levels was more short-lived compared to the sustained decrease in DOPAC levels. Amphetamines (including MA) are known to reverse the DAT and inhibit monoamine oxidase (MAO), thereby inhibiting DA re-uptake and metabolism into DOPAC [for a review see 36]. Thus, this prolonged effect on DOPAC levels suggests MA was still present in the synapse 1.5 h after administration even though DA levels had returned to baseline.
Microinjection pretreatment of 10 nM OXO into the LDT selectively decreased MA-induced (but not basal) ACh levels in the dialysis experiment, but was related to a non-selective inhibition of locomotor activity (MA-induced and spontaneous). While we cannot establish a causal link between the neurochemical response and locomotor activity measured in these two experiments, previous experiments in our lab enable us to make some inferences about the current data. We previously reported that 2 mg/kg MA significantly increases VTA ACh levels [18]. Since 10 nM OXO inhibited the 3.5 mg/kg MA-induced increase in ACh levels in the current study, it is likely that it was able to inhibit the 2.0 mg/kg MA-induced increase in ACh in the locomotor experiment. Previous studies also suggest that neostigmine may affect the local pharmacologic milieu and ultimately have effects on behavior [29,39,40]. While we cannot rule out an effect of neostigmine on our cholinergic microdialysis data, we feel that a significant effect is unlikely given the low concentration (0.3 µM) used in the current experiments. Thus, these data suggest that inhibition of the LDT-to-VTA cholinergic pathway does not alter MA-specific locomotor activity.
There are several possible explanations for why OXO affected locomotion after saline injection independently of DA or ACh levels. First, OXO may have diffused into a neighboring region to exert its effects on activity independently of DA or ACh levels in the NAc and VTA, respectively. To address the site specificity of OXO’s effects on locomotor activity injections were made into the inferior colliculus (IC), a control site located 1 mm dorsal to the LDT. The most likely diffusion route would be in a dorsal direction via the injector tract; however, microinjections into the IC had no effect no basal or MA-induced locomotor activation. Although less likely, it is possible that OXO diffused in a lateral and anterior direction to affect the PPT. While we did not asses the contribution of PPT-derived ACh on MA-induced locomotor activity, evidence suggests that this region is not essential for locomotor activation [1,41,42]. The cholinergic neurons of the anterior PPT (aPPT) project mainly to the substantia nigra (SN), while the cholinergic neurons located in the posterior PPT (pPPT), which is closer to our LDT injection target, project to DA neurons in the VTA. If diffusion occurred in a lateral and anterior direction it is most likely that the pPPT cholinergic neurons were affected, which have the same target DA neurons as the LDT cholinergic neurons [8,42]. Thus, any limited diffusion of OXO to the pPPT would most likely result in an outcome similar to an LDT microinjection. We can extrapolate from this information that it is unlikely that diffusion of OXO into the PPT contributed to an attenuation of locomotor activity.
Second, it is also possible that a decrease in ACh or DA levels after saline injection was not detected due to a floor effect. Although a non-significant decrease in picograms of ACh was observed across OXO treatment groups when comparing levels between baseline and after saline injection, it is possible that a further decrease in ACh could have occurred but was not detected. ACh levels at baseline and after saline injection in the VTA were low (Table 2) and following the high concentration of intra-LDT OXO some ACh levels fell below detection limits. When this occurred, samples were excluded from the analysis.
Third, the different contexts and handling procedures associated with the locomotor activity and microdialysis experiments could have contributed to the different pattern of results between the two experiments. The locomotor and microdialysis experiments were performed separately and in different testing chambers. It is possible that the different chambers and basic differences in handling used in these two experiments could have contributed to the lack of an observed relationship between locomotor activity and neurotransmitter levels. Ideally, we would like to be able to measure ACh and DA during locomotor activity; however there are significant problems with performing these experiments simultaneously. Previous experiments in our lab have found that the timed collection of dialysis samples disrupts the animal’s behavior and that behavioral disruptions can artificially affect transmitter levels (unpublished observations). In both experiments mice were allowed to habituate to their chambers the day before data was collected. However, in the locomotor experiment mice were also habituated to handling and injection the day before collecting baseline activity data, while samples analyzed for ACh and DA were collected with no previous habituation to injection. This may have resulted in a stress response in the microdialysis experiment and prevented a significant decrease in ACh or DA levels. Conversely, habituation to handling and injection in the locomotor experiment may have attenuated any stress response, thereby affecting activity. Locomotor activity did tend to be higher on the first day compared to the second day of testing (data not shown). Although, it is unknown how much novelty and handling stress each contributed to the difference in activity between day one and two.
Despite the procedural differences between the dialysis and locomotor experiments, these data provide several significant and novel findings that enhance our understanding of MA’s neurochemical and behavioral effects. Although inhibition of LDT-derived ACh did not affect locomotor activity in a MA-specific fashion, this does not preclude the possibility that LDT ACh could underlie other MA-related (and non-MA-related) behaviors. Indeed, there are multiple neurochemical and behavioral changes that occur following MA and/or OXO administration, which were not measured in these experiments.
Finally, the difference in the pattern of results following OXO administration could be due to the distribution of M2 ACh receptors, the subtype of receptor to which OXO preferentially binds. This receptor subtype may be located on non-cholinergic cells, such as glutamatergic neurons, so that activation of these receptors may mediate basal locomotor activity independent of an effect on cholinergic neurons. Previous research has confirmed that the M2 ACh receptor is located on cholinergic neurons within the LDT and PPT and that activation of this receptor results in a tetrodotoxin (TTX)-insensitive hyperpolarization and subsequent neuronal inactivation [43–46]. It is not clear, however, if the M2 receptor is expressed on non-cholinergic neurons, such as glutamate or GABA, within the LDT and PPT. One study found more restricted labeling of choline acetyltransferase (ChAT) mRNA, a biomarker for ACh neurons, than the ACh M2 receptor mRNA [45]. Further, bath application of carbachol induced a hyperpolarization in vitro regardless of neuronal phenotype, although 79% of neurons tested were cholinergic [33]. These studies suggest that non-cholinergic neurons may express M2 receptors. There are also known glutamate projections from the LDT to the VTA [47,48, and for a review see 49]. Furthermore, application of glutamatergic agonists in the VTA enhance, while antagonists inhibit, locomotor activity and DA levels in terminal regions [50,51]. Thus, it is conceivable that the locomotor attenuation seen in the current data may in part be due to effects via the glutamate projection to the VTA. Further investigation is needed to clarify if the M2 ACh receptor is expressed on non-cholinergic neurons within the LDT and PPT.
5. Conclusions
The present findings suggest that attenuation of LDT-derived ACh in the VTA, but not NAc DA, is related to an inhibition in locomotor activity in C57BL/6 J mice, which is consistent with previous research implicating the LDT in spontaneous and drug-induced activity [52,53]. In addition, although MA stimulated LDT cholinergic neurons induce an increase in VTA ACh, this did not appear to play a significant role in the MA-induced increase in DA within the NAc nor was this specific to MA-induced locomotor activity. While the present findings significantly contribute to our understanding of MA’s neurochemical and behavioral effects, additional studies are needed to clarify if the LDT-to-VTA cholinergic pathway is involved in other MA-related behaviors.
The LDT and PPT are situated in a key area to receive sensory information and mediate locomotor output. This neurochemically diverse brainstem region is important for interpreting sensory information, likely received from the superior colliculus, to guide appropriate motor behaviors. Given the mesopontine region’s extensive and reciprocal functional connections with the mesolimbic reward circuit it is reasonable to hypothesize a role of the PPT or LDT in learning about reward-associated cues.
Acknowledgements
The research described in this paper was in part supported by a Pre-doctoral NRSA (DA272952), a grant from the American Psychological Association, the Methamphetamine Abuse Research Center (MARC; DA018165) and a scholarship from the Achievement Rewards for College Scientists (ARCS).
The authors would like to thank Jenny Luo and Dr. Dennis Koop in the Department of Pharmacology and Physiology for use of their tandem mass spectrometer and assistance in the methods and analysis of the associated data. The authors would also like to thank Dr. Matthew Lattal for use of his locomotor activity apparatus and Drs. Christopher Cunningham and Charles Meshul for their critical review of the manuscript.
Abbreviations
- ACh
acetylcholine
- DA
dopamine
- LDT
laterodorsal tegmental nucleus
- MA
methamphetamine
- OXO
oxotremorine
- PPT
pedunculopontine tegmental nucleus
References
- 1.Winn P. How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci. 2006;248:234–250. doi: 10.1016/j.jns.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 2.Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omelchenko N, Sesack SR. Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. J Comp Neurol. 2006;494:863–875. doi: 10.1002/cne.20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 6.Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev. 2008;58:265–271. doi: 10.1016/j.brainresrev.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Yeomans JS, Mathur A, Tampakeras M. Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci. 1993;107:1077–1087. doi: 10.1037//0735-7044.107.6.1077. [DOI] [PubMed] [Google Scholar]
- 10.Laviolette SR, Priebe RP, Yeomans JS. Role of the laterodorsal tegmental nucleus in scopolamine- and amphetamine-induced locomotion and stereotypy. Pharmacol Biochem Behav. 2000;65:163–174. doi: 10.1016/s0091-3057(99)00195-1. [DOI] [PubMed] [Google Scholar]
- 11.Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westerink BH, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- 13.Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S. Hyperactivity. elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:15312–15317. doi: 10.1073/pnas.261583798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess GP, Ulrich H, Breitinger HG, Niu L, Gameiro AM, Grewer C, et al. Mechanism-based discovery of ligands that counteract inhibition of the nicotinic acetylcholine receptor by cocaine and MK-801. Proc Natl Acad Sci U S A. 2000;97:13895–13900. doi: 10.1073/pnas.240459497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
- 17.Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology. 2003;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs LK, Mark GP. Comparison of systemic and local methamphetamine treatment on acetylcholine and dopamine levels in the ventral tegmental area in the mouse. Neuroscience. 2008;156:700–711. doi: 10.1016/j.neuroscience.2008.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]
- 20.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 21.Jones CD, Bartee JA, Leite-Browning ML, Blackshear MA. Methamphetamine-induced locomotor activity and behavioral sensitization: are dopamine d3 receptors involved? Cell Mol Biol (Noisy-le-Grand, France) 2007;53:15–22. [PubMed] [Google Scholar]
- 22.Itzhak Y, Martin JL, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity in mice: long-lasting sensitization to the locomotor stimulation and desensitization to the rewarding effects of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1177–1183. doi: 10.1016/s0278-5846(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council of the National Academies. Washington, DC: The National Academies Press; 2003. Guidelines for the care and use of mammals in neuroscience and behavioral research. [PubMed] [Google Scholar]
- 24.Potter LT, Ballesteros LA, Bichajian LH, Ferrendelli CA, Fisher A, Hanchett HE, et al. Evidence of paired M2 muscarinic receptors. Mol Pharmacol. 1991;39:211–221. [PubMed] [Google Scholar]
- 25.Vanderheyden P, Gies JP, Ebinger G, De Keyser J, Landry Y, Vauquelin G. Human M1-, M2- and M3-muscarinic cholinergic receptors: binding characteristics of agonists and antagonists. J Neurol Sci. 1990;97:67–80. doi: 10.1016/0022-510x(90)90099-9. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd ed. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 27.Mark GP, Kinney AE, Grubb MC, Zhu X, Finn DA, Mader S, et al. Injection of oxotremorine in the nucleus accumbens shell reduces cocaine but not food self-administration. Brain Res. 2006;1123:51–59. doi: 10.1016/j.brainres.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gililland-Kaufman KR, Tanchuck MA, Ford MM, Crabbe JC, Beadles-Bohling AS, Snelling C, et al. The neurosteroid environment in the hippocampus exerts bi-directional effects on seizure susceptibility in mice. Brain Res. 2008;1243:113–123. doi: 10.1016/j.brainres.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You ZB, Wang B, Zitzman D, Wise RA. Acetylcholine release in the mesocorticolimbic dopamine system during cocaine seeking: conditioned and unconditioned contributions to reward and motivation. J Neurosci. 2008;28:9021–9029. doi: 10.1523/JNEUROSCI.0694-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kodama T, Honda Y. Acetylcholine releases of mesopontine PGO-on cells in the lateral geniculate nucleus in sleep-waking cycle and serotonergic regulation. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20:1213–1227. doi: 10.1016/s0278-5846(96)00107-8. [DOI] [PubMed] [Google Scholar]
- 31.Koyama Y, Sakai K. Modulation of presumed cholinergic mesopontine tegmental neurons by acetylcholine and monoamines applied iontophoretically in unanesthetized cats. Neuroscience. 2000;96:723–733. doi: 10.1016/s0306-4522(00)00004-x. [DOI] [PubMed] [Google Scholar]
- 32.Wilcox KS, Grant SJ, Burkhart BA, Christoph GR. In vitro electrophysiology of neurons in the lateral dorsal tegmental nucleus. Brain Res Bull. 1989;22:557–560. doi: 10.1016/0361-9230(89)90111-1. [DOI] [PubMed] [Google Scholar]
- 33.Luebke JI, McCarley RW, Greene RW. Inhibitory action of muscarinic agonists on neurons in the rat laterodorsal tegmental nucleus in vitro. J Neurophysiol. 1993;70:2128–2135. doi: 10.1152/jn.1993.70.5.2128. [DOI] [PubMed] [Google Scholar]
- 34.Chapman CA, Yeomans JS, Blaha CD, Blackburn JR. Increased striatal dopamine efflux follows scopolamine administered systemically or to the tegmental pedunculopontine nucleus. Neuroscience. 1997;76:177–186. doi: 10.1016/s0306-4522(96)00358-2. [DOI] [PubMed] [Google Scholar]
- 35.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 36.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Lacey MG, Calabresi P, North RA. Muscarine depolarizes rat substantia nigra zona compacta and ventral tegmental neurons in vitro through M1-like receptors. J Pharmacol Exp Ther. 1990;253:395–400. [PubMed] [Google Scholar]
- 38.Mereu G, Yoon KW, Boi V, Gessa GL, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- 39.De Boer P, Abercrombie ED. Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther. 1996;277:775–783. [PubMed] [Google Scholar]
- 40.Ikemoto S, Wise RA. Rewarding effects of the cholinergic agents carbachol and neostigmine in the posterior ventral tegmental area. J Neurosci. 2002;22:9895–9904. doi: 10.1523/JNEUROSCI.22-22-09895.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maskos U. The cholinergic mesopontine tegmentum is a relatively neglected nicotinic master modulator of the dopaminergic system: relevance to drugs of abuse and pathology. Br J Pharmacol. 2008;153 Suppl 1:S438–S445. doi: 10.1038/bjp.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winn P. Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat Disord. 2008;14 Suppl 2:S194–A198. doi: 10.1016/j.parkreldis.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Yasuda RP, Wall SJ, Wellstein A, Wolfe BB. Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol Pharmacol. 1991;40:28–35. [PubMed] [Google Scholar]
- 44.Vilaro MT, Wiederhold K-H, Palacios JM, Mengod G. Muscarinic cholinergic receptors in the rat caudate-putamen and olfactory tubercle belong predominantly to the m4 class: in situ hybridization and receptor autoradiography evidence. Neuroscience. 1991;40:159–167. doi: 10.1016/0306-4522(91)90181-m. [DOI] [PubMed] [Google Scholar]
- 45.Vilaro MT, Wiederhold KH, Palacios JM, Mengod G. Muscarinic M2 receptor mRNA expression and receptor binding in cholinergic and non-cholinergic cells in the rat brain: a correlative study using in situ hybridization histochemistry and receptor autoradiography. Neuroscience. 1992;47:367–393. doi: 10.1016/0306-4522(92)90253-x. [DOI] [PubMed] [Google Scholar]
- 46.Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-d-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- 48.Blaha CD, Yang CR, Floresco SB, Barr AM, Phillips AG. Stimulation of the ventral subiculum of the hippocampus evokes glutamate receptor-mediated changes in dopamine efflux in the rat nucleus accumbens. Eur J Neurosci. 1997;9:902–911. doi: 10.1111/j.1460-9568.1997.tb01441.x. [DOI] [PubMed] [Google Scholar]
- 49.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 50.Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Bio Psychiat. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 51.Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–414. [PubMed] [Google Scholar]
- 52.Alderson HL, Latimer MP, Winn P. Involvement of the laterodorsal tegmental nucleus in the locomotor response to repeated nicotine administration. Neurosci Lett. 2005;380:335–339. doi: 10.1016/j.neulet.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 53.Nelson CL, Wetter JB, Milovanovic M, Wolf ME. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience. 2007;146:41–49. doi: 10.1016/j.neuroscience.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]