Abstract
Septins (full name: Septin; symbol name: SEPT) belong to a family of polymerizing GTP-binding proteins that are required for many cellular functions, including membrane compartmentalization, vesicle trafficking, mitosis and cytoskeletal remodeling. Two of the 14 family members in the mammalian species, Septin12 and 14 are expressed specifically in the testis. In the mouse, knockout of Septin4 and Septin12 leads to male sterility with distinctive sperm pathology (defective annulus or bent neck). In humans, sperm with abnormal expression patterns of SEPT4, 7 and 12 are more prevalent in infertile men. How septin filament is assembled/dissembled and how the SEPT-related complex regulates spermatogenesis still await further investigation.
Key words: SEPTIN, annulus, DNA damage, spermatogenic defects and male infertility
Septin Gene Family
SEPTs belong to a highly conserved family of polymerizing GTP binding proteins.1 Bioinformatic prediction indicates several potential motifs of the human SEPTs.1 The lengths of N-termini range from long (e.g., SEPT8 and SEPT9) to very short (SEPT1).2 The central GTP-binding domain is highly conserved in all human septins.1 Immediate N-terminal of the GTP-binding domain is a polybasic region which is also conserved in the eukaryotic phylogeny. Based on the C-termini sequences, human septins could be classified into four distinct groups. SEPT6, 7, 8, 10, 11, 13 and 14 have long stretch of coiled-coil sequences. They are further sub-divided into two groups: SEPT6 group (SEPT6, 8, 10, 11 and 14) and SEPT7 group (SEPT7 and SEPT13). SEPT 1, 2, 4 and 5 are similar to each other and have a short coiled-coil structure (SEPT2 group). Septins 3, 9 and 12 have no predicted C-terminal coiled-coil domain (SEPT3 group) (Table 1). It has been hypothesized that the coiled-coil domains is important for oligomerization of SEPT filament.2,4
Table 1.
The human septins and relevant summary information
| Gene name | Accession Number | Phylogeneic Relationships | Containing Domain* | Involved Diseases** |
| SEPTIN 1 | NM_052838 | Group 2 | PR, GBD, CC | Alzheimer disease |
| SEPTIN 2 | NM_004404 | Group 2 | PR, GBD, CC | MLL, Alzheimer disease, Brain tumor |
| SEPTIN 3 | NM_145733 | Group 3 | PR, GBD | Alzheimer disease |
| SEPTIN 4 | NM_004574 | Group 2 | PRD, PR, GBD, CC | Alzheimer disease, Male infertility, Down syndrome |
| SEPTIN 5 | NM_002688 | Group 2 | PR, GBD, CC | MLL, Down syndrome, Parkinsonism |
| SEPTIN 6 | NM_145799 | Group 1 | PR, GBD, CC | MLL |
| SEPTIN 7 | NM_001788 | Group 4 | PR, GBD, CC | Male infertility |
| SEPTIN 8 | NM_001098811.1 | Group 1 | PRD, PR, GBD, CC | |
| SEPTIN 9 | NM_001113491.1 | Group 3 | PRD, PR, GBD | MLL, Ovarian cancer, Infectious disease, Breast cancer, Prostate cancer |
| SEPTIN 10 | NM_144710.2 | Group 1 | PR, GBD, CC | - |
| SEPTIN 11 | NM_018243.2 | Group 1 | PR, GBD, CC | MLL |
| SEPTIN 12 | NM_144605 | Group 3 | PR, GBD | Male infertility |
| SEPTIN 13 | SEPT7/Pseduo-gene 2 | Group 4 | PR, GBD, CC | - |
| SEPTIN 14 | NM_207366.2 | Group 1 | PR, GBD, CC | - |
Physiological Roles of the Septin Gene Family
Septins are required for the completion of cytokinesis in somatic cells. Loss of SEPT function results in multinuclear phenotypes from yeast to mammals, suggesting highly conservative role of septins during evolution. The budding yeast, Saccharomyces cerevisiae, has five septins: Cdc3p, Cdc10p, Cdc11p, Cdc12p and Shs1p/Sep7p. They are localized to the ring(s) to compartmentalize mother and daughter cells.5 Mutations in any one of the five septins result in a distinctive phenotype with multi-nuclear and multi-cellular morphology.5,6 The nematode, Caenorhabditis elegans, has two septins: unc-59 and unc-61. Their mutants have normal early embryogenesis but show multiple defects, including abnormal morphogenesis of the vulva, male tail, sensory neurons and gonad in the larvae.7 There are at least 14 septin genes in the mammalian species. Some septins are expressed ubiquitously, while some are expressed only in certain types of types (e.g., neuron or male germ cells).1 In dividing cells, SEPT2, SEPT6, SEPT7 and SEPT9 have been implicated in the completion of cytokinesis, a highly conserved function during evolution.8–10 In well-differentiated cells, septins are involved in vesicle trafficking and cytoskeletal remodeling.11–13
The Role of Septins in Reproduction
Drosophila has three SEPTs: Pnut, Sep1 and Sep2. In Drosophila, septins are involved in the formation of ring canal structure between the intercellular bridge of male and female germ cells.14 The function of septins in the intercellular bridge seems to be conserved across different species. In the mouse, SEPT2, 7 and 9 are co-localized with an intercellular bridge markers of male germ cells, TEX14 (testis-expressed gene14).15 Loss of TEX14 in mice has been shown to cause disruption of intercellular bridge as well as increased apoptosis of germ cells.16 SEPT4, along with other SEPTs (SEPT1, SETP6 and SEPT7), is located at the annulus, a ring-like structure between the midpiece and the tail region of mature spermatozoa.12 During spermiogenesis, SEPT4 was found to be essential for the maintenance of proper mitochondrial architecture and establishment of the annulus. Septin4 null (Septin4−/−) mice were viable but sterile in male due to asthenozoospermia with bent neck.12,13 The immotile sperm with defective annulus also showed dis-localization of SEPT1, SEPT6 and SEPT7 from the annulus.12,13 The latter finding suggests septin complex in sperm may consist of SEPT1, 4, 6 and 7.12 Recently, Kwitny et al. found domain confinement was lost in the sperm tail of Septin4−/− mice. Their finding provides strong evidence of a role of mammalian septin structure in establishing membrane diffusion barrier.17 To address the function of Septin12, we knocked out the Septin12 locus in the mouse.18 All the chimeric mice were viable without obvious defects. The male chimeric mice were mated with C57BL/6 female mice but only few chimeric mice fathered black progeny (C57BL/6 genetic background). Most chimeric males were infertile. Semen analysis of the infertile chimeras showed decreased sperm counts, decreased sperm motility and spermatozoa with defects involving all subcellular compartments. Our findings suggested haploinsufficiency of Septin12 could disrupt spermiogenesis. Mice whose both Septin12 alleles are knocked out (Septin12−/−) have not been generated.
Expression Pattern of SEPT7 and 12 in the Mouse
SEPT12 is exclusively expressed in the mouse testis.18 Immunofluorescence staining showed SEPT7 and 12 expressions are confined to post-meitoic germ cells in the seminiferous tubules.18–20 During spermiogenesis, SETP7 and 12 filaments start to appear around the acrosome at step 7 of spermiogenesis. At step 10–11 of spermiogenesis, SEPT7 and 12 form a circular structure between the edge of acrosome and the perinuclear mantle of the manchette. With the formation of mitochondria, SEPT7 and 12 start to be localized at the sperm neck and annulus. In mature spermatozoa, the SEPT7 and 12 signals are located at the sperm head, neck and midpiece with scanty signals at the tail.18,19 Considering SEPT 7 and 12 are widely expressed and form ring-like structure in different locations of post-meiotic germ cells, including the peri-acrosome area, peri-nuclear area, midpiece and tail, we hypothesize they are structural proteins involved in the formation of subcellular compartments-head, neck, midpiece and tail, during terminal differentiation of male germ cells. Recent studies show that septins have a role in microtubule-dependent processes, such as karyokinesis, exocytosis and maintenance of cell shape. In addition, many members of the septin family have been shown to associate with the microtubule cytoskeleton. We speculate SEPTs, e.g., SEPT12, may participate in cytoskeleton remodeling during terminal differentiation of male germ line.21,22
Reproductive Phenotypes of Septin4−/− and Septin12+/− KO mice: Similarities and Differences
Septin4−/− male mice are sterile due to defective morphology and motility of the sperm flagellum. In Septin4−/− null spermatozoa, the annulus is disrupted with by a fragile segment lacking cortical material.12 In addition, Septin4−/− mutant sperm showed defects in acrosome and mitochondrial architecture and retention of the cytoplasmic droplet.12,13 Septin12+/− chimeric mice shared some common features with the Septin4−/− male: defects of annulus, mitochondrial architecture and acrosome. Retention of cytoplasmic droplet was also observed in Septin4−/− as well as Septin12+/− chimeric mice.18 However, the phenotypic effect of SEPT12 deficiency seems to be more profound than SEPT4. First, deletion of a single allele of Septin12 is sufficient to produce profound phenotypes. Second, immature germ cells exfoliated from the seminiferous tubules possibly due to maturation arrest at the round spermatid stage. Third, the acrosome defect is more apparent. There is also nuclear defect associated with haploinsufficiency of SEPT12. Electron microscopy examination of spermatozoa purified from the cauda epididymis revealed ultrastructural abnormalities only in the spermatozoa of Septin12+/−chimeras, including misshapen nuclei and broken acrosome. Some vesicles were found in the sperm nuclei, suggesting nuclear damage. In the chimeric mice with high percentage of SEPT12 deficient cells, almost all germ cells found in the cauda epididymis were round spermatids without tail formation. These findings suggest expression level of SEPT12 is critical for the formation of four major compartments (acrosome, mitochondrial, tail and nucleus) during spermiogenesis.
SEPT12 and DNA Damage
Although Septin4−/− mutant sperm could not fertilize oocytes, the sterility could be rescued by injection of the mutant sperm into oocytes, demonstrating mutant sperm carry an intact haploid genome.12,13 Consistently, oocytes fertilized by spermatozoa of Septin12+/− KO mice using intracytoplasmic sperm injection (ICSI) can't develop beyond the morula stage, possibly due to significant nuclear DNA damage.20 Given that SEPT12 is expressed at the edge of the sperm nucleus in both humans and mice, we hypothesized the vital roles of Septin12 in sperm head shaping, nuclear DNA condensation and early embryonic development.20 This finding is in line with the observation of misshapen nuclei in the sperm of Septin12+/− KO mice.18
Expression Patterns of SEPTs in Infertile Men
In humans, SEPT12 is expressed at the edge of the sperm nucleus, sperm neck, mitochondria and annulus during spermiogenesis.20 However, sperm with head, neck or tail abnormalities tended to lose their SETP12 and SEPT7 signals.18,19 In humans, disorganized annulus/SEPTIN rings were also identified in a subset of human patients with asthenozoospermia.12,23,24 These findings suggest defective synthesis, increased degradation or dysfunction of SEPTs may be causally related to both motility and morphological defects of sperm.
Septins as Sterile Genes
The causes of infertility could not be identified in the majority of cases with spermatogenic defects.25 The pathology of male infertility is diverse, including anatomic defects, gametogenesis dysfunction, endocrinopathies, immunologic problems, ejaculatory failure, environmental exposures and gene mutations.26–29 Given the distinct reproductive phenotype of the Septin12+/− chimeric mice and unique expression pattern of SEPT12, SEPTIN12 seems to be a good candidate for sterile gene in humans. Recently, Miyakawa et al. reasoned SEPTIN12 as a good candidate gene for male infertility and chose cases with Sertoli-cell-only syndrome (SCOS) to test their hypothesis.30 They compared genetic variants of 140 healthy men and 100 cases with SCOS and identified eight single-nucleotide polymorphisms (SNPs) in SEPTIN12. Among these SNPs, three synonymous variants were more prevalent in the SCOS patients, but their functional significance was not characterized. We also have found two missense mutations in men with infertility men compared with controls located in the predicted GTP-binding domain of SEPTIN12. Importantly, patients with mutations of SEPTIN12 were presented with oligo-astheno-teratozzoseprmia (OAT) and distinctive sperm morphology, including defective annulus and bent tail (unpublished data). Recently, SEPT14 was found to be a SEPT9-interacting partner and was exclusively expressed in the testis.31 The role of SEPTIN14 in spermatogenesis and male infertility also deserves further investigation.
SEPT Complex in Sperm
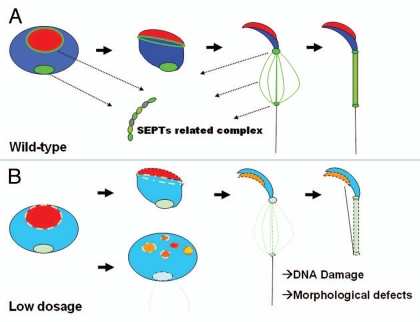
SEPTs usually mediate their cellular function through the formation of macromolecular and hetero-oligomeric filaments.3,9 Biochemical methods have been used to isolate several SEPT complexes (e.g., SEPT2/6/7, SEPT7/9b/11 and SEPT4/5/8).9,32,33 The filament-like structure was also observed in many SEPTs8,10,34 and loss of a SEPT subunit may affect the stability of the complex.9,10,35 The oligomeric core of SEPT-related complex in sperm remains to be uncovered. Since many SEPTs found in spermatids/spermatozoa are located at the annulus, there may be functional redundancy for some SEPTs. Septin12+/− KO mice shared similar phenotypes reminiscent of those for the Septin4−/− KO mice, but there are some obvious differences between mouse models of these two genes. SEPT12 has been found to interact with SEPT6 and SEPT11 and forms filaments in Hela cells.36,37 It is highly likely that SEPT12 cooperates in interactions with fixed stoichiometry with other SEPTINS (e.g., SEPT1, 4, 6, 7 or 11). Considering its dosage-sensitive effect, SEPT12 may play a pivotal role in the complex formation during spermiogenesis (Fig. 1).
Figure 1.
Working model of SEPT-related complex during mammalian spermatogenesis. (A) We reason that SEPT12-related complex consists of SEPT12, SEPT1, 4, 6 and 7. In the wild-type spermatozoa, SEPT12 coils around the arcosome and is concentrated at the neck of sperm. With the formation of mitochondria, SEPT12 starts to express at the neck and annulus. The SEPT 12 filaments also cover the mitochondrial area. (B) Decreased expression of SEPT12, results in maturation arrest at the spermatid stage, broken acrosome, bent tails, disorganized mitochondria and nuclear DNA damage.
Proteins in the GTPase superfamily usually engineer molecular switch to promote GTP binding and hydrolysis. Because the consensus GTPase domain exists in all SEPTs, it is expected that assembly of SEPT complex are mediated through GTPase signaling. So far, GTP binding/hydrolysis has not been demonstrated in the SEPT-related complex of sperm. However, we did observe decreased GTP binding/hydrolysis in two patients who carried missense mutations in the predicted GTP-binding domain of SEPTIN12 (unpublished data). Some proteins located at sperm annulus may be involved in regulating assembly/disassembly or stability of SEPT-related complex, including a novel Male Germ Cells Rab GTPase-Activating Proteins (MgcRabGAP), DNAJB13, a type 2 heart shock protein 40 (HSP40) and also a component of mouse sperm axoneme and TAT1, a new family member of Slc26 family of anion transporters.38–40 Tat1 null males were sterile due to disorganization of the midpiece-principal piece junction and abnormal mitochondrial sheath assembly, a phenotype characteristic of SPET4 and SEPT12 deficiency.40
It is also intriguing how SEPT12 deficiency results in nuclear DNA damage. In yeast, all of five septins, Cdc3p, Cdc10p, Cdc11p, Cdc12p and Shs1p/Sep7p, in the SEPT complex interacted with FHA domain of Rad 53, an important DNA damage checkpoint kinase.41 Shs1, one of these septins, appears to have an important role in the response to DNA replication stress.41 Cdc3p also interacted with BUB2, which is important to maintain a mitotic arrest during kinetochore damage.42 In mammalian cells, SEPT2/6/7 complexes regulate actin organization and are links to the DNA damage checkpoint by accumulation of adaptor protein, NCK, in nucleus.43 Whether the SEPT/SCOS7/NCK pathway is conserved during evolution deserves further investigation.
Prospects
Of all family members, four septin genes have been targeted in the mouse. Except for Sepint4 and Septin12, Septin3, Septin5 and Septin6 deficiencies do not cause overt phenotypes, suggesting a high degree of functional redundancy in the SEPTIN system.12,13,18,44–46 SEPT12 thus becomes an ideal target for male contraception. Mature spermatozoa consist of four major compartments: acrosome, nucleus, midpiece and tail. How these compartments are formed during terminal differentiation still remains obscure. Studies on the septin gene family may provide further insights into the biochemical basis of intracellular compartmentalization during spermatogenesis.
Acknowledgments
We deeply appreciated the Transgenic Mouse Models Core of National Research Program for Genome Medicine (NRPGM) for generating the knockout mice. This study was supported by grants from the National Science Council of the Republic of China. (NSC91-3112-B-006-008; NSC92-3112-B-006-002; NSC93-3112-B-006-004; NSC94-2314-B-006-075; NSC95-2314-B-006-011; NSC96-2314-B-006-003, NSC97-2622-B-006-002-CC1; NSC98-2622-B-006-004-CC1 and NSC 99-2628-B-006-027).
References
- 1.Hall PA, Jung K, Hillan KJ, Russell SE. Expression profiling the human septin gene family. J Pathol. 2005;206:269–278. doi: 10.1002/path.1789. [DOI] [PubMed] [Google Scholar]
- 2.Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204:489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 3.Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13:3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weirich CS, Erzberger JP, Barral Y. The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol. 2008;9:478–489. doi: 10.1038/nrm2407. [DOI] [PubMed] [Google Scholar]
- 5.Hartwell LH. Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp Cell Res. 1971;69:265–276. doi: 10.1016/0014-4827(71)90223-0. [DOI] [PubMed] [Google Scholar]
- 6.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/S1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113:3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- 8.Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, et al. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11:1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3:791–802. doi: 10.1016/S15345807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- 10.Spiliotis ET, Kinoshita M, Nelson WJ. A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science. 2005;307:1781–1785. doi: 10.1126/science.1106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. doi: 10.1242/jcs.114.5.839. [DOI] [PubMed] [Google Scholar]
- 12.Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–364. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Hime GR, Brill JA, Fuller MT. Assembly of ring canals in the male germ line from structural components of the contractile ring. J Cell Sci. 1996;109:2779–2788. doi: 10.1242/jcs.109.12.2779. [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum MP, Ma L, Matzuk MM. Conversion of midbodies into germ cell intercellular bridges. Dev Biol. 2007;305:389–396. doi: 10.1016/j.ydbio.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenbaum MP, Yan W, Wu MH, Lin YN, Agno JE, Sharma M, et al. TEX14 is essential for intercellular bridges and fertility in male mice. Proc Natl Acad Sci USA. 2006;103:4982–4987. doi: 10.1073/pnas.0505123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82:669–678. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YH, Lin YM, Wang YY, Yu IS, Lin YW, Wang YH, et al. The expression level of septin12 is critical for spermiogenesis. Am J Pathol. 2009;174:1857–1868. doi: 10.2353/ajpath.2009.080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao HC, Lin YH, Kuo YC, Shen CJ, Pan HA, Kuo PL. The expression pattern of SEPT7 correlates with sperm morphology. J Assist Reprod Genet. 2010;27:299–307. doi: 10.1007/s10815-010-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin YH, Chou CK, Hung YC, Yu IS, Pan HA, Lin SW, et al. SEPT12 deficiency causes sperm nucleus damage and developmental arrest of preimplantation embryos. Fertil Steril. 2011;95:363–365. doi: 10.1016/j.fertnstert.2010.07.1064. [DOI] [PubMed] [Google Scholar]
- 21.Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET. Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J Cell Biol. 2011;194:187–197. doi: 10.1083/jcb.201102076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman-Gavrila RV, Silverman-Gavrila LB. Septins: new microtubule interacting partners. ScientificWorldJournal. 2008;8:611–620. doi: 10.1100/tsw.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugino Y, Ichioka K, Soda T, Ihara M, Kinoshita M, Ogawa O, et al. Septins as diagnostic markers for a subset of human asthenozoospermia. J Urol. 2008;180:2706–2709. doi: 10.1016/j.juro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Lhuillier P, Rode B, Escalier D, Lores P, Dirami T, Bienvenu T, et al. Absence of annulus in human asthenozoospermia: case report. Hum Reprod. 2009;24:1296–1303. doi: 10.1093/humrep/dep020. [DOI] [PubMed] [Google Scholar]
- 25.Silber SJ. Evaluation and treatment of male infertility. Clin Obstet Gynecol. 2000;43:854–888. doi: 10.1097/00003081-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Matzuk MM, Lamb DJ. Genetic dissection of mammalian fertility pathways. Nat Cell Biol. 2002;4:41–49. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 27.Hirsh A. Male subfertility. BMJ. 2003;327:669–672. doi: 10.1136/bmj.327.7416.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferlin A, Raicu F, Gatta V, Zuccarello D, Palka G, Foresta C. Male infertility: role of genetic background. Reprod Biomed Online. 2007;14:734–745. doi: 10.1016/S1472-6483(10)60677-3. [DOI] [PubMed] [Google Scholar]
- 29.Cooke HJ, Saunders PT. Mouse models of male infertility. Nat Rev Genet. 2002;3:790–801. doi: 10.1038/nrg911. [DOI] [PubMed] [Google Scholar]
- 30.Miyakawa H, Miyamoto T, Koh E, Tsujimura A, Miyagawa Y, Saijo Y, Namiki M, et al. Single-Nucleotide Polymorphisms in the SEPTIN12 Gene May Be a Genetic Risk Factor for Japanese Patients with Sertoli Cell-Only Syndrome. J Androl. 2011 doi: 10.2164/jandrol.110.012146. [DOI] [PubMed] [Google Scholar]
- 31.Peterson EA, Kalikin LM, Steels JD, Estey MP, Trimble WS, Petty EM. Characterization of a SEPT9 interacting protein, SEPT14, a novel testis-specific septin. Mamm Genome. 2007;18:796–807. doi: 10.1007/s00335-007-9065-x. [DOI] [PubMed] [Google Scholar]
- 32.Nagata K, Asano T, Nozawa Y, Inagaki M. Biochemical and cell biological analyses of a mammalian septin complex, Sept7/9b/11. J Biol Chem. 2004;279:55895–55904. doi: 10.1074/jbc.M406153200. [DOI] [PubMed] [Google Scholar]
- 33.Martínez C, Sanjuan MA, Dent JA, Karlsson L, Ware J. Human septin-septin interactions as a prerequisite for targeting septin complexes in the cytosol. Biochem J. 2004;382:783–791. doi: 10.1042/BJ20040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, et al. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278:18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- 35.Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16:4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding X, Yu W, Liu M, Shen S, Chen F, Wan B, et al. SEPT12 interacts with SEPT6 and this interaction alters the filament structure of SEPT6 in Hela cells. J Biochem Mol Biol. 2007;40:973–978. doi: 10.5483/BMBRep.2007.40.6.973. [DOI] [PubMed] [Google Scholar]
- 37.Ding X, Yu W, Liu M, Shen S, Chen F, Cao L, et al. GTP Binding Is Required for SEPT12 to Form Filaments and to Interact with SEPT11. Mol Cells. 2008;25:385–389. [PubMed] [Google Scholar]
- 38.Guan J, Kinoshita M, Yuan L. Spatiotemporal association of DNAJB13 with the annulus during mouse sperm flagellum development. BMC Dev Biol. 2009;9:23. doi: 10.1186/1471-213X-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YH, Lin YM, Kuo YC, Wang YY, Kuo PL. Identification and characterization of a novel Rab GTPase-activating protein in spermatids. Int J Androl. 2010 doi: 10.1111/j.1365-2605.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 40.Touré A, Lhuillier P, Gossen JA, Kuil CW, Lhote D, Jegou B, et al. The testis anion transporter 1 (Slc26a8) is required for sperm terminal differentiation and male fertility in the mouse. Hum Mol Genet. 2007;16:1783–1793. doi: 10.1093/hmg/ddm117. [DOI] [PubMed] [Google Scholar]
- 41.Smolka MB, Chen SH, Maddox PS, Enserink JM, Albuquerque CP, Wei XX, et al. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J Cell Biol. 2006;175:743–753. doi: 10.1083/jcb.200605081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan R, Pangilinan F, Lee C, Spencer F. Saccharomyces cerevisiae BUB2 prevents mitotic exit in response to both spindle and kinetochore damage. Genetics. 2000;156:489–500. doi: 10.1093/genetics/156.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer BE, Adang LA, Macara IG. Septins regulate actin organization and cell cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell. 2007;130:837–850. doi: 10.1016/j.cell.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dent J, Kato K, Peng XR, Martinez C, Cattaneo M, Poujol C, et al. A prototypic platelet septin and its participation in secretion. Proc Natl Acad Sci USA. 2002;99:3064–3069. doi: 10.1073/pnas.052715199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono R, Ihara M, Nakajima H, Ozaki K, Kataoka-Fujiwara Y, Taki T, et al. Disruption of Sept6, a fusion partner gene of MLL, does not affect ontogeny, leukemogenesis induced by MLL-SEPT6 or phenotype induced by the loss of Sept4. Mol Cell Biol. 2005;25:10965–10978. doi: 10.1128/MCB.25.24.10965-78.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsang CW, Fedchyshyn M, Harrison J, Xie H, Xue J, Robinson PJ, et al. Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol Cell Biol. 2008;28:7012–7029. doi: 10.1128/MCB.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]