Abstract
Genetic screens in the fruit fly Drosophila melanogaster have identified a class of neoplastic tumor suppressor genes (endocytic nTSGs) that encode proteins that localize to endosomes and facilitate the trafficking of membrane-bound receptors and adhesion molecules into the degradative lysosome. Loss of endocytic nTSGs transforms imaginal disc epithelia into highly proliferative, invasive tissues that fail to differentiate and display defects in cellular apicobasal polarity, adhesion and tissue architecture. As vertebrate homologs of some Drosophila nTSGs are linked to tumor formation, identifying molecular changes in signaling associated with nTSG loss could inform understanding of neoplastic transformation in vertebrates. Here, we show that mutations in genes that act at multiple steps of the endolysosomal pathway lead to autonomous activation of the Sav/Wts/Hpo (SWH) transcriptional effector Yki (YAP/TAZ in vertebrates) and the Jun N-terminal kinase (JNK), which is known to promote Yki activity in cells with disrupted polarity. Yki and JNK activity are elevated by mutations at multiple steps in the endolysosomal pathway, including mutations in the AP-2σ gene, which encodes a component of the AP-2 adaptor complex that recruits cargoes into clathrin-coated pits for subsequent internalization. Moreover, reduction of JNK activity can decrease elevated Yki signaling caused by altered endocytosis. These studies reveal a broad requirement for components of the endocytic pathway in regulating SWH and JNK outputs and place Drosophila endocytic nTSGs into a network that involves two major signaling pathways implicated in oncogenesis.
Key words: Drosophila, endocytic tumor suppressor, Yki, JNK, Tsg101, AP-2, Hippo
Introduction
Genetic screens have identified an assortment of genes that are required to restrict growth of developing epithelia in the fruit fly Drosophila melanogaster. Among these are a relatively small subset of genes, termed neoplastic tumor suppressor genes (nTSGs), whose inactivation transforms imaginal disc epithelia into highly proliferative, invasive tissues that fail to differentiate and display defects in cellular apicobasal polarity, adhesion and tissue architecture.1–3 These phenotypes indicate that nTSGs affect many cellular processes, including proliferation, differentiation, polarity control and adhesion. Understanding these nTSG phenotypes will allow for a greater understanding of how these processes are coupled in cells. Moreover, as a number of studies have linked the vertebrate homologs of these Drosophila nTSGs to tumor formation (reviewed in refs. 4 and 5), identifying molecular changes in signaling associated with nTSG loss could inform understanding of neoplastic transformation in vertebrates.
The erupted (ept) nTSG is a member of a subgroup of nTSGs that encode factors required for targeting of internalized trans-membrane and membrane-associated proteins to the lysosome.4–7 The Ept protein is a homolog of the S. cerevisiae vacuolar protein sorting 23 (Vps23) and vertebrate tumor susceptibility gene 101 (TSG101) proteins, which are components of the endosomal-sorting complex required for transport-I (ESCRT-I) complex. ESCRT-1 functions sequentially with the ESCRT-2 and -3 complexes to promote multivesicular body (MVB) biogenesis of late endosomes, a step required for complete exposure of cargo proteins to the proteolytic environment of the lysosome (reviewed in ref. 8). The mechanisms by which defects in endolysosomal trafficking elicit such strong growth phenotypes are only partially understood. Interestingly, mutations in genes that act at multiple steps of the endolysosomal pathway, including for example syntaxin-7/avalanche,9 ept/tsg101,4 vps25 5,7 and vps22,10 produce overtly similar neoplastic disc phenotypes, indicating that they may share a regulatory target(s) or pathway. Genetic and molecular studies of ept mutant cells show central roles of the Notch and JAK-STAT signaling pathways in ept phenotypes.4,11,12 However, because the phenotypes of these animals cannot be fully rescued by reducing Notch and JAK/STAT signaling, and because neoplasia ensues in endocytic mutants which do not activate both of these pathways (e.g., syx7/avl9), it is likely that other signaling pathways are altered in these genetic backgrounds.
The Salvador-Warts-Hippo (SWH) pathway is emerging as a central integrator of signals from membrane proteins that control cell proliferation and survival in metazoans (reviewed in ref. 13). The pathway consists of a core cassette composed of two kinases Hippo (Hpo) and Warts (Wts) and the scaffolding protein Salvador (Sav). Hpo activation by upstream signals promotes its association with Sav, allowing for Hpo-dependent phosphorylation and activation of Wts. Active Wts phosphorylates the pro-growth transcription factor Yorkie (Yki), rendering it inactive via 14-3-3-dependent cytosolic sequestration. SWH signal strength is modulated in response to a variety of upstream inputs,14 including those involving transmembrane proteins such as the cadherins Fat and Dachsous,15–18 the apicobasal polarity factor Crumbs19–23 and the Dpp receptor Thickveins.24 As trans-membrane proteins are likely to be trafficked through the endolysosomal system, defects in internalization and/or trafficking of these proteins in cells lacking endocytic nTSGs may deregulate SWH signaling and contribute to nTSG overgrowth phenotypes.
In order to better understand how mutations in endocytic nTSGs promote neoplastic growth of Drosophila imaginal discs, we explored the state of Yki activity in the background of ept-deficient cells. We find that Yki activity is autonomously elevated in ept mutant cells, and that this phenotype is strongly enhanced when ept loss is combined with a block in cell death. Unlike Notch and JAK-STAT signaling, which are only activated by later blocks in the endolysosomal pathway (e.g., ept and vps25), we find that Yki activity is elevated by manipulating genes at each step in the endolysosomal pathway including AP-2σ, which encodes a component of the AP-2 adaptor complex that recruits cargoes into clathrin-coated pits for subsequent internalization (reviewed in ref. 25). Moreover, we show that Yki activation in endocytosis-defective cells is accompanied by activation of the JNK signaling pathway, a MAP kinase pathway that has been linked to Drosophila neoplasia and control of Yki activity.26–29 Additionally, we find that elevated JNK activity is required for the altered Yki signaling observed in endocytic mutants, as reduction of JNK activity can suppress the elevated Yki signaling observed in the background of a block in endocytosis. These studies highlight a requirement for multiple endocytic factors in regulating SWH outputs and place Drosophila endocytic nTSGs into a network that involving two major signaling pathways implicated in oncogenesis.
Results
ept mutant cells display altered yki activity.
Drosophila imaginal discs composed of ept mutant cells overgrow as neoplastic masses.4,11,12 Because SWH signaling is linked with two key features of neoplasia, altered contact inhibition and excessive proliferation (reviewed in ref. 13), we examined whether activity of the SWH transcriptional effector Yki was modified in ept-deficient cells. To test this model, we generated ept mutant clones in the Drosophila larval eye disc and analyzed the expression of the Yki transcriptional reporter expanded-lacZ (ex-lacZ).
The pattern of ex-lacZ expression is variable in ept mosaic eye discs, but a portion ept mutant clones display non-cell autonomous activation of ex-lacZ in a ring of normal cells immediately surrounding mutant clones (Fig. 1A–A″). This non-autonomous effect has been previously associated with Yki-dependent regenerative growth of normal cells elicited by high rates of apoptosis within ept mutant clones.27,30 To assess whether blocking apoptotic signals in ept cells might reveal an autonomous effect on Yki activity, we tested ex-lacZ activity in clones of cells doubly mutant for ept and the small deletion Df(3L)H99 (or H99), which removes genes (reaper, grim and hid) required for developmental apoptosis in Drosophila.31
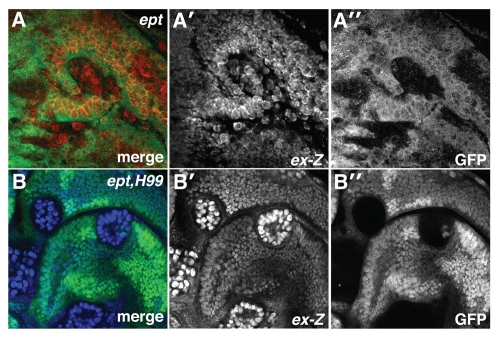
Figure 1.
ept loss elevates Yki activity. α-β-gal staining to detect the ex-lacZ reporter (red in A; blue in B) in (A) ept or (B) ept,H99 mosaic imaginal eye discs (clones are marked by the absence of GFP).
We have previously shown that ept,H99 clones overgrow aggressively and produce large neoplastic eye discs.11 ept,H99 cells show strong cell-autonomous activation of ex-lacZ (Fig. 1B–B″). The H99 genes are not themselves required to restrict ex-lacZ expression,32 indicating that blocking apoptosis of ept mutant cells leads to strong cell-autonomous inactivation of Yki.
Loss of both early and late endocytic nTSGs elevates Yki activity in disc cells.
As a component of the ESCRT-I complex, ept acts at a relatively late step in the endolysosomal pathway. To test whether earlier blocks within the endolysosomal pathway also autonomously elevate Yki activity, specific endolysosomal pathway members were depleted in the posterior domain of the larval wing by engrailed-Gal4 (en>) expression of UAS-inverted repeat (IR) RNA-interference constructs and tested for effect on expression of ex-lacZ.
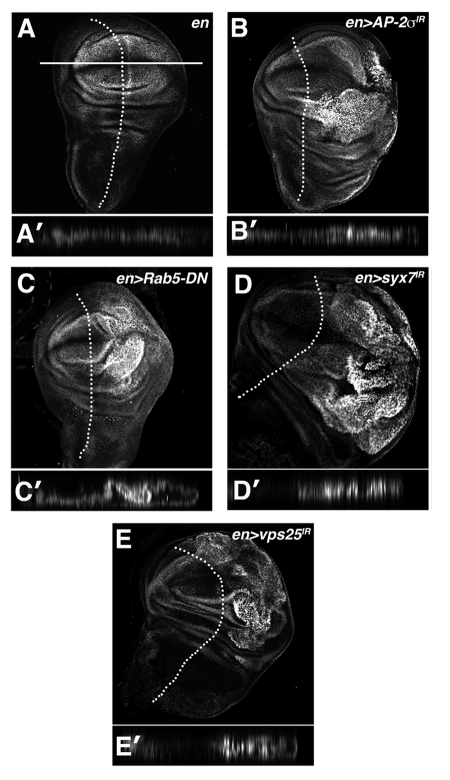
Reduction of the clathrin-adaptor protein AP-2σ, which is required for cargo recruitment and internalization (reviewed in ref. 25), activates ex-lacZ and causes the posterior compartment of the wing disc to overgrow (Fig. 2B). Similarly, depletion of Syntaxin-7 (syx7/avl) or Rab-5, which promote early endosome formation in Drosophila,9 results in strong activation of the ex-lacZ Yki-reporter and tissue overgrowth (Fig. 2C and D). Overgrowth caused by depletion of the ESCRT-II complex member Vps25, which acts after Ept/ESCRT-I, is also associated with activation of ex-lacZ (Fig. 2E). Interestingly, AP-2σ-depleted cells showed very mild activation of a second Yki reporter, thread-lacZ (th-lacZ) (Fig. S1), suggesting that endocytic blocks affect some Yki targets more so than others. This result parallels the finding that mutations in the endocytic adaptor and Yki-binding protein Myopic elevate ex-lacZ but not th-lacZ.32 These data indicate that reduction of several members of the endolysosomal pathway can elevate expression of a SWH reporter gene in the nucleus. Moreover, this link between defective endocytosis and SWH signaling appears to map to multiple steps along the pathway including cell-surface internalization, early endosome formation and MVB biogenesis.
Figure 2.
Multiple blocks in the endolysosomal pathway elevate Yki activity. α-β-gal staining of larval imaginal wing discs in which the ex-lacZ SWH-reporter has been placed in the background of (A) en, (B) en > AP-2σ-IR (C) en > Rab5DN (D) en > syx7-IR and (E) en > vps25-IR animals. Tangential and lateral sections are shown for each genotype. Lateral sections were obtained from the dorsal-ventral margin of the wing epithelium as indicated by the line in (A). Posterior compartment is to the right of the dotted line.
Endocytic neoplastic tumor suppressor genes require yki to overgrow.
Given the effect of altered endocytosis on ex-lacZ, we next tested whether Yki, the pro-growth effector of the SWH-pathway, was required for phenotypes observed in endolysosomal mutants. One hallmark of mutations that produce disc neoplasia is a strong reduction and/or absence in pupariation.6 Therefore, we utilized the eyeless-FLP system to generate eyeantennal discs composed of mostly syx7/avl mutant cells and analyzed whether overexpression of the SWH scaffolding protein Sav, which antagonizes Yki activity,33,34 could affect pupariation rates among animals with syx7/avl mutant eye discs. While animals bearing syx7/avl mutant eye discs normally die during an extended larval stage and rarely form pupae (reviewed in ref. 9), those that also express Sav in syx7/avl mutant eye discs pupariate more frequently and a fraction can eclose as adult animals with small, roughened eyes (Fig. 3A and B). Importantly, Sav overexpression itself has only a mild effect on control eyes [FRT/M(3)], suggesting that syx7/avl-deficient cells are especially sensitive to the dosage of sav activity.
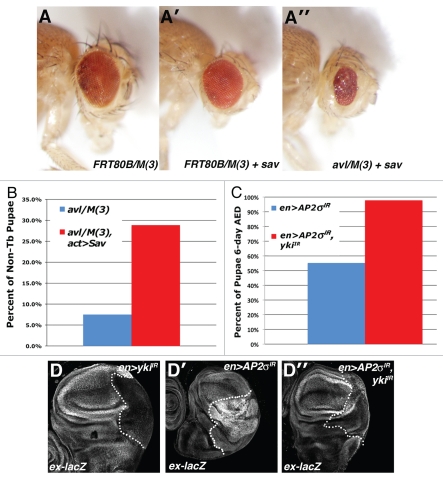
Figure 3.
Endolysosomal growth phenotypes are sensitive to the genetic dose of Yki. Light microscopic images of (A) eyFLP;FRT80B/M(3), (A′) eyFLP;Act > CD2 > Gal4, UAS-sav;FRT80B/M(3) and (A″) eyFLP,Act > CD2 > Gal4,UAS-sav;avl1/M(3) adult eyes, in which sav is overexpressed using the Act > CD2 > Gal4 ‘Flp-out technique’. (B and C) Quantitative analysis showing the percent of pupae at 6 d AED (after egg deposition) in the indicated genotypes. A minimum of 50 animals was counted per genotype. (D) α-β-gal staining to detect ex-lacZ reporter in the background of the indicated genotypes: (D) en > yki-IR (D′) en > AP-2σ-IR and (D″) en > AP-2σ-IR,yki-IR. Posterior compartment is to the right of the dotted line.
In parallel experiments, we co-depleted AP-2σ and Yki from cells by en-GAL4-driven expression of UAS-AP-2σ-IR and UAS-yki-IR transgenes. We were unable to assess whether Yki depletion could rescue AP-2σ-IR-driven lethality as en-GAL4 expression of the UAS-yki-IR transgene led to significant lethality alone (data not shown). However, as with the syx/avl-deficient animals noted above, co-depletion of Yki led to a significant reduction in pupariation delay in the en > AP-2σ-IR background (Fig. 3C). Moreover, depletion of Yki blocks the effect of AP-2σIR on ex-lacZ expression and prevents overgrowth of the posterior domain of the wing disc (Fig. 3D–D″). Thus yki is required for the effect of AP-2σIR loss on disc growth and the SWH transcriptional program.
Endocytic neoplastic tumor suppressor genes elevate JNK activity in vivo.
The transmembrane polarity protein Crumbs (Crb) is a target of ESCRT-dependent endolysosomal turnover.4,5,7 Crb also controls the levels and localization apical SWH-pathway component Expanded (Ex) by recruiting it to the apical membrane and stimulating its phosphorylation and proteasomal turnover.19–22 As Ex inhibits Yki by direct binding to it and by promoting its phosphorylation by Wts (reviewed in ref. 13), this Crb-Ex link could contribute to Yki activation in endolysosomal mutants that block Crb turnover (e.g., ept). Depletion of syx7/avl, rab7 or vps25 caused Crb to accumulate, as has been reported previously in references 5, 7 and 9, although depletion of AP-2σ had a more minor effect on Crb (Fig. S2). Depletion of AP-2σ also led to mild reduction in the polarity factor aPKC (Fig. S3), indicating a subtle but reproducible effect of AP-2σ loss on polarity proteins. Consistent with a previous report showing that vps25 mutant cells express reduced Ex,35 we found that ept-deficient cells also contain reduced levels of Ex (Fig. S4A). Ex levels are elevated in AP-2σ mutant cells (Fig. S4B), which is consistent the well-established role of ex as a Yki target gene (reviewed in ref. 34). Thus ept loss and AP-2σ loss have opposite effects on Ex levels, but nonetheless elevate Yki activity to a similar degree. Accordingly, if a common mechanism links AP-2σ and ept to Yki, then it is unlikely to be exclusively via control of the Crb/Ex arm of the SWH pathway.
Cells lacking either of the apical-basal polarity factors lethal(2) giant larvae (lgl) or discs-large (dlg) elevate Yki activity in part via the c-Jun N-terminal kinase (JNK) signaling pathway.27,30 JNK activity also promotes Yki-activation in Drosophila intestinal stem cells,28,29 and in vertebrates Yap1 (e.g., Yki) is a target of JNK1/2 activity in cultured cells.36 We therefore analyzed JNK signaling in cells lacking early, middle and late endocytic proteins. JNK-pathway signaling involves upstream activation of a MAPKKK, which leads to the eventual phosphorylation and activation of Basket (Bsk), the terminal JNK kinase in Drosophila.37 Active p-Bsk then phosphorylates the transcription factor Jun-related antigen (Jra),38 which heterodimerizes with the transcription factor Fos to form an active AP-1 transcriptional complex in the nucleus. Several transcriptional targets of active AP-1 have been identified in Drosophila, including matrix-metalloprotease-1 (MMP-1) and the puckered (puc) dual-specificity phosphatase.39,40
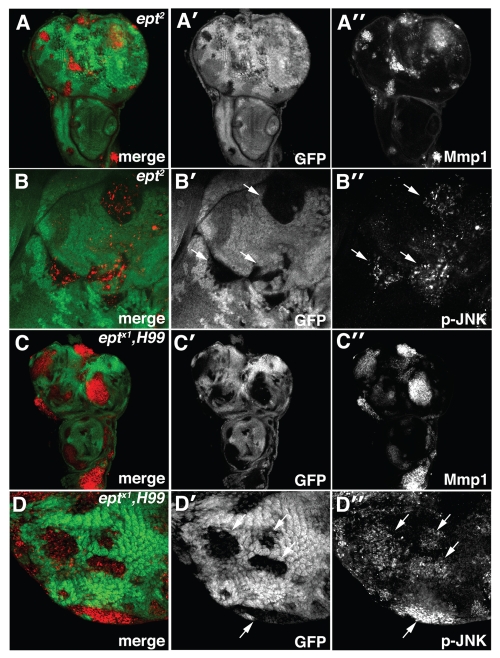
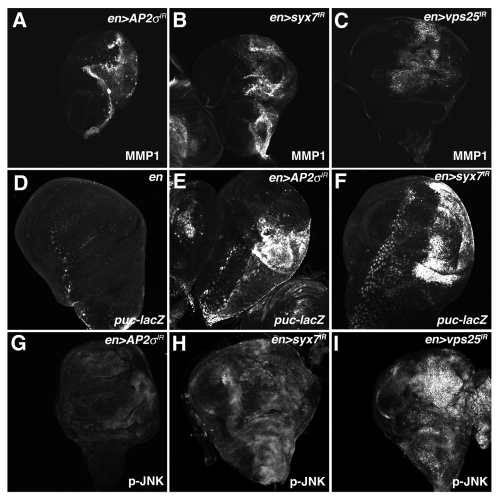
MMP-1 protein levels are strongly elevated in ept and ept,H99 clones mutant clones compared with surrounding wild type tissue (Fig. 4A–A″ and C–C″). MMP-1 expression is also elevated in the posterior compartment of en > AP-2σ-IR, en > syx7/avl-IR and en > vps25-IR wing discs (Fig. 5A–C). Similar results were observed with the JNK-reporter puc-lacZ, which is elevated in en > AP-2σ-IR and en > syx/avl7-IR wing discs relative to an en > control (Fig. 5D–F). We also analyzed levels of phospho-JNK in endocytic mutants. As with MMP-1, increased p-JNK staining was evident in ept clones, in ept,H99 clones (Fig. 4B–B″ and D-D'') and in the posterior compartment of en > syx7/avl-IR and en > vps25-IR animals (Fig. 5H–I). Although en > AP-2σ-IR cells showed robust elevation of the JNK reporters puc-lacZ and MMP-1, these cells showed only mild elevation of p-JNK levels (Fig. 5G). In summary, these data suggest that altered endocytosis is accompanied by elevations in Yki activity and JNK signaling.
Figure 4.
Loss of ept elevates JNK activity. Confocal images of (A–B) ept2 and (C–D) eptx1,H99 clones marked by the absence of GFP stained for (A and C) MMP1 and (B and D) phosphorylated-JNK (p-JNK). Arrows denote p-JNK in mutant clones.
Figure 5.
Depletion of AP-2σ, Syx7 or Vps25 elevates JNK activity. Confocal images of larval imaginal wing discs from (A and G) en > AP-2σ-IR, (B and H) en > syx7-IR and (I) en > vps25-IR flies stained for (A–C) MMP1 and (G–I) p-JNK. (D–F) α-β-Gal staining of larval imaginal wing discs in which the puc-lacZ JNK-pathway reporter has been placed into the background of (D) control en >, (E) en > AP-2σ-IR or (F) en > syx7-IR flies. Wing discs are oriented with the posterior compartment to the right.
Endocytic neoplastic tumor suppressor genes require bsk to activate Yki.
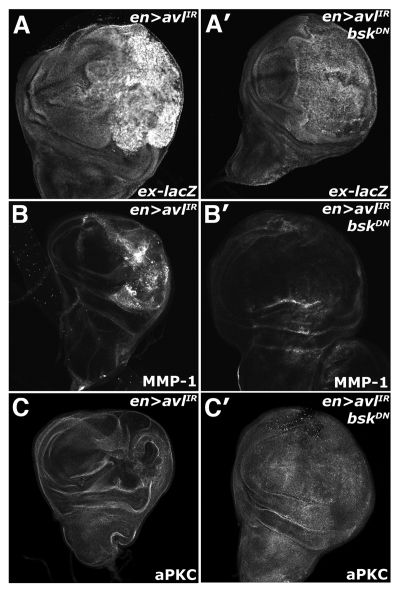
The established role of JNK upstream of Yki suggests that the activation of these two pathways could be linked in cells with defective endocytic nTSGs. To test this, we analyzed the effect of expression of a dominant-negative allele of bsk (DN-Bsk) on Yki activity in the background of en > syx7/avl-IR animals. Co-expression of DN-Bsk with avl-IR reduces both the elevated ex-lacZ and MMP-1 expression observed in en > avl-IR discs (Fig. 6A and B). Additionally, we found that expression of the DN-bsk transgene was sufficient to rescue the disorganized morphology associated with a block in endocytosis (Fig. 6C), paralleling similar results from studies that found a reduction of JNK activity could restore defective tissue architecture and polarity in lgl mutant animals.41
Figure 6.
Endolysosomal activation of Yki requires Bsk activity. Confocal images of larval imaginal wing discs in which the ex-lacZ reporter has been placed in the background and of (A–C) en > syx7-IR and (A′–C′) en > syx7-IR, DN-Bsk animals and stained for (A) α-β-gal, (B) MMP1 and (C) aPKC to show disc morphology. Wing discs are oriented with the posterior compartment to the right.
Discussion
The link between neoplastic transformation and mutations in genes required for the endolysosomal trafficking in Drosophila is well established (reviewed in refs. 42 and 43). Here we provide insight into this link further by highlighting a connection between endocytic neoplastic tumor suppressor genes (nTSGs) and two major proliferation control pathways, the JNK pathway and the SWH pathway. We show that loss of genes encoding factors that act at early (AP-2σ), middle (syx7/avl, rab5), or late (ept, vps25) steps in the endolysosomal pathway is associated with elevated nuclear activity of the SWH component Yki, which controls expression of genes involved in cell division, cell growth and cell death (reviewed in ref. 13). Moreover, we show that yki is required for overgrowth of discs lacking the clathrin adaptor gene AP-2σ and for the elevated expression of the Yki transcriptional target ex. In parallel to these effects on Yki, we find that loss of endocytic nTSGs is accompanied with significant upregulation of JNK-pathway activity, which is linked to disc neoplasia and SWH-regulation, and that reduction of JNK activity in this background can suppress ectopic Yki activation in cells.
The data presented here reinforce an emerging role for JNK signaling in driving Yki-mediated proliferative phenotypes in cells with disrupted polarity and/or endocytosis. JNK signaling is required for disc neoplasia phenotypes produced by loss of the basolateral polarity components lgl and scrib,30,44–46 and this has been proposed to occur through Yki. JNK1/2 can phosphorylate Yap1 in cultured cells,36 and JNK-activation also promotes Yki activity in Drosophila gut intestinal stem cells,28,29 indicating that Yki can be a target of JNK signaling in normal physiologic contexts as well. The mechanism by which JNK is activated by defective polarity/endocytosis is not clear. The data presented here do not indicate whether JNK activation elicited by loss of endocytic nTSGs occurs independent of altered apicobasal polarity. Studies of dlg and lgl alleles demonstrate a clear role for polarity factors in control of JNK signaling in imaginal discs cells.27,47 Moreover, recent evidence of a pathway involving the polarity factor Cdc42, the Rho1 GTPase, and JNK suggests that altered cell polarity triggers JNK fairly directly,48 and that polarity defects in endocytic nTSG mutants such as ept and vps25 are the primary driver of JNK hyperactivity. However, the polarity kinase aPKC, which is a component of the Cdc42/Par6/aPKC complex,49 is a regulator of endocytic trafficking from the apical membrane.50 Defective polarity could disrupt endocytic internalization and trafficking of apical regulators of the JNK-pathway, such as the TNF receptor Wengen.51 Indeed, the finding that loss of the clathrin-adaptor protein AP-2σ is sufficient to activate the Jun reporter puc-lacZ and the Yki reporter ex-lacZ with relatively subtle effects on the polarity markers Crb and aPKC (Figs. S2 and S3) seems to suggest that altered clathrin-mediated endocytosis (CME) of membrane receptors could contribute to Yki activation by endocytic blocks. Some signaling receptors such as EGF and TGFβ are internalized via alternate CME and non-CME pathways that produce distinct downstream signaling outputs.43,52 This type of mechanism could conceivably play an important role in controlling receptor signaling in normal Drosophila epithelial cells and in those lacking proteins required for various steps in the CME endolysosomal degradation pathway.
Our data also provide insight into phenotypes of ept and perhaps TSG101 mutant cells. First, the proliferation of ept mutant cells has been linked to JAK-STAT and Notch signaling,4,11,12 but our findings suggest that JNK-stimulated activation of Yki may also act as a pro-proliferative factor in these cells. This pro-proliferative role of JNK only becomes apparent when apoptosis is blocked. Uhlirova and Bohmann (2006) noted a similar conditional relationship between a block in apoptosis at the appearance of JNK-dependent invasive phenotypes.40 Second, a pro-death role for JNK in ept mutant cells very much mirrors recent work identifying a pro-apoptotic role for JNK in cells mutant for the ESCRT-III component vps4,53 and raises the possibility that JNK plays an equivalent role in apoptosis of mouse cells lacking the ept vertebrate ortholog TSG101,54–56 which is a candidate tumor suppressor (reviewed in ref. 57). If so, then blockade of JNK-driven death may be necessary for the survival and overproliferation of TSG101 mutant cells in vertebrate tumors.
Materials and Methods
Genetics.
Crosses were performed at 25°C unless otherwise noted. For analysis of larval wing discs, crosses were maintained at at 28°C to enhance GAL4 activity. For analysis of adult wings, crosses were maintained at 20°C. Alleles used: ept;2 eptx1,Df(3L)H99 (M. Gilbert); UAS-sav (G. Halder); ex697;58,59 thjc58 (B. Hay); UAS-AP-2σ-IR (Bloomington Stock Center, stock 27322); UAS-syx7/avl-IR (Bloomington Stock Center, stock 29546); UAS-rab7-IR (Bloomington Stock Center, stock 27501); UAS-vps25-IR (Bloomington Stock Center); avl1 (D. Bilder); UAS-Rab5-DN (M. Scott); UAS-Yorkie-IR (Vienna Drosophila RNAi Center); UAS-Bsk-DN (Bloomington Stock Center, stock 6409). Clonal analysis was performed using: eyFLP;ubi-GFP,FRT80B. ‘Flp-out’ analysis was performed using: eyFLP;Act > CD2 > Gal4;Rps17,4 FRT80B.
Immunohistochemistry and immunoblotting.
Immuno-staining and confocal microscopy were performed as described previously in reference 60. Antibodies used: mouse α-β-Gal 1/1,000 (Promega); mouse α-MMP-1, 1/100 of 1:1:1 aliquot of clones 5H7B11, 3B8D12, 3A6B4 (Developmental Studies Hybridoma Bank); rabbit α-aPKC 1/1,000 (Santa Cruz, C-20); guinea pig α-Ex 1/5,000 (R. Fehon); rabbit α-phospho-JNK 1/1,000 (Promega); rat α-Crb-Extra 1/500 (U. Tepass and E. Knust).
Pupariation analysis.
Briefly, adults were allowed to deposit embryos for a span of 24 h, after which their progeny was tracked and the number of pupae and larvae were compared at 144 h AED. A minimum of 50 flies was analyzed per genotype.
Acknowledgments
We thank M. Gilbert, D. Bilder, G. Halder, M. Scott, R. Fehon, U. Tepass and E. Knust for gifts of fly stocks and antibodies. We also thank the Bloomington Drosophila Stock Center and the Developmental Studies Hybridoma Bank for fly stocks and antibodies. We are grateful to R. Jones and members of the Moberg laboratory for helpful discussions. This work was supported by NIH R01CA123368 to K.H.M. and the Emory BCDB and MTSP Training Grants (T32 GM008367 and T32 GM008169) to B.S.R.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Vaccari T, Bilder D. At the crossroads of polarity, proliferation and apoptosis: the use of Drosophila to unravel the multifaceted role of endocytosis in tumor suppression. Mol Oncol. 2009;3:354–365. doi: 10.1016/j.molonc.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 3.Martinez AM, Cavalli G. Uncovering a tumor-suppressor function for Drosophila polycomb group genes. Cell Cycle. 2010;9:215–216. doi: 10.4161/cc.9.2.10518. [DOI] [PubMed] [Google Scholar]
- 4.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Thompson BJ, Mathieu J, Sung HH, Loeser E, Rorth P, Cohen SM. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Menut L, Vaccari T, Dionne H, Hill J, Wu G, Bilder D. A mosaic genetic screen for Drosophila neoplastic tumor suppressor genes based on defective pupation. Genetics. 2007;177:1667–1677. doi: 10.1534/genetics.107.078360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/S0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Bilder D. Endocytic control of epithelial polarity and proliferation in Drosophila. Nat Cell Biol. 2005;7:1232–1239. doi: 10.1038/ncb1324. [DOI] [PubMed] [Google Scholar]
- 10.Vaccari T, Rusten TE, Menut L, Nezis IP, Brech A, Stenmark H, et al. Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants. J Cell Sci. 2009;122:2413–2423. doi: 10.1242/jcs.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert MM, Robinson BS, Moberg KH. Functional interactions between the erupted/tsg101 growth suppressor gene and the DaPKC and rbf1 genes in Drosophila imaginal disc tumors. PLoS ONE. 2009;4:7039. doi: 10.1371/journal.pone.0007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert MM, Beam CK, Robinson BS, Moberg KH. Genetic interactions between the Drosophila tumor suppressor gene ept and the stat92E transcription factor. PLoS ONE. 2009;4:7083. doi: 10.1371/journal.pone.0007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:574–582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 15.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/ippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–3100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 19.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 21.Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci USA. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci USA. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGFβ-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rappoport JZ. Focusing on clathrin-mediated endocytosis. Biochem J. 2008;412:415–423. doi: 10.1042/BJ20080474. [DOI] [PubMed] [Google Scholar]
- 26.Vidal M. The dark side of fly TNF: an ancient developmental proof reading mechanism turned into tumor promoter. Cell Cycle. 2010;9:3851–3856. doi: 10.4161/cc.9.19.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350:139–151. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011;350:255–266. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 31.White K, Grether ME, Abrams JM, Young L, Farrell K, Steller H. Genetic control of programmed cell death in Drosophila. Science. 1994;264:677–683. doi: 10.1126/science.8171319. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert MM, Tipping M, Veraksa A, Moberg KH. A screen for conditional growth suppressor genes identifies the Drosophila homolog of HD-PTP as a regulator of the oncoprotein Yorkie. Dev Cell. 2011;20:700–712. doi: 10.1016/j.devcel.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tapon N, Harvey KF, Bell DW, Wahrer DCR, Schiripo TA, Haber DA, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/S0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 34.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 35.Herz HM, Chen Z, Scherr H, Lackey M, Bolduc C, Bergmann A. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development. 2006;133:1871–1880. doi: 10.1242/dev.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomlinson V, Gudmundsdottir K, Luong P, Leung KY, Knebel A, Basu S. JNK phosphorylates Yes-associated protein (YAP) to regulate apoptosis. Cell Death Dis. 2010;1:29. doi: 10.1038/cddis.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev. 2002;12:14–21. doi: 10.1016/S0959-437X(01)00258-1. [DOI] [PubMed] [Google Scholar]
- 38.Perkins KK, Dailey GM, Tjian R. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988;7:4265–4273. doi: 10.1002/j.1460-2075.1988.tb03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martín-Blanco E, Gampel A, Ring J, Virdee K, Kirov N, Tolkovsky AM, et al. puckered encodes a phosphatase that mediates a feedback loop regulating JNK activity during dorsal closure in Drosophila. Genes Dev. 1998;12:557–570. doi: 10.1101/gad.12.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu M, Xin T, Weng S, Gao Y, Zhang Y, Li Q, et al. Activation of JNK signaling links lgl mutations to disruption of the cell polarity and epithelial organization in Drosophila imaginal discs. Cell Res. 2010;20:242–245. doi: 10.1038/cr.2010.2. [DOI] [PubMed] [Google Scholar]
- 42.Giebel B, Wodarz A. Tumor suppressors: control of signaling by endocytosis. Curr Biol. 2006;16:91–92. doi: 10.1016/j.cub.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–219. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463:545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Igaki T, Pagliarini RA, Xu T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr Biol. 2006;16:1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 46.Brumby AM, Richardson HE. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 2003;22:5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grzeschik NA, Parsons LM, Richardson HE. Lgl, the SWH pathway and tumorigenesis: It's a matter of context & competition! Cell Cycle. 2010;9:3202–3212. doi: 10.4161/cc.9.16.12633. [DOI] [PubMed] [Google Scholar]
- 48.Warner SJ, Yashiro H, Longmore GD. The Cdc42/Par6/aPKC polarity complex regulates apoptosis-induced compensatory proliferation in epithelia. Curr Biol. 2010;20:677–686. doi: 10.1016/j.cub.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henrique D, Schweisguth F. Cell polarity: the ups and downs of the Par6/aPKC complex. Curr Opin Genet Dev. 2003;13:341–350. doi: 10.1016/S0959-437X(03)00077-7. [DOI] [PubMed] [Google Scholar]
- 50.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 51.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M. Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem. 2002;277:28372–28375. doi: 10.1074/jbc.C200324200. [DOI] [PubMed] [Google Scholar]
- 52.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGFbeta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 53.Rodahl LM, Haglund K, Sem-Jacobsen C, Wendler F, Vincent JP, Lindmo K, et al. Disruption of Vps4 and JNK function in Drosophila causes tumour growth. PLoS ONE. 2009;4:4354. doi: 10.1371/journal.pone.0004354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carstens MJ, Krempler A, Triplett AA, Van Lohuizen M, Wagner KU. Cell cycle arrest and cell death are controlled by p53-dependent and p53-independent mechanisms in Tsg101-deficient cells. J Biol Chem. 2004;279:35984–35994. doi: 10.1074/jbc.M400408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krempler A, Henry MD, Triplett AA, Wagner KU. Targeted deletion of the Tsg101 gene results in cell cycle arrest at G1/S and p53-independent cell death. J Biol Chem. 2002;277:43216–43223. doi: 10.1074/jbc.M207662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruland J, Sirard C, Elia A, MacPherson D, Wakeham A, Li L, et al. p53 accumulation, defective cell proliferation and early embryonic lethality in mice lacking tsg101. Proc Natl Acad Sci USA. 2001;98:1859–1864. doi: 10.1073/pnas.98.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herz HM, Bergmann A. Genetic analysis of ESCRT function in Drosophila: a tumour model for human Tsg101. Biochem Soc Trans. 2009;37:204–207. doi: 10.1042/BST0370204. [DOI] [PubMed] [Google Scholar]
- 58.Boedigheimer M, Bryant P, Laughon A. Expanded, a negative regulator of cell proliferation in Drosophila, shows homology to the NF2 tumor suppressor. Mech Dev. 1993;44:83–84. doi: 10.1016/0925-4773(93)90058-6. [DOI] [PubMed] [Google Scholar]
- 59.Boedigheimer M, Laughon A. Expanded: a gene involved in the control of cell proliferation in imaginal discs. Development. 1993;118:1291–1301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- 60.Moberg KH, Mukherjee A, Veraksa A, Artavanis-Tsakonas S, Hariharan IK. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol. 2004;14:965–974. doi: 10.1016/j.cub.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 61.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 62.Javier RT, Cell polarity proteins: common targets for tumorigenic human viruses. Oncogene. 2008;27:7031–7046. doi: 10.1038/onc.2008.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hariharan IK, Bilder D. Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. Annu Rev Genet. 2006;40:335–361. doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.