Abstract
Membrane-bound cytochrome c oxidase catalyzes cell respiration in aerobic organisms and is a primary energy transducer in biology. The two halves of the catalytic cycle may be studied separately: in an oxidative phase, the enzyme is oxidized by O2, and in a reductive phase, the oxidized enzyme is reduced before binding the next O2 molecule. Here we show by time-resolved membrane potential and pH measurements with cytochrome oxidase liposomes that, with both phases in succession, two protons are translocated during each phase, one during each individual electron transfer step. However, when the reductive phase is not immediately preceded by oxidation, it follows a different reaction pathway no longer coupled to proton pumping. Metastable states with altered redox properties of the metal centers are accessed during turnover and relax when external electron donors are exhausted but recover after enzyme reduction and reoxidation by O2. The efficiency of ATP synthesis might be regulated by switching between the two catalytic pathways.
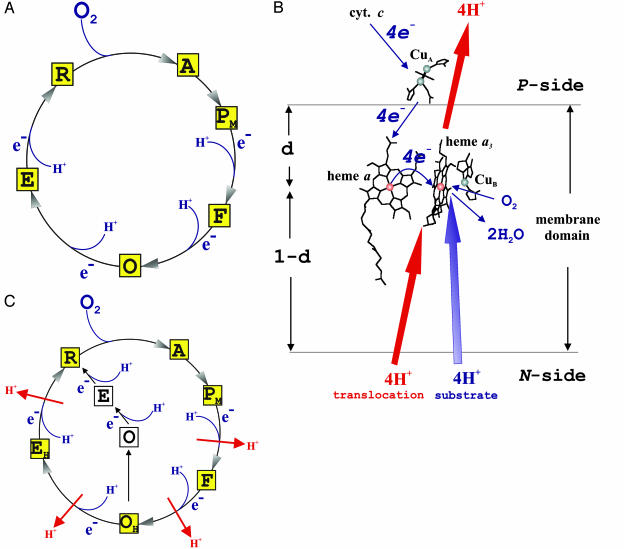
The reduction of oxygen by cytochrome c oxidase is linked to translocation of four protons across the membrane (1), generating protonmotive force for the synthesis of ATP. The catalytic cycle (Fig. 1A) is often described as a series of states of the enzyme's oxygen-binding heme a3–CuB center, which receives one electron at a time from the donor, cytochrome c, via the metal centers CuA and heme a (Fig. 1B). In the oxidative phase, the reduced center (R) binds O2 to form a dioxygen adduct, A (2–4). Then the O—O bond is broken, and dioxygen is reduced (5, 6) by three electrons from heme a3 and CuB and a fourth from a local tyrosine residue (7), yielding the PM state with ferryl heme iron (8) and cupric CuB. Next, electron and proton transfer to the center yields the F state, and finally, after another electron and proton transfer, an oxidized ferric/cupric state (O) is formed, which is often considered to be the state of the enzyme as isolated. The cycle is completed by the reductive phase, in which two electrons (and two protons) are delivered to the heme a3–CuB site to reduce it back to R via the one-electron reduced intermediate E.
Fig. 1.
Cytochrome c oxidase. (A) Conventional catalytic cycle. The squares show different states of the binuclear heme a3–CuB site and how electrons and substrate protons are taken up during activity. Proton translocation is not shown. (B) Overall reaction scheme and location of redox centers. Blue arrows show the redox reaction and its orientation with respect to the membrane. Red arrows depict proton translocation coupled to the redox reaction. The heme groups and CuB lie within the membrane at a relative dielectric depth d from the positively charged P surface. Electron transfer across d, proton consumption across 1-d, and proton pumping across the entire membrane contribute to generation of electric membrane potential (adapted from ref. 9). (C) Modified catalytic cycle. Yellow squares depict the main cycle. White squares show a side path initiated by decay of the metastable OH intermediate to O. Red arrows indicate proton translocation, and blue arrows show uptake of substrate protons. For details, see text.
To understand the mechanism of proton translocation, it is imperative to identify those partial reactions in the catalytic cycle that are coupled to this process. Recent direct measurements (9) suggested that two protons are translocated during the oxidative phase. The overall pump stoichiometry of 4 H+/O2 therefore implies that the reductive phase should also be coupled to translocation of two protons. However, the low midpoint potentials (Em) of heme a3 and CuB measured in anaerobic redox titrations (10) make it virtually impossible thermodynamically to link proton pumping to reduction of the heme a3–CuB site (9). Indeed, proton translocation was not observed on reducing the oxidized enzyme, as isolated (state O) (9, 11, 12). Hence, there is a serious dilemma: the full extent of proton translocation during turnover has not been observed when the oxidative and reductive phases of the catalytic cycle are studied separately. We have conjectured (9) that the reason for this paradox may be that the intermediates involved in the reductive phase may not be the same for the oxidized enzyme as isolated, as is the case during continuous turnover. Such a remarkable property implies that there are two alternative catalytic pathways that differ in protonpumping efficiency, and this possibility is investigated here.
Materials and Methods
Proton ejection from vesicles containing cytochrome c oxidase from bovine heart was measured as described (9). The direct time-resolved electrometric measurements were made as reported in refs. 11 and 13. Enzyme from Paracoccus denitrificans was reconstituted into liposomes (11), and the resulting proteoliposomes were fused to a measuring membrane (13). Flashes of light were given by a frequency-doubled Q switched Nd-yttrium/aluminum garnet laser (pulse width 4 ns, λ = 532 nm, energy 1,250 mJ/cm2) to photolyze CO from the reduced enzyme and to photoactivate Tris(2,2′-bispyridyl)ruthenium [II] (RuBiPy) to inject electrons into the enzyme (11, 12, 14). The yield of enzyme reduction per flash was measured by using the K354M mutant enzyme as in ref. 11 and found to be ≈20% under the present conditions of RuBiPy concentration, ionic strength, and laser energy.
The theoretical curves in Fig. 2 were calculated statistically on the basis of the probabilities of enzyme populations with 0 (fully oxidized), 1, 2, 3, or 4 (fully reduced) electrons before the reaction with O2, in conditions with different amounts of added reductant (ruthenium [II] hexaammine).
Fig. 2.
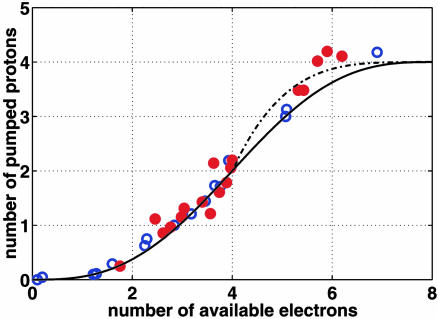
Proton ejection during oxidation and rereduction of cytochrome c oxidase. Enzyme from bovine heart was reconstituted into phospholipid vesicles and reduced to different extents anaerobically by aliquots of ruthenium [II] hexaammine under conditions described in ref. 9. To start the reaction, O2 was added stoichiometric with the enzyme as a calibrated volume of air-saturated water, and the number of protons ejected from the vesicles was measured with a sensitive pH meter. Oxidation and rereduction of the enzyme were measured simultaneously by optical spectroscopy at 445–470 nm, from which the exact amount of reductant present was determined. The results of two independent series of experiments are shown (red and blue circles). The curve shows the result expected if one proton each is ejected during the P → F and F → OH transition (Fig. 1C), and if the OH → EH transition is associated with ejection of two protons and the EH → R transition with no protons (upper curve), or when both these transitions are associated with ejection of one proton (lower curve).
Results and Discussion
Cytochrome c oxidase from bovine heart was incorporated into liposomes, supplied with different amounts of reductant, and allowed to react with a stoichiometric amount of O2 (see ref. 9). As shown in Fig. 2, two protons are ejected when the fully reduced enzyme (four electron equivalents added per enzyme molecule) is oxidized by O2. The data fit a simulation (curve) in which it is assumed that one proton each is ejected during the transitions from P to F and from F to O (see Fig. 1 A, although the reaction of the fully reduced enzyme with O2 takes place via intermediate PR where the electron in heme a has already been transferred to the binuclear center). If two additional electron equivalents are present, the oxidative phase is immediately followed by the reductive phase, and now two more protons are indeed seen to be ejected (Fig. 2). However, these data cannot make the important distinction between ejection of both protons during reduction by the first electron (upper curve) and ejection of one proton during each electron transfer (lower curve).
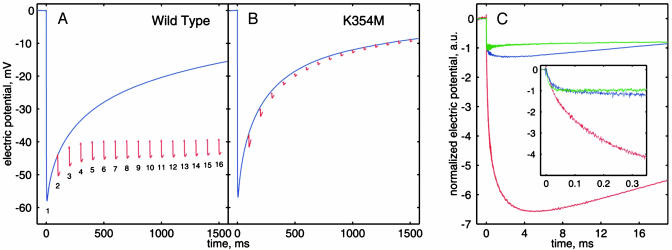
The protonmotive function of cytochrome c oxidase may be monitored independently by time-resolved measurements of membrane potential (13, 15, 16), which is generated by three different events: electron transfer from CuA to heme a, uptake of substrate protons into the heme a3–CuB site, and pumping of protons across the membrane (see Fig. 1B). Cytochrome c oxidase from P. denitrificans was incorporated in liposomes and initially fully reduced in the presence of carbon monoxide to form the CO complex of heme a3. RuBiPy was also present to allow light flash-induced electron injection into the enzyme (11, 12, 14). The novel rationale here is first to induce the oxidative phase by CO photolysis in the presence of O2 (flash no. 1; Fig. 3A) and then to induce the reductive phase by photoinjection of electrons soon after (flashes 2–16). As shown in Fig. 3A (blue), the oxidative phase is accompanied by fast generation of membrane potential (see ref. 13). A subsequent train of flashes injects electrons into the recently oxidized enzyme with a yield of ≈20% per flash (see Materials and Methods). The enzyme enters the reductive phase, which is also associated with formation of membrane potential (Fig. 3A, red; see below). Mutation of the conserved lysine-354 in subunit I to methionine (K354M mutant) or its equivalent is known to have little effect on the oxidative phase of the catalytic cycle but to dramatically inhibit the reductive phase (17–19). Membrane potential generation in the mutant enzyme is indeed not affected during the oxidative phase (Fig. 3B, blue) but is strongly inhibited during reduction (Fig. 3B, red), which confirms that the wild-type enzyme indeed enters the reductive phase during the electron photoinjections.
Fig. 3.
Membrane potential generation in cytochrome c oxidase vesicles. Experimental conditions for A–C (red and blue traces): 2 mM Hepes/4 mM Tris, pH 8/0.2 mM RuBiPy/10 mM aniline/50 mM glucose/3 mg/ml glucose oxidase/0.6 mg/ml catalase/trace amount of hexaammine ruthenium [III] chloride, 25°C. In B, 1 μM hexaammine ruthenium [III] chloride was also present. To remove trace amounts of oxygen and to achieve the fully reduced state of the enzyme, the samples were kept under 1% CO and 99% Ar atmosphere without access of air and with continuous stirring for 10–20 min before each measurement. A small volume of oxygen-saturated buffer was injected ≈1 s before the first laser flash (blue trace). In a second experiment, the first flash was followed by a train of flashes with 100-ms intervals (red traces), where each transient was recorded for 20 ms. These flashes caused photoinjection of electrons mediated by RuBiPy into the enzyme just oxidized by the first flash. Experiments were performed in the pH range 6–9 with essentially the same results. (A) Wild-type enzyme (integers below traces show flash number). (B) K354M mutant enzyme. (C) Magnified view of the response to the second flash in A (red trace) and B (blue trace). The green trace shows the response to the first flash of electron injection into fused proteoliposomes containing oxidized enzyme as isolated. Here the experimental conditions were as follows: air-saturated 2 mM Hepes/4 mM Tris, pH 8/0.08 mM RuBiPy/10 mM aniline. All three traces are normalized to the amplitude of the fast phase.
Fig. 3C shows membrane potential formation on electron injection in more detail. In wild-type enzyme that has previously undergone the oxidative phase, membrane potential is generated (second flash in Fig. 3A) in three kinetically distinguishable phases (Fig. 3C, red): an ≈17-μs event (see Fig. 3C Inset) is followed by two slower phases with characteristic time constants of 0.2 and 1.4 ms (see ref. 20) and roughly equal amplitudes. In contrast, when the first electron is injected into oxidized wild-type enzyme that has not recently undergone oxidation by O2, only the fast phase is observed (Fig. 3C, green; see ref. 11). Similarly, in the K354M mutant enzyme (Fig. 3C, blue), there is only very little formation of membrane potential beyond the fast phase. Clearly, formation of membrane potential during the reductive phase is fundamentally different depending on whether it has been preceded by an oxidative phase.
The first phase of membrane potential generation is due to electron transfer from the photoexcited dye on the P-side of the membrane via the CuA site to heme a across a relative dielectric depth d (Fig. 1B) (14, 16, 21), where d = 0.32 (9). Further electron transfer from heme a to the heme a3–CuB site takes place parallel to the membrane and is therefore not electrogenic, but it may be linked to two slower electrogenic phases caused by proton transfers: uptake of a substrate proton from the N-side into the heme a3–CuB site across 1-d, proton pumping across the membrane, or both (Fig. 1B). These protonic phases are dramatically suppressed in the oxidized wild-type enzyme that has not undergone a preceding oxidative phase (Fig. 3C, green).
The amplitude of the fast electron transfer phase serves as an internal calibration of the proton transfers. The Em values of CuA and heme a are relatively similar. Therefore, the photoinjected electron is not transferred quantitatively from CuA to heme a, but by a fraction β (= 0.7–0.8) corresponding to ΔEm of 22–36 mV, more positive for heme a (11). The amplitude of the fast phase is thus β*d, when expressed as the number of charge equivalents translocated across the membrane per electron injected. From several experiments such as Fig. 3C (red), we found that the sum of the amplitudes of the two protonic phases was 6.95 ± 1.2 (SD, n = 11) times larger than the amplitude of the fast phase. The electrogenic proton transfers thus correspond to translocation of 6.95*β*d or 1.6–1.8 electrical charge equivalents across the membrane. Because the amplitude of the fast electron transfer phase does not decrease with flash number in Fig. 3A, we conclude that the injected electron is transferred quantitatively from heme a to the heme a3–CuB site. Hence the data suggest that electron transfer to the heme a3–CuB site is accompanied by uptake of one substrate proton to this site (across 1-d, i.e., 0.68 charges across the membrane) and pumping of one proton across the membrane.
Because Fig. 3C (red) is the first reductive flash and the yield is ≈20%, it represents injection of the first electron during the reductive phase. Together with the data in Fig. 2, this suggests that one proton each is translocated during reduction by the first and second electrons. This conclusion is verified by the observation that the amplitude of the electrometric response is virtually identical for >10 light flashes (Fig. 3A, red). If, for example, the second but not the first electron transfer had been linked to proton pumping (22), there would have been a steady increase in the amplitude of the protonic phases with increasing flash number, because the probability for the enzyme to be injected by the second electron rises with flash number. Moreover, at a high flash number, a considerable fraction of the enzyme will already undergo the PM → F and F → OH transitions due to the aerobic conditions (Fig. 1C). That the electrometric response remains essentially the same over at least 16 flashes means, therefore, that all four electron transfer steps in the catalytic cycle are linked to translocation of one proton and uptake of a substrate proton.
Using the measured yield of ≈20% per flash, it is also possible to compare the extent of membrane potential formation on electron photoinjection with the potential generated during the oxidative phase. In experiments such as Fig. 3A, the membrane potential generated during the oxidative phase was ≈10 times larger than that generated on subsequent electron photoinjection (9.98 ± 1.23 SD; n = 6). When extrapolated to 100% yield, this becomes approximately a factor of two, which is in good agreement with the notion that two proton-pumping events accompany the oxidative phase, and one such event accompanies injection of each electron during the reductive phase. This comparison, which depends neither on the values for d or β nor on deconvolution of the phases of membrane potential formation on electron injection, therefore provides independent evidence for the conclusion that injection of the first and second electrons into the recently oxidized enzyme is indeed each associated with pumping of one proton.
Our conclusion is summarized in Fig. 1C. When fully reduced cytochrome c oxidase is oxidized by O2, the oxidative reaction phase is linked to translocation of two protons. At the end of this reaction, the heme a3–CuB site attains a metastable state (OH), the reduction of which is linked to translocation of two more protons across the membrane, one during each one-electron reduction step. Note that due to the aerobic conditions, R reacts further with O2 to form PM (Fig. 1C). Thus, the proton translocation linked to the second electron could in principle occur during R → PM rather than during the preceding EH → R step. However, the former reaction is not associated with membrane potential formation when analyzed separately (13). Hence, during continuous turnover, each electron transfer to the heme a3–CuB center is coupled to a single proton-translocating event, which is of key importance when considering possible molecular mechanisms (23). However, OH may alternatively relax to a more stable form, O, which corresponds to the heme a3–CuB site in the enzyme as isolated, and the reduction of which is not linked to proton pumping. Due to experimental restrictions, we can only estimate that the lifetime of the OH state is at least ≈30 s at room temperature.
Our finding that one proton is translocated during the EH → R transition is consistent with the experiments by Ruitenberg et al. (20), who in our view produced the EH state directly from F using the two-electron donor carbon monoxide, thus avoiding the metastable OH state (Fig. 1C). However, in their work, the amplitude of the protonic phases was much smaller than shown here due to the low occupancy of enzyme in the F state, which may be deduced from the optical spectra (20).
Cytochrome c oxidase accesses metastable states during continuous turnover, which differ fundamentally in function from those involved during static conditions. This explains several enigmatic observations in the past. For example, the Em values of the heme–copper site measured in anaerobic redox titrations (10) may be considerably higher in the metastable states occupied during turnover, which makes proton pumping during the reductive phase thermodynamically feasible. The metastable and relaxed states of the oxidized enzyme may well correspond to some of the “pulsed” and “resting” states described earlier to differ with respect to electron transfer rate and conformation of the heme a3–CuB site (24–26). Further studies are called for to elucidate the structural basis of the functional difference described here, but differences in redox properties and coordination of one or both metals in the heme a3–CuB site may already be anticipated.
The bifurcation of the catalytic cycle (Fig. 1C) opens up the possibility of regulating cytochrome oxidase with respect to proton translocation efficiency. For example, at high protonmotive force, the OH state might preferably relax into O instead of being reduced to EH. This would lead to the inner cycle of Fig. 1C, in which only two protons are translocated instead of four. Such a decrease of efficiency has, in fact, been reported for isolated mitochondria under certain conditions (27). If this occurred in vivo, the maximum charge-translocating stoichiometry of the mitochondrial respiratory chain (5 charges/e–) (28) would decrease to 4.5 charges/e– on engaging the route via the O intermediate. Although small, this change could be of considerable physiological importance, because it would lower the degree of coupling between electron and proton transfer to 90%, which is close to the theoretical degree of coupling (91%) that maximizes power output of ATP synthesis (29). Moreover, such control could be important to avoid an excessively high protonmotive force across the inner mitochondrial membrane, a condition that has been shown to maximize production of reactive oxygen species in mitochondria (30).
Acknowledgments
This work was supported by Biocentrum Helsinki, the Sigrid Juselius Foundation, and the Academy of Finland (Program 44895).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: RuBiPy, Tris(2,2′-bispyridyl)ruthenium [II].
References
- 1.Wikström, M. (1977) Nature 266, 271–273. [DOI] [PubMed] [Google Scholar]
- 2.Chance, B., Saronio, C. & Leigh, J. S. (1975) Proc. Natl. Acad. Sci. USA 72, 1635–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babcock, G. T. & Wikström, M. (1992) Nature 356, 301–309. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson-Miller, S. & Babcock, G. T. (1996) Chem. Rev. 96, 2889–2907. [DOI] [PubMed] [Google Scholar]
- 5.Proshlyakov, D. A., Pressler, M. A. & Babcock, G. T. (1998) Proc. Natl. Acad. Sci. USA 95, 8020–8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabian, M., Wong, W. W., Gennis, R. B. & Palmer, G. (1999) Proc. Natl. Acad. Sci. USA 96, 13114–13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proshlyakov, D. A., Pressler, M. A., DeMaso, C., Leykam, J. F., DeWitt, D. L. & Babcock, G. T. (2000) Science 290, 1588–1591. [DOI] [PubMed] [Google Scholar]
- 8.Kitagawa, T. & Ogura, T. (1997) Prog. Inorg. Chem. 45, 431–479. [Google Scholar]
- 9.Verkhovsky, M. I., Jasaitis, A., Verkhovskaya, M. L., Morgan, J. E. & Wikström, M. (1999) Nature 400, 480–483. [DOI] [PubMed] [Google Scholar]
- 10.Dutton, P. L. & Wilson, D. F. (1974) Biochim. Biophys. Acta 346, 165–212. [DOI] [PubMed] [Google Scholar]
- 11.Verkhovsky, M. I., Tuukkanen, A., Backgren, C., Puustinen, A. & Wikstrom, M. (2001) Biochemistry 40, 7077–7083. [DOI] [PubMed] [Google Scholar]
- 12.Ruitenberg, M., Kannt, A., Bamberg, E., Ludwig, B., Michel, H. & Fendler, K. (2000) Proc. Natl. Acad. Sci. USA 97, 4632–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasaitis, A., Verkhovsky, M. I., Morgan, J. E., Verkhovskaya, M. L. & Wikström, M. (1999) Biochemistry 38, 2697–2706. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson, T. (1992) Proc. Natl. Acad. Sci. USA 89, 6497–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drachev, L. A., Kaulen, A. D., Semenov, A. Y., Severina, I. I. & Skulachev, V. P. (1979) Anal. Biochem. 96, 250–262. [DOI] [PubMed] [Google Scholar]
- 16.Zaslavsky, D., Kaulen, A. D., Smirnova, I. A., Vygodina, T. & Konstantinov, A. A. (1993) FEBS Lett. 336, 389–393. [DOI] [PubMed] [Google Scholar]
- 17.Vygodina, T. V., Pecoraro, C., Mitchell, D., Gennis, R. & Konstantinov, A. A. (1998) Biochemistry 37, 3053–3061. [DOI] [PubMed] [Google Scholar]
- 18.Ädelroth, P., Gennis, R. B. & Brzezinski, P. (1998) Biochemistry 37, 2470–2476. [DOI] [PubMed] [Google Scholar]
- 19.Wikström, M., Jasaitis, A., Backgren, C., Puustinen, A. & Verkhovsky, M. I. (2000) Biochim. Biophys. Acta 1459, 514–520. [DOI] [PubMed] [Google Scholar]
- 20.Ruitenberg, M., Kannt, A., Bamberg, E., Fendler, K. & Michel, H. (2002) Nature 417, 99–102. [DOI] [PubMed] [Google Scholar]
- 21.Zaslavsky, D. L., Smirnova, I. A., Siletsky, S. A., Kaulen, A. D., Millett, F. & Konstantinov, A. A. (1995) FEBS Lett. 359, 27–30. [DOI] [PubMed] [Google Scholar]
- 22.Michel, H. (1999) Biochemistry 38, 15129–15140. [DOI] [PubMed] [Google Scholar]
- 23.Wikström, M., Verkhovsky, M. I. & Hummer, G. (2003) Biochim. Biophys. Acta 1604, 61–65. [DOI] [PubMed] [Google Scholar]
- 24.Antonini, E., Brunori, M., Colosimo, A., Greenwood, C. & Wilson, M. T. (1977) Proc. Natl. Acad. Sci. USA 74, 3128–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson, M. T., Peterson, J., Antonini, E., Brunori, M., Colosimo, A. & Wyman, J. (1981) Proc. Natl. Acad. Sci. USA 78, 7115–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brudvig, G. W., Stevens, T. H., Morse, R. H. & Chan, S. I. (1981) Biochemistry 20, 3912–3921. [DOI] [PubMed] [Google Scholar]
- 27.Kadenbach, B. (2003) Biochim. Biophys. Acta 1604, 77–94. [DOI] [PubMed] [Google Scholar]
- 28.Hinkle, P. C., Kumar, M. A., Resetar, A. & Harris, D. L. (1991) Biochemistry 30, 3576–3582. [DOI] [PubMed] [Google Scholar]
- 29.Stucki, J. W. (1980) Eur. J. Biochem. 109, 269–283. [DOI] [PubMed] [Google Scholar]
- 30.Korshunov, S. S., Skulachev, V. P. & Starkov, A. A. (1997) FEBS Lett. 416, 15–18. [DOI] [PubMed] [Google Scholar]