The human body is formed by trillions of individual cells. These cells work together with remarkable precision, first forming an adult organism out of a single fertilized egg, and then keeping the organism alive and functional for decades. To achieve this precision, one would assume that each individual cell reacts in a reliable, reproducible way to a given input, faithfully executing the required task. However, a growing number of studies investigating cellular processes on the level of single cells revealed large heterogeneity even among genetically identical cells of the same cell type. Here we discuss the sources of heterogeneity in mammalian systems; how cells ensure reliable processing of information despite fluctuations in their molecular components; and what could be the benefit of cell-to-cell variability for mammalian cells.
Origin of heterogeneity
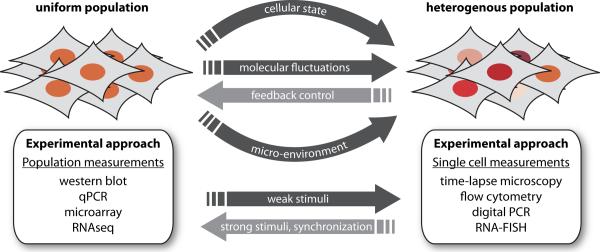
Mammalian cells have to deal with double trouble when it comes to heterogeneity. Part of the heterogeneity emerges from inside the cell, as a result of fluctuations in cellular components and changes in cellular state, while a dynamically changing microenvironment contributes additional uncertainty (Figure 1). Fluctuations in internal components are the consequence of the discrete nature of basic cellular processes. Main culprits are the low number of participating components, the burst-like nature of eukaryotic transcription, and random segregation at cell division, leading to stochastic changes in mRNA number and protein levels [1–3]. These fluctuations can affect how individual cells respond to external signals and influence cellular decision-making. For example, fluctuations of regulatory proteins were shown to determine the probability and timing of ligand-induced apoptosis [4] and the outcome of stem cell differentiation [5].
Figure 1.
Multiple internal and external factors increase heterogeneity between individual genetically identical cells of the same type. Experimental techniques that average the behavior of many cells together do not allow observing these variations and can result in misleading assumptions about cellular responses.
In addition to stochastic fluctuations, cellular states (such as cell-cycle phase or stress from intrinsic sources) have great influence on the cellular response. Intrinsic damage, for example, triggers a pulse of the tumor suppressor p53 in proliferating cells. In unsynchronized cells, the p53 response appears to happen spontaneously, giving the impression of a stochastic process. However, when the cell-cycle phase is taken into account, it becomes clear that p53 pulses are correlated with cellular activities that cause intrinsic damage, for example DNA replication [6]
Finally, the microenvironment of a cell is an important determinant of the cellular response. Parameters like local cell density, number of cell-cell contacts or free space per cell varies dramatically between individual cells. These parameters can influence cellular state and phenotype such as efficiency of endocytosis or susceptibility for viral infection [7]. Studying how fluctuation of internal components, cellular states and changes in the microenvironment cause heterogeneity in individual mammalian cells is essential for our ability to understand and predict cellular responses [8,9].
Observing and measuring heterogeneity in mammalian cells
Historically, cellular signal processing has been investigated with experiments based on populations of cells. While these experiments produce robust and reproducible data, their results only represent the average behavior of cells. This can be sufficient when all cells respond unanimously, for example when cells are treated with strong inputs that stimulate them in a synchronize manner despite their basal variations. However, when cells encounter weak stimuli, or when they lose synchrony during sustained activation, the response becomes more dependent on an individual's cellular state. For example, p53 dynamics in response to DNA damage were originally characterized as damped oscillations [10]. We later showed in single-cell experiments that individual cells show varying numbers of p53 pulses of fixed amplitude and duration [11]. The appearance of damped oscillations resulted from averaging the pulses across many cells (Figure 2). There are multiple additional examples known where the actual dynamical behavior of a signaling pathway is lost in the average response of the population [6,12–13]. In such cases experimental techniques that allow quantification of the cellular response with single cell resolution are essential.
Figure 2.
Single Cell observations: Population studies suggested that p53 levels are low in basal conditions and show damped oscillations in response to DNA damage [10]]. Single live-cell analysis using fluorescent reporter for p53 revealed that individual cells show a series of undamped p53 pulses in response to DNA damage [11] and similar, but less frequent pulses in basal conditions [6]. Averaging across a population of cells masked these behaviors of p53 as the timing of pulses varies between cells in basal conditions and different cells show different number of pulses in response to DNA damage.
Insights: Prior to the single cell studies, the general view was that the delayed negative feedback loop between p53 and Mdm2 is sufficient for triggering p53's damped oscillations [10,51]. However, this simple mechanism was insufficient in explaining the undamped pulses seen in single cells. Therefore, a new model was developed, in which the p53 pulses are driven by pulses in the upstream signaling molecules ATM-P and Chk2-P through a negative feedback loop from p53 to ATM [36]. In this model, p53 pulses are the result of repeated initiation from recurring examination of damaged DNA by ATM. The spontaneous bursts that were found in basal conditions taught us that activation of p53 is excitable; transient DNA damage during normal growth triggers similar pulses as high sustained damage. However, transient damage is not sufficient for changing p53 modifications from an inactive to an active state. Therefore, p53 pulses in basal conditions do not induce a cellular response such as cell cycle arrest [6].
A powerful technique for measuring cell to cell variability in protein abundance and dynamics is live cell time-lapse microscopy. Proteins of interest are fused with fluorescent proteins, which allow quantitative measurements of their levels and subcellular localization with high temporal and spatial resolution [14]. Various strategies have been used for generating live cell reporters, including transiently transfecting cells with vectors expressing the fluorescent reporters [12]; or the establishment of stable clonal cell lines [6,11,13]. Another approach is to integrate a fluorescent protein in the endogenous gene locus, either on bacterial artificial chromosomes [15] or in the genome by random viral insertion and screening [16] or by targeted somatic recombination [17]. The advantage of this approach is that the reporter is under the exact same transcription and translational regulation as the endogenous gene. For quantifying protein activity in single living cells, specialized biosensors have been developed [18]. They are often based on modification-dependent interaction of protein domains, which can be measured by Foerster Resonance Energy Transfer (FRET). This allows, for example, determining the activities of kinases [19–21], and second messengers like calcium [22]. Similarly, loss of FRET signal can be used to monitor the cleavage of defined peptide sequences by proteases [23].
One limitation of live-cell imaging is the time it takes for generating reliable reporter lines. And even after such a line exists, not all proteins can be successfully fused to a fluorescent tag while keeping them functional and without perturbing the natural system. To overcome these challenges, the levels and post-translational modification of proteins can be observed at the single cell level by flow-cytometry [24,25] or immunofluorescent microscopy [26]. While these methods are powerful in revealing heterogeneity in a large number of cells, they are limited by the inability to follow the same cell over time. A big effort is being made in developing new computational analysis for extracting dynamical information from protein distributions at stationary state.
mRNA distributions can be monitored in fixed cells using single cell RT-PCR for measuring high abundance transcripts [27], and with digital PCR for measuring mRNA at low concentrations [28]. In addition RNA levels have been followed successfully in fixed mammalian cells using Fluorescent in situ hybridization (FISH) [29–31]. This approach allows measurements of multiple mRNAs using distinct fluorophores. Although genome-wide transcription profiling in single mammalian cells is still challenging and associated with high costs, there are a few successful examples for such studies especially in neurons [32,33]. mRNA molecules can also be measured in real time using fluorescent RNA-binding proteins [34]. The strength of this approach is that it allows monitoring RNAs without delay, as the fluorescent proteins are constitutively expressed, giving them ample time to mature. In addition, both nascent mRNA at the genomic locus and mature mRNA in the cytoplasm, including its subcellular location, can be visualized with up to single molecule resolution. However, addition of the corresponding binding sites to the untranslated regions may alter the turnover rates of the RNA. Alternatively, indirect measurements of gene activity can be achieved through expressing a destabilized fluorescent protein under defined promoter sequences [6].
Gaining insights from studying non-genetic heterogeneity
Data collected from single cell measurements can lead to new insights into the structure and behavior of the studied system. First, determining which properties of the response are robust and which vary between cells can help better understand the wiring of the network and the parameters controlling it. For example, nuclear NFkB activity was found to be constant after stimulation with varying concentrations of ligand; however, the number of reacting cells and the duration of the response varied significantly between cells [13]. This suggested that cells are activated in response to TNFα in a digital manner and led to the development of an improved model of the NF-kB pathway under TNFα stimulation. Similarly, the finding that p53 pulses show noisy amplitude, but tightly controlled timing led to new understanding about the source of noise in this system and to the suggestion of a new model [35], which was later verified experimentally [36] (Figure 2).
Heterogeneity between cells can also be used to better understand the underlying principles of complex biological circuits. For example, the finding that IL-2 receptor levels vary significantly between T cells revealed how flexibility is achieved in the immune system between regulatory and effectors T cells [37]. In addition, studying single cells with no extrinsic stimulations helped understand how sensitivity and tolerance is achieved in the p53 network (Figure 2). Previous studies based on populations of cells led to the assumption that p53 levels are low at basal conditions. Single cell analysis revealed spontaneous pulses of p53 accumulation that had the same amplitude and duration as the pulses after DNA damage. These observations led to two main insights about the p53 network. First, that the p53 network behaves as an excitable system where low transient damage during normal growth leads to a full p53 pulse, similar to the pulses after severe damage [6,38]. Second, when damage is transient, a filtering mechanism keeps p53 pulses inactive (Figure 2). Specifically, for transcriptional activity, p53 requires a defined set of modifications, which occur only when damage persists. Such a mechanism enables cells to distinguish between transient damage, which does not justify arrest or cell death, and severe sustained damage that requires induction of the full stress response. The combination between an excitable system and a slower filtering mechanism is therefore powerful for cells as it allows a fast sensitive response and tolerance against noise [6] (Figure 2).
Potential benefits of non-genetic heterogeneity
Is heterogeneity beneficial or harmful for cells? In other words, has heterogeneity evolved as part of an optimized response [39] or is it just a side effect of noisy responses, which would otherwise require tight controls and high costs for correcting [40]? When considering unicellular organisms the answer is pretty clear; they often employ bethedging strategies for maximizing survival [41] [42]. In multi-cellular organisms the emerging picture is more complicated. On one hand, a predictable, synchronized cellular response is desirable for ensuring the whole tissue is functioning properly in a uniform way. Indeed, several reoccurring control circuits (e.g negative feedbacks, feed forward and autoregulatory loops) have been shown to eliminate or tolerate noise [42–45] and a significant increase in cell to cell variation in gene expression and splicing has been associated with ageing [46] and cancer [31]. On the other hand, several recent studies suggested that cell to cell variation can, in some cases, contributes to the proper responses of mammalian cells. Heterogeneity in PI3K activity was suggested to be beneficial for normal human cells by maintaining a subset of non-responding cells, preventing the risk of senescence or cancer of the whole population [25]. Another recent study showed that a dual negative feedback motif is optimized to increase variability in the timing of NFkB oscillations between cells, which minimizes fluctuations of the paracrine signaling at the tissue level [47]. Lastly, in the case of the p53 response, different sensitivities of individual cells, resulting from activating p53 in response to intrinsic stress in basal conditions [6] may be used to eliminate “weak” cells, while “healthy” undamaged cells survive.
It is also intriguing to ask whether heterogeneity is the “norm”; does it exist in most (or all) pathways in human cells or only in a small subset of them? Much attention has been given to heterogeneity in specific systems such as the p53 pathway, NFkB and the immune response, mainly since these are the systems that have been looked at the single cell level. We suspect that further single cell analyses will reveal heterogeneity in many additional pathways in mammalian cells, while the extent of heterogeneity may be dependent on the pathway, tissue or the specific input. New and improved tools for accurately measuring cellular responses at the single cell level will allow us to look at many other pathways in human cells and ask much more precise questions about the relationship between the initial cell state, the network behavior and the final outcome. We believe that in the long term, this kind of approach will also be used in the clinic. It has already been shown that cell-to-cell variation can cause a differential response to therapeutic drugs [48]. With improved imaging technologies and computational tools we may be able to measure individual cells' states and responses in tissues [49,50], and thus make decisions on the most likely therapy to be effective.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barkai N, Shilo BZ. Variability and robustness in biomolecular systems. Mol Cell. 2007;28:755–760. doi: 10.1016/j.molcel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Raj A, van Oudenaarden A. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell. 2008;135:216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huh D, Paulsson J. Non-genetic heterogeneity from stochastic partitioning at cell division. Nat Genet. 2011;43:95–100. doi: 10.1038/ng.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spencer SL, Gaudet S, Albeck JG, Burke JM, Sorger PK. Non-genetic origins of cell-to-cell variability in TRAIL-induced apoptosis. Nature. 2009;459:428–432. doi: 10.1038/nature08012. ++ Through measurements and modeling, Spencer et al. elegantly show that variability in the levels of regulatory proteins can determine cellular decisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang HH, Hemberg M, Barahona M, Ingber DE, Huang S. Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. Nature. 2008;453:544–547. doi: 10.1038/nature06965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loewer A, Batchelor E, Gaglia G, Lahav G. Basal dynamics of p53 reveals transcriptionally attenuated pulses in cycling cells. Cell. 2010 doi: 10.1016/j.cell.2010.05.031. in press. ++ In this study, time-lapse microscopy of cells in basal conditions was used to show how the p53 pathway achieves sensitivity in a noisy environment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snijder B, Sacher R, Ramo P, Damm EM, Liberali P, Pelkmans L. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature. 2009;461:520–523. doi: 10.1038/nature08282. ++ While many studies focus on stochastic processes as the sources of heterogeneity, Snijder et al. aim to understand how deterministic factors in the microenvironment cause variation in cellular responses. [DOI] [PubMed] [Google Scholar]
- 8.Snijder B, Pelkmans L. Origins of regulated cell-to-cell variability. Nat Rev Mol Cell Biol. 2011;12:119–125. doi: 10.1038/nrm3044. ++ While many studies focus on stochastic processes as the sources of heterogeneity, Snijder et al. aim to understand how deterministic factors in the microenvironment cause variation in cellular responses. [DOI] [PubMed] [Google Scholar]
- 9.Balazsi G, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–925. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proc Natl Acad Sci U S A. 2000;97:11250–11255. doi: 10.1073/pnas.210171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- 12.Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science. 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- 13.Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature. 2010;466:267–271. doi: 10.1038/nature09145. + Using microfluidics, the authors profile NFkB activity in response to a range of ligand concentrations in single cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ankers JM, Spiller DG, White MR, Harper CV. Spatio-temporal protein dynamics in single living cells. Curr Opin Biotechnol. 2008;19:375–380. doi: 10.1016/j.copbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Poser I, Sarov M, Hutchins JR, Heriche JK, Toyoda Y, Pozniakovsky A, Weigl D, Nitzsche A, Hegemann B, Bird AW, et al. BAC TransgeneOmics: a high-throughput method for exploration of protein function in mammals. Nat Methods. 2008;5:409–415. doi: 10.1038/nmeth.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Alaluf I, Swerdlin N, Perzov N, Danon T, Liron Y, et al. Dynamic proteomics in individual human cells uncovers widespread cell-cycle dependence of nuclear proteins. Nat Meth. 2006;3:525–531. doi: 10.1038/nmeth892. [DOI] [PubMed] [Google Scholar]
- 17.Issaeva I, Cohen AA, Eden E, Cohen-Saidon C, Danon T, Cohen L, Alon U. Generation of double-labeled reporter cell lines for studying co-dynamics of endogenous proteins in individual human cells. PLoS ONE. 2010;5:e13524. doi: 10.1371/journal.pone.0013524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta S, Zhang J. Reporting from the field: genetically encoded fluorescent reporters uncover signaling dynamics in living biological systems. Annu Rev Biochem. 2011;80:375–401. doi: 10.1146/annurev-biochem-060409-093259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ting AY, Kain KH, Klemke RL, Tsien RY. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci U S A. 2001;98:15003–15008. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–15002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsou P, Zheng B, Hsu CH, Sasaki AT, Cantley LC. A fluorescent reporter of AMPK activity and cellular energy stress. Cell Metab. 13:476–486. doi: 10.1016/j.cmet.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albeck JG, Burke JM, Aldridge BB, Zhang M, Lauffenburger DA, Sorger PK. Quantitative analysis of pathways controlling extrinsic apoptosis in single cells. Mol Cell. 2008;30:11–25. doi: 10.1016/j.molcel.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irish JM, Kotecha N, Nolan GP. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6:146–155. doi: 10.1038/nrc1804. [DOI] [PubMed] [Google Scholar]
- 25.Yuan TL, Wulf G, Burga L, Cantley LC. Cell-to-cell variability in PI3K protein level regulates PI3K-AKT pathway activity in cell populations. Curr Biol. 2011;21:173–183. doi: 10.1016/j.cub.2010.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo L-H, Wu LF, Altschuler SJ. Image-based multivariate profiling of drug responses from single cells. Nat Methods. 2007;4:445–453. doi: 10.1038/nmeth1032. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson M, Stahlberg A, Rorsman P, Kubista M. Gene expression profiling in single cells from the pancreatic islets of Langerhans reveals lognormal distribution of mRNA levels. Genome Res. 2005;15:1388–1392. doi: 10.1101/gr.3820805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren L, Bryder D, Weissman IL, Quake SR. Transcription factor profiling in individual hematopoietic progenitors by digital RT-PCR. Proc Natl Acad Sci U S A. 2006;103:17807–17812. doi: 10.1073/pnas.0608512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Femino AM, Fay FS, Fogarty K, Singer RH. Visualization of single RNA transcripts in situ. Science. 1998;280:585–590. doi: 10.1126/science.280.5363.585. [DOI] [PubMed] [Google Scholar]
- 30.Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods. 2008;5:877–879. doi: 10.1038/nmeth.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waks Z, Klein AM, Silver PA. Cell-to-cell variability of alternative RNA splicing. Mol Syst Biol. 2011;7:506. doi: 10.1038/msb.2011.32. + This study is the first to investigate variation in alternative splice form selection. While the variation is at the theoretical limit in healthy cells, it increases significantly in cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamme F, Salunga R, Yu J, Tran DT, Zhu J, Luo L, Bittner A, Guo HQ, Miller N, Wan J, et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J Neurosci. 2003;23:3607–3615. doi: 10.1523/JNEUROSCI.23-09-03607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tietjen I, Rihel JM, Cao Y, Koentges G, Zakhary L, Dulac C. Single-cell transcriptional analysis of neuronal progenitors. Neuron. 2003;38:161–175. doi: 10.1016/s0896-6273(03)00229-0. [DOI] [PubMed] [Google Scholar]
- 34.Tyagi S. Imaging intracellular RNA distribution and dynamics in living cells. Nat Meth. 2009;6:331–338. doi: 10.1038/nmeth.1321. [DOI] [PubMed] [Google Scholar]
- 35.Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, Yarnitzky T, Liron Y, Polak P, Lahav G, et al. Oscillations and variability in the p53 system. Mol Syst Biol. 2006;2:0033. doi: 10.1038/msb4100068. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Mol Cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinerman O, Jentsch G, Tkach KE, Coward JW, Hathorn MM, Sneddon MW, Emonet T, Smith KA, Altan-Bonnet G. Single-cell quantification of IL-2 response by effector and regulatory T cells reveals critical plasticity in immune response. Mol Syst Biol. 2010;6:437. doi: 10.1038/msb.2010.90. + Feinerman et al. show that heterogeneity in IL- 2 receptor levels is crucial for the function of regulatory T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batchelor E, Loewer A, Mock C, Lahav G. Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol. 2011;7:488. doi: 10.1038/msb.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010;467:167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lestas I, Vinnicombe G, Paulsson J. Fundamental limits on the suppression of molecular fluctuations. Nature. 2010;467:174–178. doi: 10.1038/nature09333. + A theoretical study showing the limits to which fluctuations can be suppressed in biological systems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 42.Acar M, Mettetal JT, van Oudenaarden A. Stochastic switching as a survival strategy in fluctuating environments. Nat Genet. 2008;40:471–475. doi: 10.1038/ng.110. [DOI] [PubMed] [Google Scholar]
- 43.Bluthgen N. Transcriptional feedbacks in mammalian signal transduction pathways facilitate rapid and reliable protein induction. Mol Biosyst. 2010;6:1277–1284. doi: 10.1039/c002598d. [DOI] [PubMed] [Google Scholar]
- 44.Bleris L, Xie Z, Glass D, Adadey A, Sontag E, Benenson Y. Synthetic Incoherent Feed-forward Circuits Show Adaptation to the Amount of their Genetic Template. Mol Syst Biol. 2011 doi: 10.1038/msb.2011.49. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goentoro L, Shoval O, Kirschner MW, Alon U. The incoherent feedforward loop can provide fold-change detection in gene regulation. Mol Cell. 2009;36:894–899. doi: 10.1016/j.molcel.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. doi: 10.1038/nature04844. ++ First study to show that variations in mRNA levels increase in ageing cells through increased genome damage. [DOI] [PubMed] [Google Scholar]
- 47.Paszek P, Ryan S, Ashall L, Sillitoe K, Harper CV, Spiller DG, Rand DA, White MR. Population robustness arising from cellular heterogeneity. Proc Natl Acad Sci U S A. 2010;107:11644–11649. doi: 10.1073/pnas.0913798107. + Using single cell reporters for studying NFkB dynamics this study suggests that highly heterogeneous single cell processes contribute to stability in the tissue level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science. 2008;322:1511–1516. doi: 10.1126/science.1160165. + This study shows that the variable susceptibility of cells to a chemotherapeutic drug can be mainly explained by fluctuations in two proteins. [DOI] [PubMed] [Google Scholar]
- 49.Orth JD, Kohler RH, Foijer F, Sorger PK, Weissleder R, Mitchison TJ. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 71:4608–4616. doi: 10.1158/0008-5472.CAN-11-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb Perspect Biol. 2:a003848. doi: 10.1101/cshperspect.a003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monk NA. Oscillatory expression of Hes1, p53, and NF-kappaB driven by transcriptional time delays. Curr Biol. 2003;13:1409–1413. doi: 10.1016/s0960-9822(03)00494-9. [DOI] [PubMed] [Google Scholar]