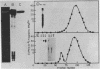
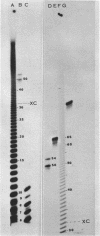
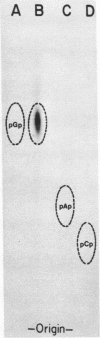
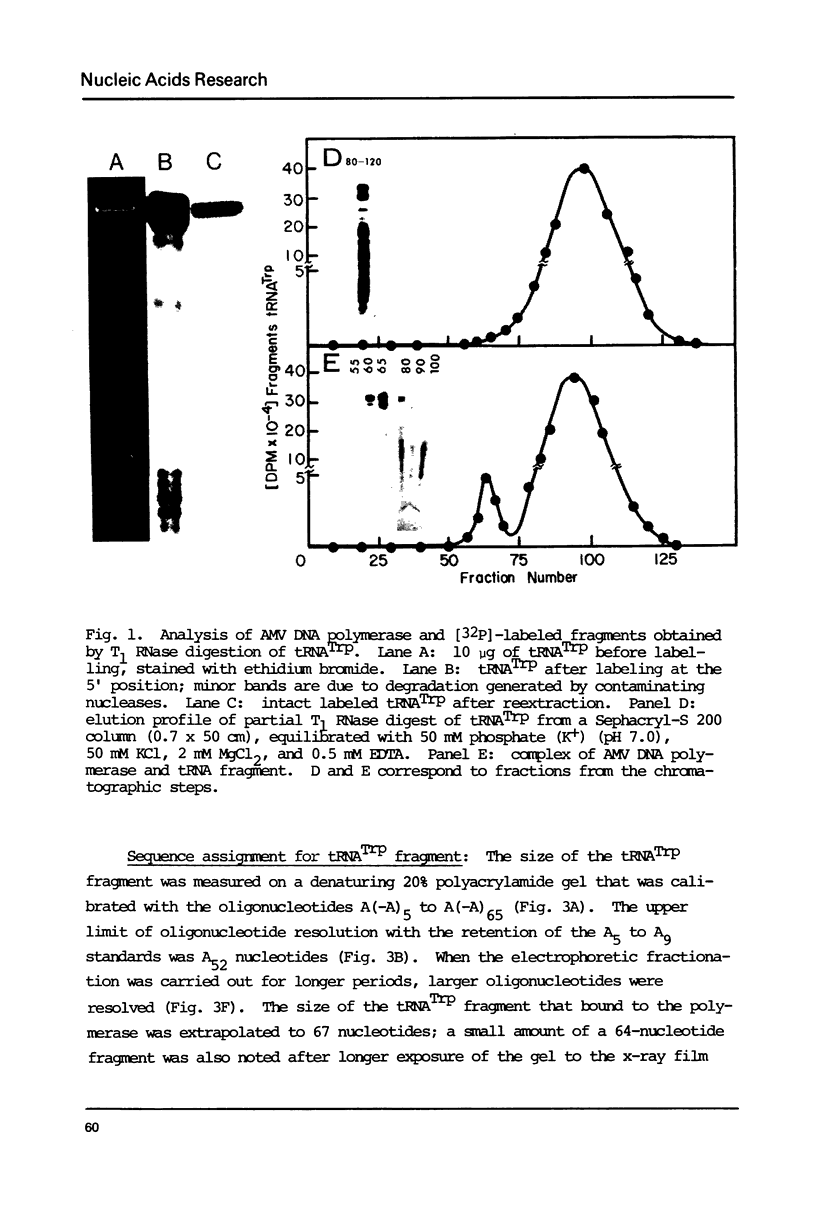
Abstract
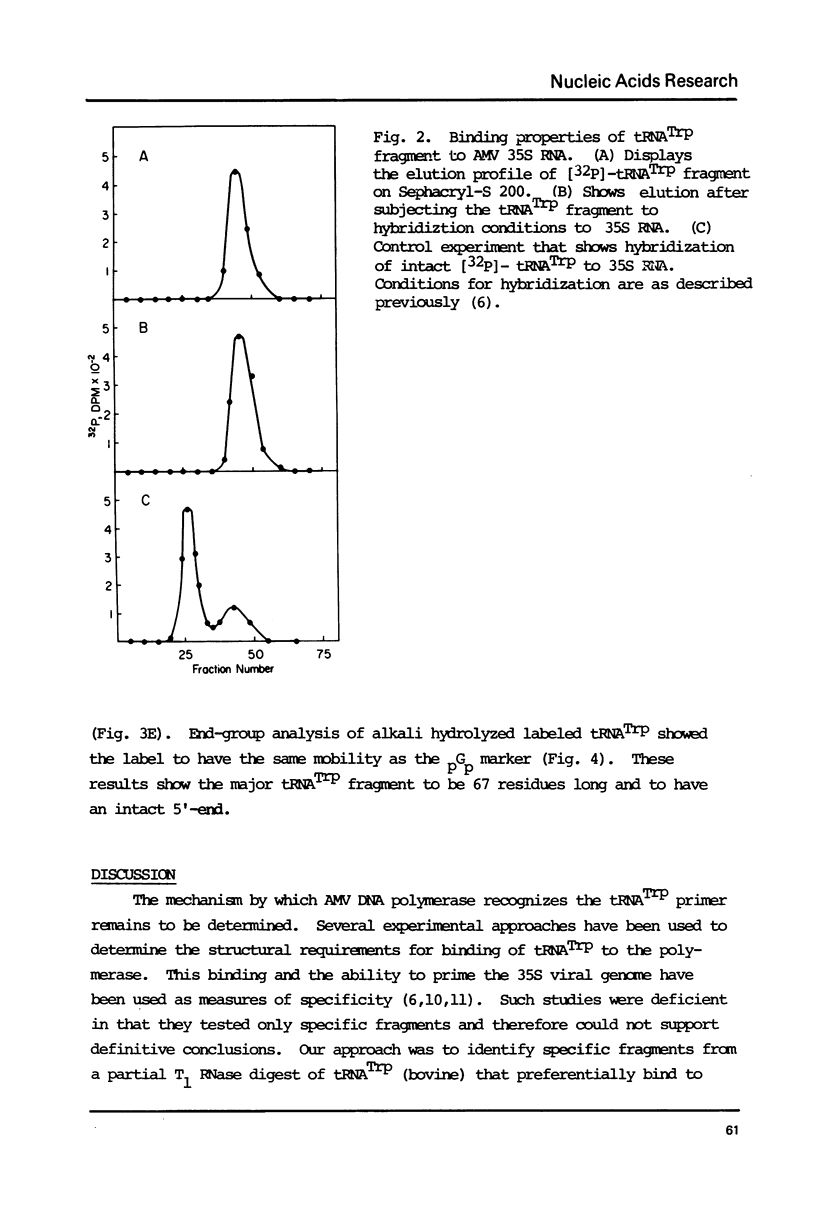
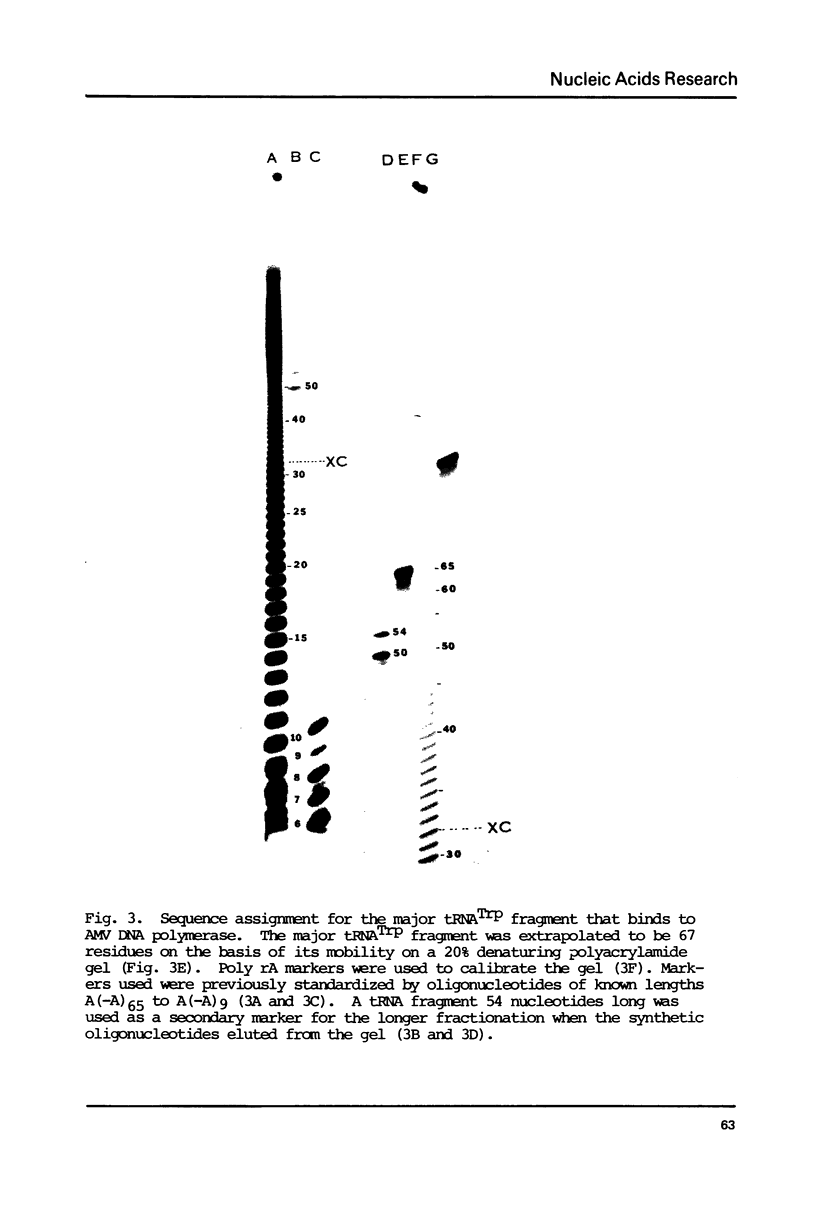
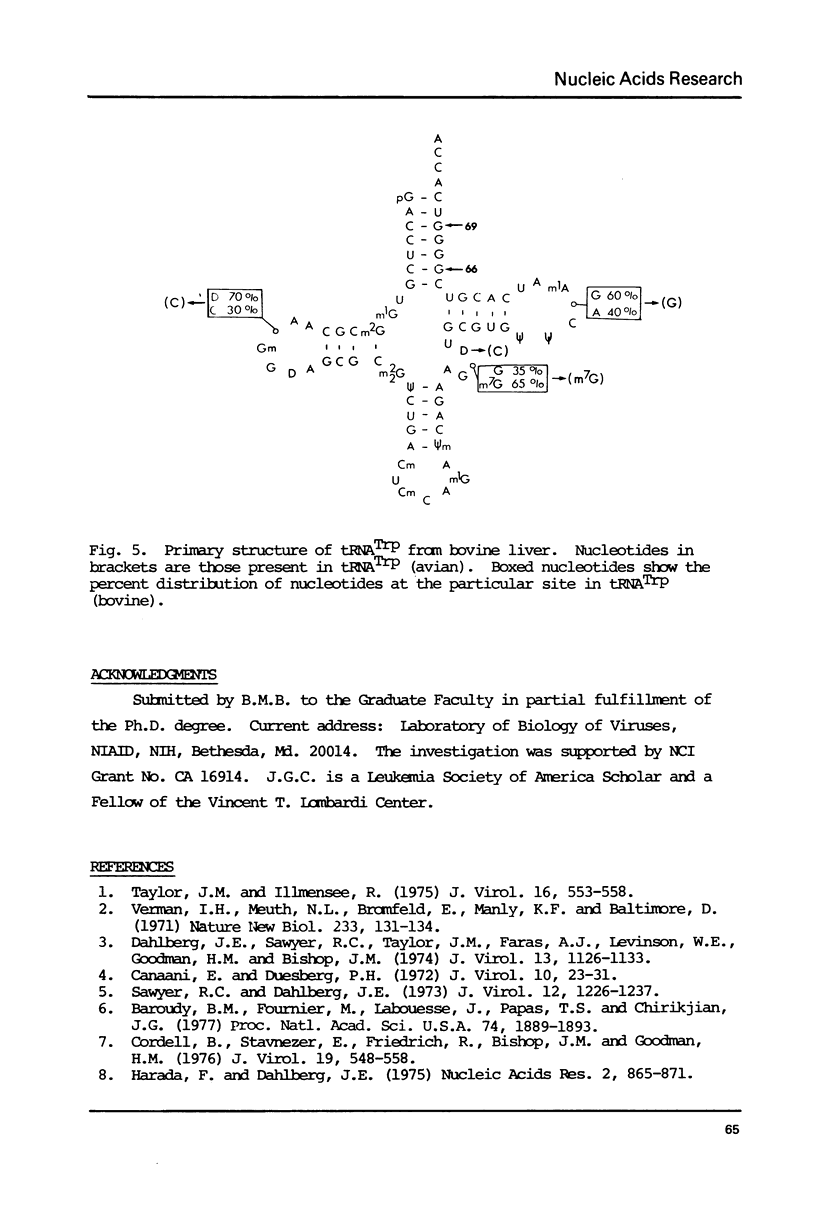
Avian DNA polymerases use host tRNATrp as the primer for transcription. Bovine tRNATrp has been previously shown to be a biologic substitute for the avian primer. A bovine tRNATrp fragment has been identified as having a high binding affinity for the polymerase. The fragment is assigned to be 67 nucleotides, and contains most of the elements required to maintain the secondary and tertiary structure of tRNATrp.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Fournier M., Labouesse J., Papas T. S., Chirikjian J. G. tRNATrp (bovine) binding to the reverse transcriptase of avian myeloblastosis virus and function as a heterologous primer. Proc Natl Acad Sci U S A. 1977 May;74(5):1889–1893. doi: 10.1073/pnas.74.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirikjian J. G., Rye L., Papas T. S. Affinity chromatography of viral DNA polymerases on pyran-sepharose. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1142–1146. doi: 10.1073/pnas.72.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Stavnezer E., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence that binds primer for DNA synthesis to the avian sarcoma virus genome. J Virol. 1976 Aug;19(2):548–558. doi: 10.1128/jvi.19.2.548-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Swanstrom R., Goodman H. M., Bishop J. M. tRNATrp as primer for RNA-directed DNA polymerase: structural determinants of function. J Biol Chem. 1979 Mar 25;254(6):1866–1874. [PubMed] [Google Scholar]
- Dahlberg J. E., Sawyer R. C., Taylor J. M., Faras A. J., Levinson W. E., Goodman H. M., Bishop J. M. Transcription of DNA from the 70S RNA of Rous sarcoma virus. I. Identification of a specific 4S RNA which serves as primer. J Virol. 1974 May;13(5):1126–1133. doi: 10.1128/jvi.13.5.1126-1133.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Quade K., Nichols J. L. Interaction of tryptophan transfer RNA with Rous sarcoma virus 35S RNA. Nature. 1976 Jan 22;259(5540):245–247. doi: 10.1038/259245a0. [DOI] [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Golomb M., Grandgenett D. P. Endonuclease activity of purified RNA-directed DNA polymerase from avian myeloblastosis virus. J Biol Chem. 1979 Mar 10;254(5):1606–1613. [PubMed] [Google Scholar]
- Harada F., Dahlberg J. E. Specific cleavage of tRNA by nuclease S1. Nucleic Acids Res. 1975 Jun;2(6):865–871. doi: 10.1093/nar/2.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas T. S., Chirigos M. A., Chirikjian J. G. Protection of avian myeloblastosis virus (AMV) DNA polymerase by substrates against heat inactivation. Nucleic Acids Res. 1974 Nov;1(11):1399–1409. doi: 10.1093/nar/1.11.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papas T. S., Marciani D. J., Samuel K., Chirikjian J. G. Mechanism of release of active alpha subunit from dimeric alpha beta avian myeloblastosis virus DNA polymerase. J Virol. 1976 Jun;18(3):904–910. doi: 10.1128/jvi.18.3.904-910.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel K. P., Papas T. S., Chirikjian J. G. DNA endonucleases associated with the avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2659–2663. doi: 10.1073/pnas.76.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Base pairing and fidelity in codon-anticodon interaction. Nature. 1976 Sep 23;263(5575):289–293. doi: 10.1038/263289a0. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Meuth N. L., Bromfeld E., Manly K. F., Baltimore D. Covalently linked RNA-DNA molecule as initial product of RNA tumour virus DNA polymerase. Nat New Biol. 1971 Sep 29;233(39):131–134. doi: 10.1038/newbio233131a0. [DOI] [PubMed] [Google Scholar]