Abstract
Spermatogenesis is the process of production of male gametes from SSCs. The SSCs are the stem cells that differentiate into male gametes in the testis. in the mean time, the Spg are remarkable for their potential multiple trans-differentiations, which make them greatly invaluable for clinical applications. However, the molecular mechanism controlling differentiation of the Spg is still not clear. Among the discovered spermatogenesis-related genes, c-kit seems to be expressed first by the Spgs thus may play a central role in switching on the differentiation process. Expression of Kit and the activation of the Kit/Kitl pathway coincide with the start of differentiation of Spgs. Several genes have been discovered to be related to the Kit/Kitl pathway. in this review, we have summarized the recent discoveries of c-kit and the Kit/Kitl pathway-related genes in the spermatogenic cells during different stages of spermatogenesis.
Key words: c-kit, spermatogenesis, sperm, proliferation, restoration, differentiation, meiosis, vitamin A, BMP4, FSH
Introduction
It has long been believed that “differentiating” Spg are irreversibly committed to differentiation. In other words, the c-kit positive differentiated Spg can't reverse to c-kit negative stem cells. But in male and female Drosophila melanogaster, it is shown that differentiating germ cells can revert to functional stem cells that can restore germinal lineage.1,2 A study by Barroca et al. reported that in mouse, purified c-kit positive Spg, although committed to differentiate, can repopulate when transplanted into the γ-irradiated germ-cell-depleted adult mice testes. GDNF and FGF2 are found to be able to reprogram in vitro Spg for reverse differentiation.3 As a marker of Spg differentiation, functions of c-kit include anti-apoptosis in PGCs, promoting cell replication in PGCs and Spg, and initiating the entry of Spg into meiosis.4 As the fate of germ cell lineage in mammals is defined by a chain of inductions, we will discuss the role of c-kit and its signaling pathway (Kit/Kitl pathway) in Spg differentiation by structure, function and interacting factors.5
Spermatogenesis and c-kit
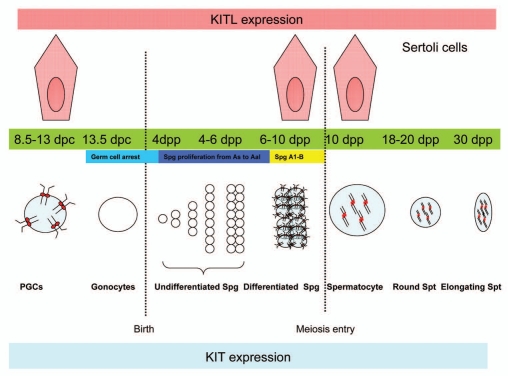
Spermatogenesis is a highly regulated process of differentiation and complex morphologic alterations that leads to the formation of sperm in the seminiferous epithelium. In the adult male mammals, it can be subdivided into three main phases: spermatogonial proliferation, meiosis of spermatocytes and spermiogenesis of haploid spermatids. In the adult testes, spermatogenesis starts from diploid SSCs. The SSCs, also called type As Spg, are located on the basal membrane of the seminiferous tubules. The As Spg can self-renew or produce the type Ap Spg. After successive divisions, Ap Spg differentiate and form chains of 4, 8 or 16 type Aal Spg and migrate along the basal membrane. According to morphological criteria, the SSCs, Ap and Aal Spg are classically called undifferentiated Spg. Aal Spg differentiate into more committed A1 Spg that will further divide and differentiate into A2, A3, A4, intermediate and B Spg, which will undergo meiosis after a final mitosis.3 The “undifferentiated” (As, Ap and Aal) and the “differentiating” (A1, A2, A3, A4, intermediate and B) Spg differ in the expression of c-kit.6 The transition of undifferentiated Spg into differentiating Spg coincides with the gain of Kit. The presence of Kit in Spg has been routinely used as a marker to identify differentiating Spg.7,8 Figure 1 summarizes c-kit expression during spermatogenesis. Kit continues to be expressed until meiosis and play essential roles in the survival of the Kit-expressing cells.6,9 Kitl is expressed in the Sertoli cells which there have extensive contacts with the germ cells. Kit is activated only after binding with Kitl and the Kit/Kitl pathway is considered to be crucial for the proliferation, migration, survival and maturation of the germ cells both in the embryonic and the postnatal gonads.9–18 Mice carrying a mutation rendering a constitutively active Kit kinase have an interrupted transition from the round into the elongating spermatids.19
Figure 1.
Schematic representation of expression of c-kit in mouse germ cells from fetus to 30 days postpartum. PGCs from 8.5–13 dpc express full-length Kit which is located on the membrane. From 13.5 dpc to 3 dpp, the PGCs lose Kit expression and turn into c-kit- gonocytes which are arrested. At 3 dpp, the gonocytes start to proliferate into As and Aal Spg, which are undifferentiated without expressing Kit. Expression of c-kit is re-started at around 7 dpp when the A1–B stage Spg appear. A truncated isoform of c-kit starts to be expressed (Tr-Kit) in the cytocol of the spermatocytes and is kept expressed in later stage germ cells (spermatocytes, spermatids and spermatozoa). Sertoli cells express Kitl from 8.5 dpc to 13.5 dpc and 6–10 dpp only. Entry of germ cells into meiosis occurs around 10 dpp. Expression of Kit and Kitl in the spermatogenic cells and Sertoli cells respectively is represented by the red color in the membrane, cytosol and nucleus.
c-kit plays a key role in maintaining the ratio between self-renewal and differentiation of SSCs.20 In normal seminiferous epithelium, the ratio between self-renewal and differentiation of spermatogonial stem cells is maintained at 1.0. Alterations in this ratio entail greater Spg self-renewal leading to Kit+ tumor cells in the seminiferous epithelium.21 In the mean time, the alterations also entail stem cell depletion resulting in Sertoli cells only syndrome.22
Transcription and Translation of c-kit and its Ligand in Spermatogenic Cells
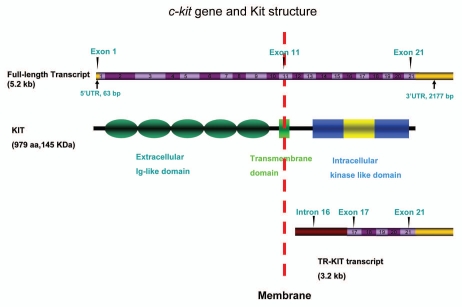
c-kit is allelic to the W locus on mouse chromosome 5.23 The 21-exon gene encodes a 5,150 bp transcript which is translated into a product of 145 kDa protein with 979 amino acid residues which is called Kit.24 c-kit mRNA and protein synthesis are regulated separately possibly by circulating hormones as the undifferentiated Spg contains only c-kit mRNA but not protein.15 Kit belongs to a family of growth factor receptors with intrinsic tyrosine kinase activity that transduces growth regulatory signals across the plasma membrane. Kit has three main functional regions: the extracellular domain, the trans-membrane region and the intracellular domain. The extracellular domain consists of five immunoglobin-like repeats with about 520 amino acids which are required for ligand binding and dimerization.25 The transmembrane region is a 23 amino acid hydrophobic domain, which anchors the receptor to the cell membrane. The 433 amino acid intracellular domain consists of three domains, with a proximal kinase region for ATP binding, a 70–100 amino acid non-conserved insert and a distal phosphotransferase kinase region.26 Binding to the Kitl induces a rapid and complete receptor dimerization that involves activation by autophosphorylation of the tyrosine kinase residues (Fig. 2).27 The phosphoTyrosine (pTyr) residues in the intracellular juxtamembrane domain subsequently serve as docking sites for signal transduction molecules.28
Figure 2.
c-kit gene and Kit structure. The c-kit DNA is 5,150 bp in length, consists of 21 exons and encodes a 150 KDa full-length protein with 979 amino acids. Exons 1–10 encode the extracellular components with 5 Ig-like domains and the signal sequences. Exon 11 encodes the transmembrane segment. Exons 12–20 encodes the intercellular segments including the juxtamembrane segment, proximal kinase domain, kinase insert domain and distal kinase domain. Exon 21 encodes the C-terminal tail. Truncated mRNA from intron 16 to exon 21 encodes the TR-Kit.
Kit has function not only during spermatogenesis, but also throughout all stages of male germ cell development before and after birth. Northern blot analysis of germ cells at different developmental stages has shown the presence of two alternative mRNA of c-kit, 3.2 and 2.3 kb in length respectively, in the haploid cells of the mouse testis.29 With an Open Reading Frame (ORF) that starts in the intron 16th mouse c-kit, the alternative spermatid-specific c-kit transcripts contain all the downstream exons, and encode for a truncated form of the Kit protein (∼30 kDa), called Tr-Kit, including 12 hydrophobic amino acids followed by the last 190 carboxy terminal residues of the Kit.30,31 Tr-Kit derived from alternative promoter in the intron 16 of c-kit and encodes part of the non-conserved insert from the C-terminal tail region and the distal phosphotransferase kinase region, and it lacks the entire extracellular and the transmembrane domain.32 This intronic promoter of the c-kit is only active in the late stages of spermatogenesis, suggesting a role for this truncated protein either during spermatid differentiation or for the function of mature sperm.33 Tr-Kit is found in the residual sperm cytoplasm and there is evidence for its serving a function in the activation of oocyte at fertilization in mice.31
Kitl and Spermatogenesis
Kitl, also called SCF, is produced in the Sertoli cells and is a cytokine essential for haematopoiesis, melanogenesis and development of germ cells. Kitl has been identified as an analogue of the murine steel (Sl) locus and is located on chromosome 12 in humans. Two isoforms of Kitl are generated from the same gene by alternative splicing—a soluble (Kitls) and a transmembrane (Kitlm) form.34,35 The soluble form arises after proteolytic cleavage of a membrane-bound precursor.36 In the Spg proliferating stage, the membrane isoform is a predominant one; whereas in the Spg quiescent stage, the soluble form dominates. Actions of Kit/Kitl system include stimulation of PGC migration, enhancement of proliferation of PGCs/Spg and anti-apoptosis of PGCs.4 However, the down-stream signaling pathways involved on Spg proliferation and anti-apoptosis seem to be different. In vitro addition of Kitl to A1-A4 Spgs results in a transient activation of Erk1/2 and PI3K which lead to Spg proliferation.37 Kitl existed in the seminal plasma in human significantly correlate with the sperm count.38 Mutation in Kitl in human has significant association of idiopathic male infertility.39 Together with the soluble growth factors including GDNF and FGF2, Kitl might contribute to construct the potential niche which stimulates stem cell divisions.40 Kitl is also found to be able to increase the percentage of sperm undergoing acrosome reaction when cultured in vitro.41
Kit/Kitl-Dependent Mechanisms during Spermatogenesis
Four pathways are known to be activated in response to Kit/Kitl activation in the Spg. The PI3K pathway results in cell survival (via AKT and BAD regulation), adhesion (via c-JUN and c-FOS activation) and proliferation (via AKT and p70S6K). The PI3K/AKT pathway appears to be critical exclusively in postnatal stage spermatogenesis. Mice with a mutant form of Kit are incapable of PI3K recruiting and are sterile caused by reduced proliferation and increased apoptosis in the Spg.42 Through PI3K pathway, Kit/Kitl facilitate the upregulation and nuclear accumulation of cyclin D3 as well as Spg proliferation suggesting that cyclin might be one of the targets of Kit/Kitl pathway within the testis.37,43 Secondly, the SRC pathway involves the association of SRC family proteins with the intracellular juxtamembrane domain of Kit and affects cell migration and AKT phosphorylation in mice PGCs.44 Thirdly, Tr-Kit activated PLCG through the PLCG pathway, mediates the resumption of meiosis of the fertilized eggs.45 Lastly, the MAPK cascade is activated by RAS with the binding of Kit and GRB2. MAPK directly mediates gene transcription in PGCs and proliferation in Spg.37,44,46
Roles of c-kit in Embryonic and Neonatal Spermatogenic Cells
c-kit and spermatogenic cell proliferation and restoration.
In mouse, at around 7.2 dpc, somatic signals earmark a small cohort of proximal epiblast cells as potential germ cell precursors.47 This group of cells moves into the extraembryonic tissue at the base of the allantois, where a second round of selection occurs which results in a group of about 45 cells specified to be germ cell precursors or PGCs. After specification, the germ cells become transcriptionally silent at 9.5 dpc and are subject to an extensive reprogramming of their genomes by histone modifications and alterations in the state of DNA methylation.48 C-kit mRNA is first detected in the PGCs at 6.5–7 dpc and persists during their subsequent proliferation and migration to the genital ridge (7.5–13.5 dpc).49,50 In the mean time, the somatic cells along the migratory pathway and genital ridges synthesize Kitl.16,17,51,52 In the absence of either Kit or Kitl, mice are sterile and with a reduced number of PGCs.17 Kitl secreted by the somatic cells seems to be an attractant for germ cells migration and are required for their adhesion, proliferation, migration and survival prior to 10 dpc after which downregulation of Kitl is associated with switching on the intrinsic apoptotic pathway in ectopic germ cells.16,44,53 We wonder if Kit/Kitl pathway may facilitate SSCs survival by suppressing apoptosis as that in the ES cells.54
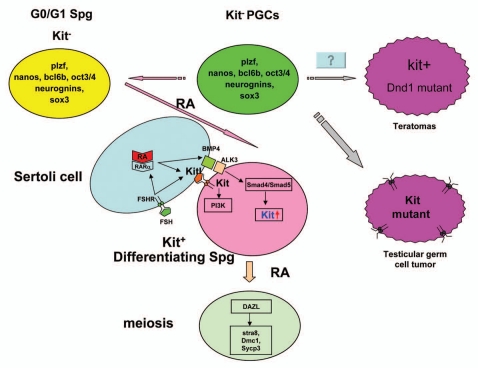
Unlike in the female where kit expression in oogonia continues into meiosis, the male PGCs arrest in G0/G1 of the mitotic cycle around 13.5 dpc and resume mitosis around 3 days post partum (dpp) during which Kit expression is markedly reduced in mice (Fig. 3).49 At around 3 dpp, expression of Kit is still low when the male PGCs actively proliferate again. The transition from c-kit independent type to c-kit dependent type occurs at about 5 dpp when the competence to enter meiosis is reached.12,55,56 Expression of Kit (3 dpp) is before the expression of Kitl (6–8 dpp) and their expression is closely coordinated.57–59
Figure 3.
Summary of Kit/Kitl signaling during spermatogenesis. The c-kit+ PGCs lose c-kit expression and turn into the undifferentiated Spg which characteristically express Plzf, Nanos, Bcl6b, Oct3/4, neurogenin3 and Sox3. Most of those undifferentiated Spg will go into c-kit- mitotic arrest (G0/G1 stage) under control of meiosis-inhibiting factor Cyp26b1. Undifferentiated Spg arrested during mitosis can return to normal spermatogenesis triggered by various differentiation signals. The lack of c-kit negative mitotic arrest disorders will lead to teratomas. RA, FSH and BMP4 may facilitate Spg differentiation as well as the activation of the Kit/Kitl signal pathway. Active RA acts on Sertoli cells through the RARα to stimulate the synthesis of growth factors Kitl, which bind to Kit to activate kit/Kitl/PI3K pathway. ALK3, receptor of BMP4, is specifically expressed in the mitotic Spg during the first week after birth. BMP4 action is mediated by a rapid nuclear translocation of Smad4 and Smad5 which make the Spg start to express c-kit. Mutations of c-kit (resulting in self-activation of Kit) at post-migration stage of Spg will cause bilateral testicular germ cell tumors. Differentiated Spg will go into meiosis after the activation of Kit/Kitl signaling. Dazl is a competence factor for meiosis initiation and germ cells start to express Stra8, Dmc1 and Sycp3 once get into meiosis.
Even though its activation represents the start of differentiation in Spg, c-kit is an important stem cell marker for many types of stem cells such as hematopoietic stem cells.60 Expression of Kit in the SSCs is contradictory. In the early studies, Kit expression in the adult testis is detected by immunohistochemical analysis and in situ hybridization in the differentiating type A (A1–A4), intermediate, and type B spermatogonia, as well as preleptotene spermatocytes and interstitial Leydig cells, but not in the undifferentiated spermatogonia and Sertoli cells.6,9 Hence, activation of the Kit/Kitl signaling pathway is not required for SSCs self-renewal.20,61 More recent studies demonstrate that both Kit− and Kit+ cells showed comparable levels of stem cell activity after germ cell transplantation.3,20,62 As SSCs can change their phenotype according to their microenvironment, Kit+ cells might be an intermediate state during SSCs self-renewal.20 Izadyar et al. further characterized the Kit+ SSCs and find that the POU5F1+/Kit+ subset of mouse SSCs generates cell lines that express pluripotent ES markers and can differentiate into multiple lineages. But in vivo testes regeneration assay shows that only the POU5F1+/Kit− SSCs will regenerate the spermatogenesis of the recipient tests.63 Inactivation of c-kit by Imatinib caused an impairment of Spg self-renewal.64 Therefore, Kit seems not to affect SSCs self-renewal directly, instead, it might affect the size of SSCs pool by playing a role during the phenotypic transitions of SSCs (Fig. 4). Intriguingly, Kit is expressed by SSCs such as As, Apr and Aal even though activation of the Kit/Kitl signaling pathway is not required for their self-renewal which demonstrates the comlex dynamics of spermatogonial differentiation.20,61,65–67
Figure 4.
c-kit and phenotypic transition of SSCs during spermatogenesis. There are two kinds of SSCs including the Kit+ and Kit− SSCs in the early stage of postnatal spermatogenesis in mice. The Kit− SSCs is the performer of normal spermatogenesis. In order to complete self-renewal, Kit− SSCs change their phenotype to Kit+ SSCs according to their microenvironment. Unlike the Kit− SSCs, Kit+ SSCs have limited pluripotence and cannot re-enter spermatogenesis after in vivo transplantation to the recipient mice.
Role of c-kit in onset of meiosis.
Activation of Kit signaling before meiosis and inactivation of Kit after meiosis are important for proper spermatogenesis. Synthesis of c-kit mRNA and protein is concordant with the first appearance of differentiating Spg, and persists at relatively lower levels in meiotic pachytene spermatocytes.15 Followed by c-kit activation, early meiotic markers such as Dmc1 and Scp3 are activated.68 It has been demonstrated that the timing of meiosis entry is indirectly controlled by the Sertoli cells through the activation of Kit/Kitl system when they are induced by RA.11,13,69–71 In vitro experiment has proved that the addition of RA to the Kit expressing Spg will induce the onset of spermatogenesis but not the Kit negative Spg.70 Kit/Kitl activation causes a transient activation of ERK1/2 and PI3K-dependent AKT kinase. These events are followed by a rapid nuclear redistribution of cyclin D3 and accumulation of cyclin E that promotes cell cycle progression via the PI3K/p70 S6 kinase pathway.37,43 Hyperphosphorylation of retinoblastoma protein Rb by cyclin E/cdk2 is followed by the release of Rb-associated transcription factor E2F, which elicits a timely induction of other genes required for S-phase progression.72 Silencing c-kit expression by siRNA in the Spg induces cell cycle arrest thus confirming the role of Kit on meiosis entrance.73 Transcriptome analysis of the Spg treated with Kitl indicates that Kitl stimulates their entrance of meiotic program by upregulating the G1/S transition inhibitors and G2/M promoters and by downregulating the G1/S promoters.74
Kit and mature sperm.
c-kit gene translates to two kinds of proteins, Kit and Tr-Kit, during spermatogenesis. Tr-Kit protein is expressed during human spermiogenesis and maintained in human spermatozoa. Tr-Kit begins to be expressed in the post-meiotic haploid germ cells.30,31 Cytometric analysis of several human sperm samples from volunteer donors, showed variable degrees of the Tr-Kit-specific immunolabeling, and a significant inverse correlation of the Tr-Kit positivity with markers of sperm damage, i.e., DNA fragmentation, as revealed by TUNEL analysis and the intense clusterin positivity. So, the maintaining of Tr-Kit in the haploid spermatogenic cells appears to correlate with the next stage spermatozoa DNA integrity.75 Whether Kit is present in these haploid germ cells is under debate. Muciaccia et al. found that Kit and its coding mRNA was not detected in the spermatozoa.75 Feng et al. show that the mature human spermatozoa express Kit and its presence appears to be correlated with sperm capacitation and the acrosomal reaction. The percentage of sperm underwent acrosomal reaction declined and the percentage of head-to-head agglutination increased following blocking with Kit antibodies.76
Mechanisms Controlling the Activation of Kit/Kitl Pathway
The mechanisms controlling c-kit expression during spermatogenesis are not very clear. However, several upstream regulating factors have been discovered in the studies of PGCs, oogenesis and other organisms. Here we will discuss 3 factors which directly or indirectly relate to Kit/Kitl pathway activation in the Spg.
Vitamin A and its derivatives.
Retinoic acid (RA), an active metabolite of vitamin A, is a vital signaling molecule for normal fetal development, pattern formation, cell proliferation and differentiation, and apoptosis.77 RA is synthesized by the mesonephroi to which the gonads are attached.78 ES cells will differentiate to PGCs when culture with 2 µM RA for 5 days.79,80 RA may regulate proliferation and differentiation of Spg mainly through RARα mediated signal pathway. During post-natal development, RARα and RXRβ are confined to Sertoli cells, whereas RARγ is expressed in Spg followed by a colocalization of RARβ, RXRα and RXRγ to the step 7–8 spermatids.81 RA acts to initiate meiosis both in male and in female. In male, exogenous RA can induce XY Aal staged germ cells in a cultured mouse fetal testis to enter meiotic prophase.82 It is not yet known whether the action of RA in inducing differentiation and c-kit expression is directly, or indirectly via Kitl in the Sertoli cell. It is accepted that RA regulates the timing of meiosis indirectly via juxtacrine signaling by Sertoli cells.70 Some studies show that RA directly act on spermatogenic cells by stimulating Stra8 and c-kit gene expression, whereas others show exogenous RA could not stimulate c-kit expression in spermatogenic cells but cause apoptosis of the Aal Spg.83–85 Besides, RA also upregulates Kitl levels in the Sertoli cells, resulting in increased levels of the early meiotic cell markers. This activation is independent of germ cell viability and occurs through the phosphatidylinositol-3-kinases (PI3K) and MAP kinase (MAPK) pathways.70
BMP4/ALK3/SMAD5 signaling pathway.
BMP4, one of the TGFβ-BMP superfamily growth factor, is produced by Sertoli cells very early in the postnatal life and is downregulated during peri-pubertal phase. BMP4 treatment of the PGCs, SSCs and Spg increases Kit levels and causes a mitogenic response to Kitl.86–88 BMP4 expression is significantly upregulated in the testes of VAD mice and is downregulated in freshly isolated germ cells treated by retinol. This reflects a direct requirement for retinoid by germ cells for the resumption of spermatogenesis in VAD animals via mechanisms that involve the suppression of BMP4 expression.89 Receptors of BMP4 (ALK3 and BMPIIR) are specifically expressed in mitotic Spg during the first week after birth. BMP4 action is mediated by a rapid nuclear translocation of Smad4 and Smad5, where the Smad4/Smad5 complexes are able to recruit the transactivating factor CBP and to bind Smad-responsive DNA sequences.
Another member of the TGFβ-BMP superfamily growth factor is BMP8b which stimulate both PGCs and Spg to proliferate. BMP8b-/- mice show impairment of PGC commitment, defects of Spg proliferation and spermatocyte apoptosis.90,91
FSH.
FSH, LH and the testis androgen are involved in the process of orchestrated control of spermatogenesis. FSH is not essential for spermatogenesis but is required for quantitatively normal sperm production in both mice and human.92–96 FSH works directly on Sertoli cells via FSHR. Kitl is expressed by Sertoli cells under FSH stimulation. So, the Sertoli cells of the genetic mutant mice lacking FSH receptor will produce less Kitl. These mutant mice exhibit reduced testis, epididymis, and seminal vesicle as well as low levels of testosterone. A significant increase in the percentage of c-kit positive Spg and non-germ cells and a significant decrease in the percentage of elongated spermatids are observed in these mice. The increase in the percentage of c-kit-positive cells and decrease in the testosterone values of FSH receptor mutant mice may be due to the reduced levels of Kitl available for intercellular communication in the absence of FSH receptor signaling.97 In the Sertoli cells, FSH can regulate transcriptional function of the RARα, thus controlling cell proliferation and differentiation.98 Therefore, it appears that it is FSH that determines the expression of c-kit in the Spg via Sertoli cell factors including Kitl and RARα.
Roles of Kit/Kitl Pathway and Related Genes in Spg Maintenance
Genes involved in Spg maintenance.
Plzf, Nanos, Bcl6b, Oct3/4, neurogenin3 and Sox3 are markers of the undifferentiated Spg. The DNA sequence-specific transcriptional repressor, Plzf, is considered to be involved in stem cell maintenance. Loss of Plzf function shifts the balance between spermatogonial stem cell self-renewal and differentiation toward differentiation at the cost of self-renewal and leads to an increase of post-meiosis apoptotic cells.99,100 It is shown that Plzf directly represses the transcription of Kit.101 Nanos encodes for a zinc-finger RNA-binding protein and shows a translational repression activity requiring the interaction with the ubiquitously expressed protein Pumilio. The Nanos-Pumilio protein complex binds to NRE in the 3′ UTR of target mRNAs and represses their translation.102 It has been indicated that Nanos3 is required to prevent PGCs from undergoing apoptosis during migratio.103 Overexpression of Nanos3 causes an increase of G1 stage undifferentiated Spg. RA significantly decreases the expression of Nanos3 in the undifferentiated Spg.104,105 So, Nanos3 is important for maintaining the undifferentiated stage of Spg. Nanos2 suppresses meiosis by preventing stra8 expression. Nanos2-/- male PGCs go into apoptosis at 16.5 dpp and are completely lost before birth.106 Oct3/4 is the stem cell and germ line specific marker encoding DNA binding domain POU107 and it is also called Pou5f1. When RA binds to RARs, expression of Oct3/4 is inhibited. In the germ cell-specific nulls of Pou5f1, XX and XY germ cells undergo apoptosis between 9.5–10.5 dpc, before colonization of the gonad.108
At around 13.5 dpc, germ cells in the testis enter mitotic arrest in G0 until near birth.109 At this stage, low level expression of meiosis-associated genes, such as Sycp3 and Dmc1, indicates that all these germ cells are capable of entering meiosis.68 It is hyphthesized that the mitotic arrest is induced by a testis-derieved meiosis-inhibiting factor.110–112 Cyp26b1 seems to be one of the meiosis-inhibiting factors which regulate the amount of RA in the prenatal gonads.113 Germ cells that fail to enter into mitotic arrest forms teratomas in the Dnd1 mutant male mice and a finely tuned temporal expression of c-kit appears critical for normal spermatogenesis and surrpession of testicular tumors.114–116
Dazl is a competence factor for meiosis initiation.
The Dazl gene family encodes potential RNA binding proteins that are expressed in prenatal and postnatal germ cells of males and females. In the testis, this protein is localized to the nucleus of Spg but relocates to the cytoplasm during meiosis where it persists in spermatids and spermatozoa. Dazl is expressed from 11.5–12.5 dpc and is important for successful completion of meiotic prophase. Expression of Dazl is required for stra8 responses to RA stimulation as RA could not stimulate stra8 in Dazl-/- testis.78,117,118 In male germ cells, Dazl is expressed in the Aal to A1 Spg.119,120 The prime spermatogenic defect in the Dazl-/- mice is a failure of the great majority of the Aal Spg to differentiate into A1 Spg though most of the A Spg of Dazl-/- mice were positively stained for the c-kit protein. Most seminiferous tubules of Dazl-/ mice only contain actively proliferating As, Apr and Aal Spg, with no further successful differentiation due to apoptosis of subsequent cell types.120
Stra8, Dmc1 and Sycp3 are meiotic marker genes.
Stra8 is a vertebrate-specific gene that encodes a cytoplasmic protein whose expression is induced by retinoic acid.84,121 It is required for premeiotic DNA replication, chromosome condensation and subsequence events of meiotic prophase in germ cells of embryonic ovaries.122 Located only in both male and female premeiotic germ cells (specifically in the Spg and preleptotene-stage spermatocytes in males), Stra8 may play a role in the premeiotic phase of spermatogenesis.>121,123
In male mice, Stra8 is expressed postnatally, in the mitotically active cells of the Spg and their immediate descendants (preleptotene spermatocytes).121,124 The peak of Stra8 mRNA expression coincides with the onset of meiosis in postnatal testes. Stra8 is detected as early as 5 dpc and its expression in the neonatal testes was not uniform among Spg. In adult testes, the highest level of Stra8 mRNA and protein was found in seminiferous epithelial stages VI–VIII. In normal adult testes, RA stimulated Stra8 mRNA expression. Stra8 expression in adult Spg is induced by RA stimulation, suggesting its role in spermatogonial differentiation.84,124 Retinoic acid also increases the number of preleptotene spermatocytes exhibiting 5-bromo-2-deoxyuridine incorporation, indicating a more synchronized premeiotic DNA replication.124 Mice lacking Stra8 function produces no sperm; most spermatogenic cells undergo apoptosis at a developmental stage when they normally would have progressed through meiotic prophase.122 Figure 3 concludes the possible network of genes controlling the normal Spg differentiation towards meiosis.
Conclusion and Prospect of Spg Studies
Mechanisms controlling spermatogenesis is mammals are still unclear. So far, we have known that the SSCs proliferate by stimulation of soluble growth factors (GDNF and FGF etc.,) and cell structural gene products (Plzf, Nanos, Bcl6b, Oct, Neurogenin 3 and Sox 3, etc.). Competence of entering of meiosis should be obtained (expression of Dazl) before the SSCs could respond the meiosis-promoting factors such as Syp3, Dmc1 and Stra8. Meiosis is regulated not only by the meiosis-promoting factors, but also by meiosis-inhibiting factors (such as Cyp26b1). The prospect of Spg researches should be focused on the mechanisms driving SSCs differentiation toward a haploid male germ cell which will help those infertile men who can't produce sperm.
Acknowledgments
This work is supported by Hong Kong RGC grant (2140638) to Y.B. Han.
Abbreviations
- As A single
Aal, A aligned
- Apr
A paired
- ADH
alcohol dehydrogenase
- ALK3 (also called BMPR1A)
bone morphogenetic protein receptor, type 1A
- AKT
thymoma viral proto-oncogene 1
- BAD
BCL2-associated agonist of cell death
- BMP4
bone morphogenetic protein 4
- BMP8b
bone morphogenetic protein 8b
- CBP
sarcoplasmic calcium-binding protein
- cdk2
cyclin-dependent kinase 2
- c-Fos
FBJ osteosarcoma oncogene
- c-Jun
Jun oncogene
- CRABP
cellular retinoic acid binding protein
- CYP26B1
cytochrome enzyme P450
- Dmc1
DMC1 dosage suppressor of mck1 homolog
- dpc
days post coitum
- dpp
days post partum
- E2F
E2F transcription factor
- ERK
elk-related tyrosine kinase
- FGF2
fibroblast growth factor 2
- FSH
follicular stimulating hormone
- FSHR
FSH receptor
- GDNF
glial cell line-derived neurotrophic factor
- GRB2
growth factor receptor-bound protein 2
- Kit
Kit receptor
- Kitl
Kit ligand
- Kitm
membrane form of Kit
- Kits
soluble Kit
- Kitlm
membrane form of Kitl
- Kitls
soluble kit ligand
- LH
luteinizing hormone
- MAPK
mitogen-activated protein kinases
- NRE
nanos-responsive element
- p70S6K
Rps6kb1, ribosomal protein S6 kinase
- PGCs
primordial germ cells
- PI3K
phosphoinositide-3-kinase
- PLCG
phospholipase Cgamma
- RA
retinoic acid
- RALDH
retinaldehyde dehydrogenase
- RAR
retinoic acid receptor
- RARE
retinoic acid responding element
- RAS
RAt Sarcoma
- Rb
retinoblastoma protein
- RBP
Retinoic acid binding protein
- SCF
stem cell factor
- SCP3
synaptonemal complex protein 3
- SiRNA
small interfering RNA
- Smad5
MAD homolog 5
- Spg
spermatogonia
- SRC
rous sarcoma oncogene
- Sry
Sex-determining region Y
- SSC
spermatogonial stem cell
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-nick end labeling
- VAD
vitamin A deficient
Genes
- Aldh1a2
also called Raldh 2, aldehyde dehydrogenase family 1, subfamily A2
- Bcl-2
B-cell leukemia/lymphoma 2
- Bcl6b
B-cell CLL/lymphoma 6, member B
- c-kit
also called CD117, kit oncogene
- Dazl
deleted in azoospermia-like
- Dmc1
also called Dmc1 h, dosage suppressor of mck1 homolog
- Kitl
also called SCF, kit ligand
- Mvh
also called Ddx4, DEAD (Asp-Glu-Ala-Asp) box polypeptide 4
- Nanos2
nanos homolog 2
- Nanos3
nanos homolog 3
- Neurogenin3
also called neurogenin3
- Oct3/4
also called Pou5f1, POU domain, class 5, transcription factor 1
- Sox3
SRY-box containing gene 3
- Stra8
stimulated by retinoic acid gene 8
- Sycp3
also called Scp3 and Cor1 Synaptonemal complex protein
References
- 1.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 2.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 3.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11:190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 4.Mauduit C, Hamamah S, Benahmed M. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update. 1999;5:535–545. doi: 10.1093/humupd/5.5.535. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, de Sousa Lopes SM, Surani MA. Germ cell specification in mice. Science. 2007;316:394–396. doi: 10.1126/science.1137545. [DOI] [PubMed] [Google Scholar]
- 6.Schrans-Stassen BH, de Kant HJ, v de Rooij DG, van PAM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 7.Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 10.Dym M, Jia MC, Dirami G, Price JM, Rabin SJ, Mocchetti I, Ravindranath N. Expression of c-kit receptor and its autophosphorylation in immature rat type A spermatogonia. Biol Reprod. 1995;52:8–19. doi: 10.1095/biolreprod52.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Packer AI, Besmer P, Bachvarova RF. Kit ligand mediates survival of type A spermatogonia and dividing spermatocytes in postnatal mouse testes. Mol Reprod Dev. 1995;42:303–310. doi: 10.1002/mrd.1080420307. [DOI] [PubMed] [Google Scholar]
- 12.Ohta H, Yomogida K, Dohmae K, Nishimune Y. Regulation of proliferation and differentiation in spermatogonial stem cells: the role of c-kit and its ligand SCF. Development. 2000;127:2125–2131. doi: 10.1242/dev.127.10.2125. [DOI] [PubMed] [Google Scholar]
- 13.Yan W, Suominen J, Toppari J. Stem cell factor protects germ cells from apoptosis in vitro. J Cell Sci. 2000;113:161–168. doi: 10.1242/jcs.113.1.161. [DOI] [PubMed] [Google Scholar]
- 14.Guerif F, Cadoret V, Rahal-Perola V, Lansac J, Bernex F, Panthier JJ, et al. Apoptosis, onset and maintenance of spermatogenesis: evidence for the involvement of Kit in Kit-haplodeficient mice. Biol Reprod. 2002;67:70–79. doi: 10.1095/biolreprod67.1.70. [DOI] [PubMed] [Google Scholar]
- 15.Prabhu SM, Meistrich ML, McLaughlin EA, Roman SD, Warne S, Mendis S, et al. Expression of c-Kit receptor mRNA and protein in the developing, adult and irradiated rodent testis. Reproduction. 2006;131:489–499. doi: 10.1530/rep.1.00968. [DOI] [PubMed] [Google Scholar]
- 16.Runyan C, Schaible K, Molyneaux K, Wang Z, Levin L, Wylie C. Steel factor controls midline cell death of primordial germ cells and is essential for their normal proliferation and migration. Development. 2006;133:4861–4869. doi: 10.1242/dev.02688. [DOI] [PubMed] [Google Scholar]
- 17.Gu Y, Runyan C, Shoemaker A, Surani A, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136:1295–1303. doi: 10.1242/dev.030619. [DOI] [PubMed] [Google Scholar]
- 18.Orth JM, Qiu J, Jester WF, Jr, Pilder S. Expression of the c-kit gene is critical for migration of neonatal rat gonocytes in vitro. Biol Reprod. 1997;57:676–683. doi: 10.1095/biolreprod57.3.676. [DOI] [PubMed] [Google Scholar]
- 19.Schnabel D, Ramirez L, Gertsenstein M, Nagy A, Lomeli H. Ectopic expression of KitD814Y in spermatids of transgenic mice, interferes with sperm morphogenesis. Dev Dyn. 2005;233:29–40. doi: 10.1002/dvdy.20292. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto H, Kanatsu-Shinohara M, Takashima S, Chuma S, Nakatsuji N, Takehashi M, Shinohara T. Phenotypic plasticity of mouse spermatogonial stem cells. PLoS One. 2009;4:7909. doi: 10.1371/journal.pone.0007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokemeyer C, Kuczyk MA, Dunn T, Serth J, Hartmann K, Jonasson J, et al. Expression of stem-cell factor and its receptor c-kit protein in normal testicular tissue and malignant germ-cell tumours. J Cancer Res Clin Oncol. 1996;122:301–306. doi: 10.1007/BF01261407. [DOI] [PubMed] [Google Scholar]
- 22.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–354. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 23.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335:88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 24.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, et al. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blechman JM, Lev S, Barg J, Eisenstein M, Vaks B, Vogel Z, et al. The fourth immunoglobulin domain of the stem cell factor receptor couples ligand binding to signal transduction. Cell. 1995;80:103–113. doi: 10.1016/0092-8674(95)90455-7. [DOI] [PubMed] [Google Scholar]
- 26.Blechman JM, Lev S, Givol D, Yarden Y. Structure-function analyses of the kit receptor for the steel factor. Stem Cells. 1993;11:12–21. doi: 10.1002/stem.5530110804. [DOI] [PubMed] [Google Scholar]
- 27.Lev S, Yarden Y, Givol D. Dimerization and activation of the kit receptor by monovalent and bivalent binding of the stem cell factor. J Biol Chem. 1992;267:15970–15977. [PubMed] [Google Scholar]
- 28.Roskoski R., Jr. Signaling by Kit protein-tyrosine kinase—the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 29.Sorrentino V, Giorgi M, Geremia R, Besmer P, Rossi P. Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene. 1991;6:149–151. [PubMed] [Google Scholar]
- 30.Rossi P, Marziali G, Albanesi C, Charlesworth A, Geremia R, Sorrentino V. A novel c-kit transcript, potentially encoding a truncated receptor, originates within a kit gene intron in mouse spermatids. Dev Biol. 1992;152:203–207. doi: 10.1016/0012-1606(92)90172-d. [DOI] [PubMed] [Google Scholar]
- 31.Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, Rossi P. Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development. 1997;124:2267–2274. doi: 10.1242/dev.124.11.2267. [DOI] [PubMed] [Google Scholar]
- 32.Albanesi C, Geremia R, Giorgio M, Dolci S, Sette C, Rossi P. A cell- and developmental stage-specific promoter drives the expression of a truncated c-kit protein during mouse spermatid elongation. Development. 1996;122:1291–1302. doi: 10.1242/dev.122.4.1291. [DOI] [PubMed] [Google Scholar]
- 33.Sette C, Dolci S, Geremia R, Rossi P. The role of stem cell factor and of alternative c-kit gene products in the establishment, maintenance and function of germ cells. Int J Dev Biol. 2000;44:599–608. [PubMed] [Google Scholar]
- 34.Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 35.Flanagan JG, Chan DC, Leder P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- 36.Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolci S, Pellegrini M, Di AS, Geremia R, Rossi P. Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem. 2001;276:40225–40233. doi: 10.1074/jbc.M105143200. [DOI] [PubMed] [Google Scholar]
- 38.Fujisawa M, Kanzaki M, Okuda Y, Okada H, Arakawa S, Kamidono S. Stem cell factor in human seminal plasma as a marker for spermatogenesis. Urology. 1998;51:460–463. doi: 10.1016/s0090-4295(97)00627-4. [DOI] [PubMed] [Google Scholar]
- 39.Galan JJ, De Felici M, Buch B, Rivero MC, Segura A, Royo JL, et al. Association of genetic markers within the KIT and KITLG genes with human male infertility. Hum Reprod. 2006;21:3185–3192. doi: 10.1093/humrep/del313. [DOI] [PubMed] [Google Scholar]
- 40.Ebata KT, Yeh JR, Zhang X, Nagano MC. Soluble growth factors stimulate spermatogonial stem cell divisions that maintain a stem cell pool and produce progenitors in vitro. Exp Cell Res. 2011;317:1319–1329. doi: 10.1016/j.yexcr.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Feng H, Sandlow JI, Sandra A. The c-kit receptor and its possible signaling transduction pathway in mouse spermatozoa. Mol Reprod Dev. 1998;49:317–326. doi: 10.1002/(SICI)1098-2795(199803)49:3<317::AID-MRD12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 42.Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, Hunter T. Kit/stem cell factor receptor-induced activation of phosphatidylinositol-3′-kinase is essential for male fertility. Nat Genet. 2000;24:157–162. doi: 10.1038/72814. [DOI] [PubMed] [Google Scholar]
- 43.Feng LX, Ravindranath N, Dym M. Stem cell factor/c-kit upregulates cyclin D3 and promotes cell cycle progression via the phosphoinositide-3-kinase/p70 S6 kinase pathway in spermatogonia. J Biol Chem. 2000;275:25572–25576. doi: 10.1074/jbc.M002218200. [DOI] [PubMed] [Google Scholar]
- 44.Farini D, La Sala G, Tedesco M, De Felici M. Chemoattractant action and molecular signaling pathways of Kit ligand on mouse primordial germ cells. Dev Biol. 2007;306:572–583. doi: 10.1016/j.ydbio.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 45.Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 2002;21:5386–5395. doi: 10.1093/emboj/cdf553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mithraprabhu S, Loveland KL. Control of KIT signalling in male germ cells: what can we learn from other systems. Reproduction. 2009;138:743–757. doi: 10.1530/REP-08-0537. [DOI] [PubMed] [Google Scholar]
- 47.Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 48.Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278:440–458. doi: 10.1016/j.ydbio.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Orr-Urtreger A, Avivi A, Zimmer Y, Givol D, Yarden Y, Lonai P. Developmental expression of c-kit, a protooncogene encoded by the W locus. Development. 1990;109:911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- 50.Manova K, Bachvarova RF. Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev Biol. 1991;146:312–324. doi: 10.1016/0012-1606(91)90233-s. [DOI] [PubMed] [Google Scholar]
- 51.Matsui Y, Zsebo KM, Hogan BL. Embryonic expression of a haematopoietic growth factor encoded by the Sl locus and the ligand for c-kit. Nature. 1990;347:667–669. doi: 10.1038/347667a0. [DOI] [PubMed] [Google Scholar]
- 52.De Felici M, Di CA, Pesce M. Role of stem cell factor in somatic-germ cell interactions during prenatal oogenesis. Zygote. 1996;4:349–351. doi: 10.1017/s0967199400003373. [DOI] [PubMed] [Google Scholar]
- 53.Godin I, Deed R, Cooke J, Zsebo K, Dexter M, Wylie CC. Effects of the steel gene product on mouse primordial germ cells in culture. Nature. 1991;352:807–809. doi: 10.1038/352807a0. [DOI] [PubMed] [Google Scholar]
- 54.Bashamboo A, Taylor AH, Samuel K, Panthier JJ, Whetton AD, Forrester LM. The survival of differentiating embryonic stem cells is dependent on the SCFKIT pathway. J Cell Sci. 2006;119:3039–3046. doi: 10.1242/jcs.03038. [DOI] [PubMed] [Google Scholar]
- 55.Tajima Y, Sawada K, Morimoto T, Nishimune Y. Switching of mouse spermatogonial proliferation from the c-kit receptor-independent type to the receptor-dependent type during differentiation. J Reprod Fertil. 1994;102:117–122. doi: 10.1530/jrf.0.1020117. [DOI] [PubMed] [Google Scholar]
- 56.Ohta H, Tohda A, Nishimune Y. Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biol Reprod. 2003;69:1815–1821. doi: 10.1095/biolreprod.103.019323. [DOI] [PubMed] [Google Scholar]
- 57.Rossi P, Albanesi C, Grimaldi P, Geremia R. Expression of the mRNA for the ligand of c-kit in mouse Sertoli cells. Biochem Biophys Res Commun. 1991;176:910–914. doi: 10.1016/s0006-291x(05)80272-4. [DOI] [PubMed] [Google Scholar]
- 58.Tajima Y, Sakamaki K, Watanabe D, Koshimizu U, Matsuzawa T, Nishimune Y. Steel-Dickie (Sld) mutation affects both maintenance and differentiation of testicular germ cells in mice. J Reprod Fertil. 1991;91:441–449. doi: 10.1530/jrf.0.0910441. [DOI] [PubMed] [Google Scholar]
- 59.Tajima Y, Onoue H, Kitamura Y, Nishimune Y. Biologically active kit ligand growth factor is produced by mouse Sertoli cells and is defective in SId mutant mice. Development. 1991;113:1031–1035. doi: 10.1242/dev.113.3.1031. [DOI] [PubMed] [Google Scholar]
- 60.Wilson A, Oser GM, Jaworski M, Blanco-Bose WE, Laurenti E, Adolphe C, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann NY Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 61.Kubota H, Avarbock MR, Schmidt JA, Brinster RL. Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation. Biol Reprod. 2009;81:293–301. doi: 10.1095/biolreprod.109.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trefil P, Bakst MR, Yan H, Hejnar J, Kalina J, Mucksová J. Restoration of spermatogenesis after transplantation of c-Kit positive testicular cells in the fowl. Theriogenology. 2010;74:1670–1676. doi: 10.1016/j.theriogenology.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Izadyar F, Pau F, Marh J, Slepko N, Wang T, Gonzalez R, et al. Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction. 2008;135:771–784. doi: 10.1530/REP-07-0479. [DOI] [PubMed] [Google Scholar]
- 64.Heim C, Minniear K, Dann CT. Imatinib has deleterious effects on differentiating spermatogonia while sparing spermatogonial stem cell self renewal. Reprod Toxicol. 2011;31:454–463. doi: 10.1016/j.reprotox.2010.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki H, Sada A, Yoshida S, Saga Y. The heterogeneity of spermatogonia is revealed by their topology and expression of marker proteins including the germ cell-specific proteins Nanos2 and Nanos3. Dev Biol. 2009;336:222–231. doi: 10.1016/j.ydbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di CAD, Travia G, De Felici M. The meiotic specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. Int J Dev Biol. 2000;44:241–244. [PubMed] [Google Scholar]
- 69.Vincent S, Segretain D, Nishikawa S, Nishikawa SI, Sage J, Cuzin F, Rassoulzadegan M. Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: a Kit-KL interaction critical for meiosis. Development. 1998;125:4585–4593. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 70.Pellegrini M, Filipponi D, Gori M, Barrios F, Lolicato F, Grimaldi P, et al. ATRA and KL promote differentiation toward the meiotic program of male germ cells. Cell Cycle. 2008;7:3878–3888. doi: 10.4161/cc.7.24.7262. [DOI] [PubMed] [Google Scholar]
- 71.Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, et al. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rossi P, Dolci S, Sette C, Geremia R. Molecular mechanisms utilized by alternative c-kit gene products in the control of spermatogonial proliferation and sperm-mediated egg activation. Andrologia. 2003;35:71–78. doi: 10.1046/j.1439-0272.2003.00539.x. [DOI] [PubMed] [Google Scholar]
- 73.Sikarwar AP, Reddy KV. siRNA-mediated silencing of c-kit in mouse primary spermatogonial cells induces cell cycle arrest. Oligonucleotides. 2008;18:145–160. doi: 10.1089/oli.2008.0108. [DOI] [PubMed] [Google Scholar]
- 74.Rossi P, Lolicato F, Grimaldi P, Dolci S, Di Sauro A, Filipponi D, Geremia R. Transcriptome analysis of differentiating spermatogonia stimulated with kit ligand. Gene Expr Patterns. 2008;8:58–70. doi: 10.1016/j.modgep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Muciaccia B, Sette C, Paronetto MP, Barchi M, Pensini S, D'Agostino A, et al. Expression of a truncated form of KIT tyrosine kinase in human spermatozoa correlates with sperm DNA integrity. Hum Reprod. 2010;25:2188–2202. doi: 10.1093/humrep/deq168. [DOI] [PubMed] [Google Scholar]
- 76.Feng HL, Sandlow JI, Zheng LJ. c-kit receptor and its possible function in human spermatozoa. Mol Reprod Dev. 2005;70:103–110. doi: 10.1002/mrd.20186. [DOI] [PubMed] [Google Scholar]
- 77.Livera G, Rouiller-Fabre V, Pairault C, Levacher C, Habert R. Regulation and perturbation of testicular functions by vitamin A. Reproduction. 2002;124:173–180. [PubMed] [Google Scholar]
- 78.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 79.Vogel G. Embryonic stem cells. Scientists make sperm in a dish. Science. 2003;302:1875–1. doi: 10.1126/science.302.5652.1875a. [DOI] [PubMed] [Google Scholar]
- 80.Eguizabal C, Shovlin TC, Durcova-Hills G, Surani A, McLaren A. Generation of primordial germ cells from pluripotent stem cells. Differentiation. 2009;78:116–123. doi: 10.1016/j.diff.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, Mark M. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 82.Snyder EM, Small C, Griswold MD. Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod. 2010;83:783–790. doi: 10.1095/biolreprod.110.085811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, Culty M. Identification and distribution of a novel platelet-derived growth factor receptor beta variant: effect of retinoic acid and involvement in cell differentiation. Endocrinology. 2007;148:2233–2250. doi: 10.1210/en.2006-1206. [DOI] [PubMed] [Google Scholar]
- 84.Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod. 2008;78:537–545. doi: 10.1095/biolreprod.107.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to Retinoic Acid in the Neonatal but Not Adult Mouse Results in Synchronous Spermatogenesis. Biol Reprod. 2011 doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carlomagno G, van Bragt MP, Korver CM, Repping S, de Rooij DG, van Pelt AM. BMP4-induced differentiation of a rat spermatogonial stem cell line causes changes in its cell adhesion properties. Biol Reprod. 2010;83:742–749. doi: 10.1095/biolreprod.110.085456. [DOI] [PubMed] [Google Scholar]
- 87.Pellegrini M, Grimaldi P, Rossi P, Geremia R, Dolci S. Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–3372. doi: 10.1242/jcs.00650. [DOI] [PubMed] [Google Scholar]
- 88.Pesce M, Gioia KF, De Felici M. Derivation in culture of primordial germ cells from cells of the mouse epiblast: phenotypic induction and growth control by Bmp4 signalling. Mech Dev. 2002;112:15–24. doi: 10.1016/s0925-4773(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 89.Baleato RM, Aitken RJ, Roman SD. Vitamin A regulation of BMP4 expression in the male germ line. Dev Biol. 2005;286:78–90. doi: 10.1016/j.ydbio.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 90.Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol Endocrinol. 2000;14:1053–1063. doi: 10.1210/mend.14.7.0479. [DOI] [PubMed] [Google Scholar]
- 91.Zhao GQ, Deng K, Labosky PA, Liaw L, Hogan BL. The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 1996;10:1657–1669. doi: 10.1101/gad.10.13.1657. [DOI] [PubMed] [Google Scholar]
- 92.Kumar TR, Palapattu G, Wang P, Woodruff TK, Boime I, Byrne MC, Matzuk MM. Transgenic models to study gonadotropin function: the role of follicle-stimulating hormone in gonadal growth and tumorigenesis. Mol Endocrinol. 1999;13:851–865. doi: 10.1210/mend.13.6.0297. [DOI] [PubMed] [Google Scholar]
- 93.Vaskivuo TE, Aittomäki K, Anttonen M, Ruokonen A, Herva R, Osawa Y, et al. Effects of follicle-stimulating hormone (FSH) and human chorionic gonadotropin in individuals with an inactivating mutation of the FSH receptor. Fertil Steril. 2002;78:108–113. doi: 10.1016/s0015-0282(02)03148-5. [DOI] [PubMed] [Google Scholar]
- 94.Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15:201–204. doi: 10.1038/ng0297-201. [DOI] [PubMed] [Google Scholar]
- 95.Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141:1795–1803. doi: 10.1210/endo.141.5.7456. [DOI] [PubMed] [Google Scholar]
- 97.Krishnamurthy H, Danilovich N, Morales CR, Sairam MR. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol Reprod. 2000;62:1146–1159. doi: 10.1095/biolreprod62.5.1146. [DOI] [PubMed] [Google Scholar]
- 98.Santos NC, Kim KH. Activity of retinoic acid receptor-alpha is directly regulated at its protein kinase A sites in response to follicle-stimulating hormone signaling. Endocrinology. 2010;151:2361–2372. doi: 10.1210/en.2009-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 100.Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 101.Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sonoda J, Wharton RP. Recruitment of Nanos to hunchback mRNA by Pumilio. Genes Dev. 1999;13:2704–2712. doi: 10.1101/gad.13.20.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki H, Tsuda M, Kiso M, Saga Y. Nanos3 maintains the germ cell lineage in the mouse by suppressing both Bax-dependent and -independent apoptotic pathways. Dev Biol. 2008;318:133–142. doi: 10.1016/j.ydbio.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 104.Asaoka-Taguchi M, Yamada M, Nakamura A, Hanyu K, Kobayashi S. Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat Cell Biol. 1999;1:431–437. doi: 10.1038/15666. [DOI] [PubMed] [Google Scholar]
- 105.Lolicato F, et al. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–738. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 106.Tsuda M, et al. Conserved role of nanos proteins in germ cell development. Science. 2003;301:1239–1241. doi: 10.1126/science.1085222. [DOI] [PubMed] [Google Scholar]
- 107.Scholer HR, Ciesiolka T, Gruss P. A nexus between Oct-4 and E1A: implications for gene regulation in embryonic stem cells. Cell. 1991;66:291–304. doi: 10.1016/0092-8674(91)90619-a. [DOI] [PubMed] [Google Scholar]
- 108.Kehler J, et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004;5:1078–1083. doi: 10.1038/sj.embor.7400279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durcova-Hills G, Capel B. Development of germ cells in the mouse. Curr Top Dev Biol. 2008;83:185–212. doi: 10.1016/S0070-2153(08)00406-7. [DOI] [PubMed] [Google Scholar]
- 110.Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- 111.Francavilla S, Zamboni L. Differentiation of mouse ectopic germinal cells in intra- and perigonadal locations. J Exp Zool. 1985;233:101–109. doi: 10.1002/jez.1402330114. [DOI] [PubMed] [Google Scholar]
- 112.McLaren A. Meiosis and differentiation of mouse germ cells. Symp Soc Exp Biol. 1984;38:7–23. [PubMed] [Google Scholar]
- 113.Trautmann E, et al. Retinoic acid prevents germ cell mitotic arrest in mouse fetal testes. Cell Cycle. 2008;7:656–664. doi: 10.4161/cc.7.5.5482. [DOI] [PubMed] [Google Scholar]
- 114.Cook MS, Munger SC, Nadeau JH, Capel B. Regulation of male germ cell cycle arrest and differentiation by DND1 is modulated by genetic background. Development. 2011;138:23–32. doi: 10.1242/dev.057000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mol CD, et al. Structure of a c-kit product complex reveals the basis for kinase transactivation. J Biol Chem. 2003;278:31461–31464. doi: 10.1074/jbc.C300186200. [DOI] [PubMed] [Google Scholar]
- 116.Biermann K, et al. c-KIT is frequently mutated in bilateral germ cell tumours and downregulated during progression from intratubular germ cell neoplasia to seminoma. J Pathol. 2007;213:311–318. doi: 10.1002/path.2225. [DOI] [PubMed] [Google Scholar]
- 117.Koubova J, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Y, Gill ME, Koubova J, Page DC. Germ cell-intrinsic and -extrinsic factors govern meiotic initiation in mouse embryos. Science. 2008;322:1685–1687. doi: 10.1126/science.1166340. [DOI] [PubMed] [Google Scholar]
- 119.Ruggiu M, et al. The mouse Dazla gene encodes a cytoplasmic protein essential for gametogenesis. Nature. 1997;389:73–77. doi: 10.1038/37987. [DOI] [PubMed] [Google Scholar]
- 120.Schrans-Stassen BH, Saunders PT, Cooke HJ, de Rooij DG. Nature of the spermatogenic arrest in Dazl-/- mice. Biol Reprod. 2001;65:771–776. doi: 10.1095/biolreprod65.3.771. [DOI] [PubMed] [Google Scholar]
- 121.Oulad-Abdelghani M, et al. Characterization of a pre-meiotic germ cell-specific cytoplasmic protein encoded by Stra8, a novel retinoic acid-responsive gene. J Cell Biol. 1996;135:469–477. doi: 10.1083/jcb.135.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baltus AE, et al. In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet. 2006;38:1430–1434. doi: 10.1038/ng1919. [DOI] [PubMed] [Google Scholar]
- 123.Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- 124.Zhou Q, et al. Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod. 2008;79:35–42. doi: 10.1095/biolreprod.107.066795. [DOI] [PMC free article] [PubMed] [Google Scholar]