Abstract
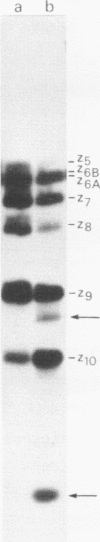
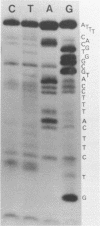
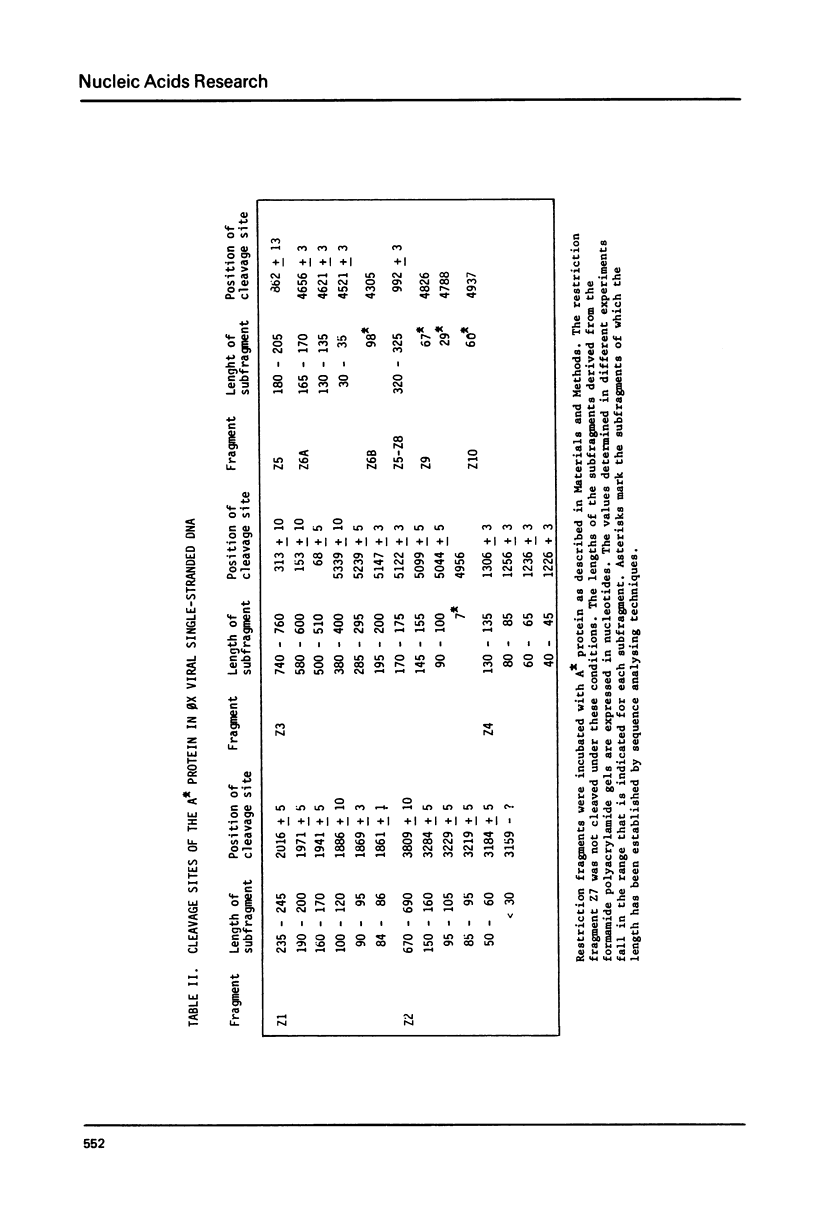
The A* protein of bacteriophage phi X174 is a single-stranded DNA specific nuclease. It can cleave phi X viral ss DNA in many different places. The position of these sites have been determined within the known phi X174 nucleotide sequence (1). From the sequences at these sites it is clear that the A* protein recognizes and cleaves at sites that show only partial homology with the origin of RF DNA replication in the phi X DNA. Different parts of the origin sequence can be deduced that function as a signal for recognition and cleavage by the A* protein. We conclude that different parts within the DNA recognition domain of the A* protein are functional in the recognition of the origin sequence in single-stranded DNA. The existence of different DNA recognition domains in the A* protein, and therefore also in the A protein, leads to a model that can explain how the A protein performs its multiple function in the phi X174 DNA replication process (2).
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakesley R. W., Wells R. D. 'Single-stranded' DNA from phiX174 and M13 is cleaved by certain restriction endonucleases. Nature. 1975 Oct 2;257(5525):421–422. doi: 10.1038/257421a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. Cleavage of phi X174 single-stranded DNA by gene A protein and formation of a tight protein-DNA complex. J Virol. 1980 Aug;35(2):409–413. doi: 10.1128/jvi.35.2.409-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Griffith J., Kornberg A. phiX174 cistron A protein is a multifunctional enzyme in DNA replication. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3198–3202. doi: 10.1073/pnas.74.8.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg S., Kornberg A. Purification and characterization of phiX174 gene A protein. A multifunctional enzyme of duplex DNA replication. J Biol Chem. 1979 Jun 25;254(12):5328–5332. [PubMed] [Google Scholar]
- Fiddes J. C., Barrell B. G., Godson G. N. Nucleotide sequences of the separate origins of synthesis of bacteriophage G4 viral and complementary DNA strands. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1081–1085. doi: 10.1073/pnas.75.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Formation of the parental replicative form DNA of bacteriophage phi-X174 and initial events in its replication. J Mol Biol. 1971 Nov 14;61(3):565–586. doi: 10.1016/0022-2836(71)90065-9. [DOI] [PubMed] [Google Scholar]
- Heidekamp F., Langeveld S. A., Baas P. D., Jansz H. S. Studies of the recognition sequence of phi X174 gene A protein. Cleavage site of phi X gene A protein in St-1 RFI DNA. Nucleic Acids Res. 1980 May 10;8(9):2009–2021. doi: 10.1093/nar/8.9.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T. J., Knippers R. Isolation and function of the gene A initiator of bacteriophage phi-chi 174, a highly specific DNA endonuclease. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1549–1553. doi: 10.1073/pnas.71.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K. Origin of DNA replication of bacteriophage f1 as the signal for termination. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5226–5229. doi: 10.1073/pnas.77.9.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Hurwitz J. Isolation and characterization of the protein coded by gene A of bacteriophage phiX174 DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2669–2673. doi: 10.1073/pnas.73.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Shimamoto N., Hurwitz J. Role of polymeric forms of the bacteriophage phi X174 coded gene A protein in phi XRFI DNA cleavage. J Biol Chem. 1979 Oct 10;254(19):9416–9428. [PubMed] [Google Scholar]
- Jacob R. J., Lebowitz J., Kleinschmidt A. K. Locating interrupted hydrogen bonding in the secondary structure of PM2 circular DNA by comparative denaturation mapping. J Virol. 1974 Jun;13(6):1176–1185. doi: 10.1128/jvi.13.6.1176-1185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld S. A., van Arkel G. A., Weisbeek P. J. Improved method for the isolation of the A and A* proteins of bacteriophage phi X174. FEBS Lett. 1980 Jun 2;114(2):269–272. doi: 10.1016/0014-5793(80)81131-8. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., Baas P. D., Jansz H. S., van Arkel G. A., Weisbeek P. J. Nucleotide sequence of the origin of replication in bacteriophage phiX174 RF DNA. Nature. 1978 Feb 2;271(5644):417–420. doi: 10.1038/271417a0. [DOI] [PubMed] [Google Scholar]
- Langeveld S. A., van Mansfeld A. D., de Winter J. M., Weisbeek P. J. Cleavage of single-stranded DNA by the A and A* proteins of bacteriophage phi X174. Nucleic Acids Res. 1979 Dec 20;7(8):2177–2188. doi: 10.1093/nar/7.8.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Godson G. N. Identification of a phiX174 coded protein involved in the shut-off of host DNA replication. Biochem Biophys Res Commun. 1975 Jul 8;65(1):323–330. doi: 10.1016/s0006-291x(75)80096-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. R., Wells R. D. Intercistronic regions in phi X174 DNA. II. Biochemical and biological analysis of mutants with altered intercistronic regions between genes J and F. J Mol Biol. 1980 Jul 25;141(1):25–41. doi: 10.1016/s0022-2836(80)80027-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Smith M., Brown N. L., Air G. M., Barrell B. G., Coulson A. R., Hutchison C. A., 3rd, Sanger F. DNA sequence at the C termini of the overlapping genes A and B in bacteriophage phi X174. Nature. 1977 Feb 24;265(5596):702–705. doi: 10.1038/265702a0. [DOI] [PubMed] [Google Scholar]
- Tessman E. S. Mutants of bacteriophage S13 blocked in infectious DNA synthesis. J Mol Biol. 1966 May;17(1):218–236. doi: 10.1016/s0022-2836(66)80104-3. [DOI] [PubMed] [Google Scholar]
- Weisbeek P. J., Borrias W. E., Langeveld S. A., Baas P. D., Van Arkel G. A. Bacteriophage phiX174: gene A overlaps gene B. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2504–2508. doi: 10.1073/pnas.74.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]