Summary
Anthrax is endemic throughout Africa, causing considerable livestock and wildlife losses and severe, sometimes fatal, infection in humans. Predicting the risk of infection is therefore important for public health, wildlife conservation and livestock economies. However, because of the intermittent and variable nature of anthrax outbreaks, associated environmental and climatic conditions, and diversity of species affected, the ecology of this multihost pathogen is poorly understood.
We explored records of anthrax from the Serengeti ecosystem in north-west Tanzania where the disease has been documented in humans, domestic animals and a range of wildlife. Using spatial and temporal case-detection and seroprevalence data from wild and domestic animals, we investigated spatial, environmental, climatic and species-specific associations in exposure and disease.
Anthrax was detected annually in numerous species, but large outbreaks were spatially localized, mostly affecting a few focal herbivores.
Soil alkalinity and cumulative weather extremes were identified as useful spatial and temporal predictors of exposure and infection risk, and for triggering the onset of large outbreaks.
Interacting ecological and behavioural factors, specifically functional groups and spatiotemporal overlap, helped to explain the variable patterns of infection and exposure among species.
Synthesis and applications. Our results shed light on ecological drivers of anthrax infection and suggest that soil alkalinity and prolonged droughts or rains are useful predictors of disease occurrence that could guide risk-based surveillance. These insights should inform strategies for managing anthrax including prophylactic livestock vaccination, timing of public health warnings and antibiotic provision in high-risk areas. However, this research highlights the need for greater surveillance (environmental, serological and case-detection-orientated) to determine the mechanisms underlying anthrax dynamics.
Keywords: Bacillus anthracis, disease ecology, exposure, infectious disease, multihost, serology, surveillance, susceptibility, zoonosis
Introduction
Bacillus anthracis (Cohn), the causative agent of anthrax, is a multihost pathogen affecting human, livestock and wildlife populations. The disease has a world-wide distribution, but has declined in many developed countries because of the implementation of livestock vaccination programmes and sanitary measures. However, anthrax is still endemic in Africa, with severe outbreaks causing significant losses in domestic and wild animal populations (Prins & Weyerhaeuser 1987; Shiferaw et al. 2002; Siamudaala 2006; Clegg et al. 2007; Wafula, Patrick & Charles 2008). Non-fatal cutaneous anthrax acquired through contact with infected carcasses or animal products accounts for >95% of all reported human cases globally (World Health Organization 2008). More severe forms, particularly gastrointestinal anthrax because of handling and consumption of inadequately cooked products from infected animals, may also exert a substantial burden that is both underreported and under-diagnosed (Sirisanthana & Brown 2002). Predicting the risk of infection is therefore important from the perspectives of public health, wildlife conservation and livestock economies.
Despite the infamous reputation of anthrax, the ecology and transmission of the disease under natural conditions are not well understood (Hugh-Jones & de Vos 2002). The difficulty lies in the intermittent and variable nature of outbreaks, with considerable variation in the species affected, and associated environmental and climatic conditions (Table 1).
Table 1.
Anthrax outbreaks in Eastern and Southern Africa for which details were available. Predominant species are emboldened, followed by species in which > 5 cases were documented
| Location | Country | Habitat | Date | Species affected | Weather/season | Reference |
|---|---|---|---|---|---|---|
| Mago NP | Ethiopia | Bushland, savanna grassland | September-December 1999 |
Lesser kudu, gerenuk, warthog, bushbuck |
Drought | Shiferaw et al. (2002) |
| Bushland, savanna grassland | September-October 2000 |
Lesser kudu, bushbuck, gerenuk, dik-dik, |
Dry season | Shiferaw et al. (2002) | ||
| Samburu | Kenya | Semi-arid savanna, grasslands, bushland |
December 2005- March 2006 |
Grevy’s zebra, plains zebra, livestock |
Drought | Muoria et al. (2007) |
| Queen Elizabeth NP | Uganda | Rivers, riverine habitat, alkaline soils |
1959 | Hippopotamus | Wafula, Patrick & Charles (2008) | |
| Rivers, riverine habitat, alkaline soils |
1962 | Hippopotamus | Wafula, Patrick & Charles (2008) | |||
| Rivers, riverine habitat, alkaline soils |
1991 | Hippopotamus | Wafula, Patrick & Charles (2008) | |||
| Rivers, riverine habitat, alkaline soils |
July 2004-March 2005 |
Hippopotamus, zebra, buffalo, kob, warthog |
Prolonged dry period |
Wafula, Patrick & Charles (2008) | ||
| Lake Mburo NP | Uganda | Short grass plains in low lying valleys, alkaline soils |
March-September 2005 | Zebra | First rains following prolonged dry period |
Wafula, Patrick & Charles (2008) |
| Serengeti NP | Tanzania | Acacia woodland, alkaline soils | January-February 1998 | Impala | Heavy rains (ENSO associated) |
Mlengeya et al. (1998) |
| Lake Manyara | Tanzania | Riverine woodland | December 1983- November 1984 |
Impala | End of the Dry season |
Prins &Weyerhaeuser (1987) |
| Luangwa Valley | Zambia | Riverine woodland | June-November 1987 |
Hippopotamus, buffalo, elephant |
Dry season | Turnbull et al. (1991) |
| Riverine woodland | August-September 1988 | Hippopotamus | Dry season | Turnbull et al. (1991) | ||
| Malilangwe WR & Save Valley Conservancy |
Zimbabwe | Riverine woodland, acacia woodland |
August-November 2004 |
Greater kudu, buffalo, bushbuck, waterbuck |
Dry season | Clegg et al. (2007) |
| Caprivi | Namibia | Bushland and riverine habitat | May-July 1960 | Kudu, Roan antelope | Drought | Pienaar (1961) |
| Chobe | Botswana & | Marshland | 1998 | Elephant | Dry season | ProMed-Mail (2000) |
| National Park + Caprivi Region |
Namibia | September-December 2004 | Buffalo, elephant, zebra | Dry season | ProMed-Mail (2004a, 2004b) | |
| August-October 2006 | Buffalo, elephant, zebra | Dry season |
Barrett (2006); Dudley (2006): ProMed-Mail (2006) |
|||
| Etosha NP | Namibia | Central eastern Etosha with ‘contaminated’ waterholes |
1968-1972 | Zebra, wildebeest, and others | Wet season | Lindeque, Brain & Turnbull (1996) |
| Widely distributed, especially in west |
November 1981-January 1982 |
Elephant | Dry season | Lindeque & Turnbull (1994) | ||
| Widely distributed, especially in west |
October-December 1989 | Elephant | Dry season | Lindeque & Turnbull (1994) | ||
| Central and eastern Etosha | March-April 1984 |
Zebra, Wildebeest, gemsbok, springbok |
End of rainy season |
Lindeque & Turnbull (1994) | ||
| Central and eastern Etosha Possible association with burning |
February 2010 | Zebra, springbok | Rainy season | Bellan (2010) | ||
| Kruger NP | South Africa | Around watering holes | October-November 1959 |
Kudu, waterbuck, gemsbok, buffalo |
Dry season | Pienaar (1960) |
| Mopane and low lying plains | June-September 1960 |
Greater kudu, roan, buffalo, waterbuck, Nyala, bushbuck, steinbuck, impala |
Dry season | Pienaar (1961); de Vos (1990) | ||
| Mopane and low lying plains | August-October 1970 |
Greater kudu, roan, buffalo and others |
Dry season | de Vos (1990) | ||
| Mopane and low lying plains - associated with |
August-October 1990 | Greater kudu, buffalo, roan, waterbuck | Dry season | de Vos & Bryden (1996) | ||
| stagnant pools | May-October 1991 | Greater kudu, buffalo, roan, waterbuck | Dry season | de Vos & Bryden (1996) | ||
| Mixed plains and knobthorn /marula savanna |
September-November 1993 | Greater kudu and others | Dry season | Bryden (1999) | ||
| Mixed plains and knobthorn /marula savanna |
June-July 1999 |
Greater kudu, wildebeest, zebra and others |
Dry season |
Bryden (1999): Pollack (1999) |
NP, National Park; WR, Wildlife Reserve.
The life cycle of B. anthracis comprises a multiplication phase in the mammalian host and a persistence phase of spores in the soil. Transmission to herbivores largely occurs indirectly, so risk factors are usually associated with exposure to spores, rather than direct animal-to-animal transmission. Survival mechanisms of the pathogen outside the host remain unclear. Bacillus anthracis has been shown to be associated with plant roots (Saile & Koehler 2006), and this is suggested to be an adaptation that increases the likelihood of infecting ungulate hosts (Raymond et al. 2010). Certain environmental factors affect long-term spore survival and thus increase the risk of anthrax (Blackburn et al. 2007). For example, the endemicity of B. anthracis in some areas has been associated with calcium-rich and neutral-to-alkaline soils (van Ness 1971; Dragon et al. 2005; Hugh-Jones & Blackburn 2009), although strain differences exist in soil chemistry preferences (Smith et al. 2000). Strain differences, in fact, may also govern spread in anthrax epidemics (Blackburn et al. 2007; Garofolo et al. 2010).
Seasonal incidence patterns suggest climatic factors (precipitation and ambient temperature) play an important role in triggering outbreaks, although these are not consistent between locations (Table 1), and therefore underlying mechanisms are debated. In some African ecosystems, outbreaks have been reported late in the dry season (Prins & Weyerhaeuser 1987; Turnbull et al. 1991; Lindeque & Turnbull 1994; de Vos & Bryden 1996; Shiferaw et al. 2002; Clegg et al. 2007; Muoria et al. 2007), leading to suggestions that transmission may be facilitated by close grazing, nutritional stress and congregation at water holes (Hugh-Jones & de Vos 2002). Elsewhere, outbreaks have been associated with heavy rains (Lindeque & Turnbull 1994; Mlengeya et al. 1998; Wafula, Patrick & Charles 2008; Bellan 2010), which are hypothesized to unearth spores and amplify vector populations (Durrheim et al. 2009; Hugh-Jones & Blackburn 2009; Lewerin et al. 2010). Experimental spore germination and vegetative growth in the rhizosphere provide a mechanism by which bursts of rainfall could spark epidemics (Saile & Koehler 2006). This would parsimoniously explain anthrax occurrences during dry summers in North America and Australia that have been preceded by rains and when animals are often in good body condition (Turner et al. 1999; Hugh-Jones & de Vos 2002; Parkinson, Rajic & Jenson 2003; Hugh-Jones & Blackburn 2009; Epp, Waldner & Argue 2010).
Although anthrax infects a wide range of species, outbreaks are typically associated with just a few (Table 1), for example impala Aepyceros melampus, kudu Tragelaphus strepsiceros, buffalo Syncerus caffer and zebra Equus spp. (Prins & Weyerhaeuser 1987; Mlengeya et al. 1998; Clegg et al. 2007; Muoria et al. 2007; Wafula, Patrick & Charles 2008). Even within a single ecosystem, different species may be affected at different times (Lindeque & Turnbull 1994). Reasons for this remain unclear, but could be attributed to host-specific differences in susceptibility and exposure as a result of behavioural and ecological traits as well as differences in pathogen strains. The overall ecological and genetic factors that contribute to environmental persistence of anthrax, and the underlying conditions that initiate outbreaks and underlie patterns of circulation are poorly known.
In the Serengeti ecosystem, anthrax has been reported in a variety of species, with sporadic outbreaks occurring in relatively localized endemic foci and mostly affecting a few focal species (Lembo et al. 2011). The ecosystem covers a large area (over 20 000 km2, Sinclair et al. 2008) encompassing a variety of habitats and environmental gradients, which may influence anthrax occurrence. Here, we draw on data gathered opportunistically including serology indicative of exposure and case reports from human, domestic and wild animal populations in the greater Serengeti ecosystem, to explore ecological factors affecting infection patterns in a range of species. Combined, the investigation into environmental and climatic predictors of anthrax and their associations with species-specific patterns of exposure and mortality provides a holistic picture of ecological drivers of anthrax dynamics and identifies priorities for further research in anthrax endemic ecosystems.
Materials and methods
STUDY AREA
Data were collected from multi-ethnic, agro-pastoralist communities to the west of Serengeti National Park (SNP), pastoralist communities in Ngorongoro District to the east of SNP and wildlife populations within and adjacent to SNP (Fig. 1). Spatial data on environmental and climatic factors including elevation, rainfall, vegetation and distances to water-bodies were available from the Serengeti Biocomplexity Project Database (http://www.serengetidata.org); additional rainfall data from Maswa Game Reserve was collected by Tanzania Game Tracker Safaris/Friedkin Conservation Fund (TGTS/FCF). Parameters of soil moisture and capacity to hold nutrients (including percentages of sand, clay, and carbon/organic matter, pH, cation exchange capacity and exchangeable sodium percentage) were compiled from the Harmonized World Soil Database (FAO/IIASA/ISRIC/ISSCAS/JRC 2009).
Fig. 1.
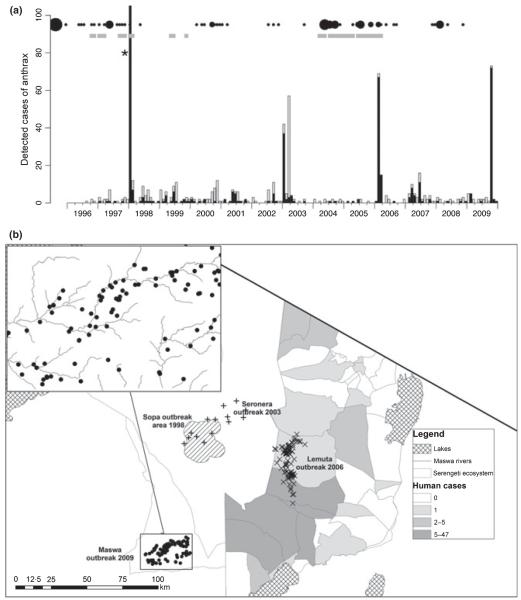
Spatiotemporal patterns of anthrax infection in the Serengeti ecosystem. (a) Time series showing continuous detection of small numbers of cases in wildlife, interspersed with occasional large outbreaks. Probable cases are shown in black and suspect cases in grey. *549 ‘probable’ and 67 ‘suspect’ cases were detected. Black circles indicate hospital records, scaled according to numbers and gray squares indicate when livestock cases were reported (data quality too poor to quantify). (b) Spatial location of cases, showing outbreak areas. Villages shaded according to hospital reports from 1995 to 2008. Exact locations of carcasses retrieved during the 1998 (Sopa) outbreak were unavailable, so the shaded area demarcates the outbreak area. For the 2003 outbreak (crosses – Seronera), the locations of cases are randomized within a 10 km radius of the outbreak area, because exact locations were not available. Inset shows the location of buffalo carcasses during the 2009 outbreak in Maswa district. LGCA, Loliondo Game Control Area; NCA, Ngorongoro Conservation Area; SNP, Serengeti National Park.
DISEASE MONITORING OPERATIONS
Detection of anthrax in wildlife relied upon passive surveillance operations established in 1996 by Tanzania National Parks (TANAPA) and Tanzania Wildlife Research Institute (TAWIRI). Sightings of carcasses were reported through a network of veterinarians, rangers, scientists and tour operators. Follow-up protocols included the collection of blood smears from carcasses, staining with methylene blue and examination by microscopy for encapsulated bacilli. Because of the relatively low proportion of carcasses from which diagnostic samples could be retrieved, a presumptive diagnosis was based on post-mortem presentation: anthrax was considered ‘suspect’ in case of unexplained death or ‘probable’ if carcasses had evidence of bloody discharge from anus, vulva, nostrils, mouth, eyes or ears and incomplete rigor mortis. ‘Probable’ cases included carcasses detected during outbreaks where at least one case was confirmed by microscopy, but which were not necessarily individually confirmed. No definitive confirmation by bacterial culture and isolation was possible because of the lack of facilities in Tanzania.
Detection of anthrax in livestock and human populations was based on passive surveillance data compiled from records in veterinary offices (Government livestock offices, TAWIRI, TANAPA and Ngorongoro Conservation Area (NCA) Authority) and hospitals.
SEROLOGICAL IN VESTIGATIONS
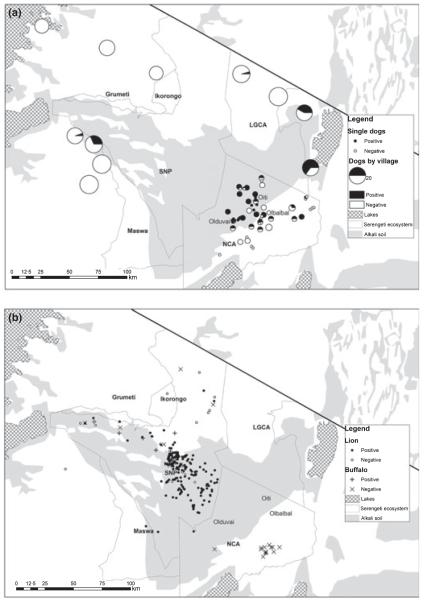
Serum samples from wildlife populations in SNP and the NCA and domestic dog Canis familiaris populations adjacent to the protected areas were obtained opportunistically during long-term epidemiological and ecological studies. These were tested with an immunoassay (QuickELISA Anthrax-PA kit; Immunetics, Inc., Boston, MA, USA) that detects antibodies to the protective antigen (PA) of B. anthracis, as described in Lembo et al. (2011). The domestic dog samples (n = 314) were collected between 1997 and 2006 during dog vaccination campaigns in 24 villages (Fig. 3a): four in Loliondo division, 13 in the NCA and seven to the west of SNP (in Serengeti, Musoma and Bariadi districts) and included 169 random samples obtained as part of other surveys and 53 samples linked to a major outbreak in pastoralist areas in 2006. Wildlife samples comprised: 286 lions Panthera leo sampled between 1984 and 2007; 53 spotted hyenas Crocuta crocuta, 49 buffalo S. caffer, 59 wildebeest Connochaetes taurinus, and 85 plains zebra Equus burchelli, sampled between 1998 and 2007. Interpretation of serological results was based on comparison of absorbance for the sample vs. an assay-defined cut-off. The QuickELISA Anthrax-PA Kit was configured to detect c. 300 ng mL−1 of PA-specific antibody at the cut-off. The assay cut-off was defined as the mean net A450 nm plus 0.1 of the negative control, which was provided in the kit as negative controls were not available from the study populations; the targeted cut-off range was 0.11–0.25.
Fig. 3.
Maps showing (a) domestic dog seroprevalence and (b) buffalo and lion serostatus in relation to soil alkalinity. LGCA, Loliondo Game Control Area; NCA, Ngorongoro Conservation Area; SNP, Serengeti National Park.
STATISTICAL ANALYSES
Randomization tests were used to explore pair-wise associations in the timing of anthrax occurrence among species. A co-occurrence index (Ii.j) incorporating the number and timing of cases in each of a pair of species (species i and species j) was constructed from the observed time series of probable cases:
The number of probable anthrax cases per month, from month 1 to month T, in species i, is denoted by ct,i. The index was recalculated for each of 1000 permutations of the monthly anthrax occurrences and compared to the true index to determine whether the association of cases between pairs of species was likely to have occurred by chance alone. A more conservative test was carried out whereby only months with at least one anthrax case (i.e. ci,t > 0) were permuted. A third test was conducted using data only on presence and absence of anthrax (i.e. ct,i was binary) and permuting all months in the time series.
An environmental risk factor analysis for seroprevalence in dogs, buffalo and lions was conducted using a Generalized Linear Mixed Model (GLMM) framework with a binary outcome variable (seronegative or seropositive), and the location (village for dogs, protected area for wildlife) modelled as a random effect. The following predictors were screened by univariate analysis: (i) animal age (in months for dogs and lions and estimated for buffalo: young = 0–5 years; mid = 5–10 years; and old = >10 years); (ii) the area in which the village/sub-village was located (the West, Loliondo and the NCA) for dogs and SNP or NCA for buffalo and no random effect for lions as all but four data points were within SNP; and (iii) soil-related predictors including acid or alkaline soil (pH ≤ 7 or pH > 7), the proportion of sand, clay, organic content and cation exchange capacity. Predictors that were significant in the univariate analysis with P < 0.05 were included in the multivariate analysis. The multivariate model was reduced by sequentially removing non-significant terms until a model was left with only terms significant at P < 0.05. Differences in seroprevalence between species (lion, hyena, buffalo, wilde-beest and zebra), populations (SNP vs. Ngorongoro Crater) and years were also tested using the GLMM framework and binomial error structure. Locations of probable buffalo carcasses (n = 81) were available from the 2009 outbreak in Maswa. The distances of these to rivers were compared to expected distances had buffalo been distributed homogeneously (using a random set of 100 000 points within the vicinity) using a t-test (2-sided).
A generalized linear model with binomial errors was used to test whether outbreak occurrence was associated with climatic conditions, and in particular prolonged weather extremes (floods and droughts). ‘Outbreak months’ were defined as those with over 10 ‘probable’ anthrax cases, including at least one confirmed by microscopy. Seasonal rainfall patterns were consistent between outbreak sites (which were within areas of similar rainfall), despite the presence of an overall precipitation gradient across the ecosystem (Holdo, Holt & Fryxell 2009). Mean monthly (across-site) rainfall was therefore calculated from a minimum of 30 months of rainfall data from each outbreak site and absolute rainfall deviation at a site from the mean monthly (across-site) average was tested as a predictor of outbreaks. Cumulative deviations from the (previous 1, 2 and 3) monthly means were also tested as predictors of outbreaks.
A linear regression was used to investigate the relationship between mean annual antibody responses to anthrax in Serengeti lions and annual rainfall, weighted by the square root of the number of lions sampled. Residuals were checked to ensure the validity of model assumptions.
All statistical analyses were implemented within the r programming language.
Results
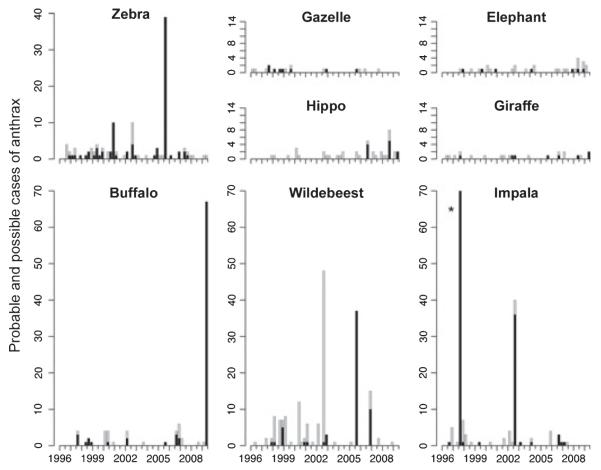
Anthrax was detected throughout the study period, but major outbreaks occurred in 1998, 2003, 2006 and 2009 (Fig. 1). Many species were affected, including wildlife, livestock and humans (for full list of affected species see Lembo et al. 2011) but the predominant species varied between outbreaks: impalas in 1998 and 2003, zebras and wildebeest in 2006 and buffalos in 2009 (Fig. 2). Significant associations in anthrax occurrence amongst these predominant species were found (Table 2), particularly among grazers such as wildebeest, zebra and livestock. Anthrax cases co-occurred in buffalo and impala, buffalo and giraffe Giraffa camelopardalis, livestock and impala, and wildebeest and gazelles (Eudorcas thomsonii and Nanger granti, grouped), but there were too few cases in these other species for statistical inference (Fig. 2). Only two carnivores fatalities, a cheetah Acinonyx jubatus and a serval cat Leptailurus serval were attributed to anthrax (during the 1998 outbreak). Seroprevalence was consistently high in carnivores (90% and 57% overall seropositivity in Serengeti and Ngorongoro Crater lions respectively and 87% seropositivity in Serengeti spotted hyenas) and significantly lower among herbivores (46% and 14% seropositivity in Serengeti and Ngorongoro Crater buffalo, 19% and 4% in Serengeti and Ngorongoro Crater wildebeest), with no seropositive zebras (for more details, see Lembo et al. 2011).
Fig. 2.
Species-specific patterns of anthrax mortality in wildlife. Only the most commonly reported species are shown. Black and grey indicate probable and suspect cases respectively, as defined in the methods. *549 ‘probable’ and 67 ‘suspect’ impala cases were detected.
Table 2.
P-values from randomization tests of anthrax co-occurrences among species that commonly succumbed to disease (>50 total cases and case detection in most years)
| Buffalo | Impala | Wildebeest | Zebra | Livestock | Humans | |
|---|---|---|---|---|---|---|
| Buffalo | 0·021* | 0·700 | 0·293 | 0·661 | 0·318 | |
| Impala | 0·009** | 0·559 | 0·198 | 0-072† | 0·115 | |
| Wildebeest | NS | 0·554 | 0·010 | NS | NS | |
| Zebra | 0·341 | 0·165 | 0·012* | 0·015* | 0·120 | |
| Livestock | 0·780 | 0-055† | 0·008** | 0·017* | NS | |
| Humans | 0·111 | 0-055† | 0-061† | 0·362 | 0·446 |
Values above the diagonal correspond to permutation tests where all positions in the time series were permuted. Values below the diagonal correspond to tests where only non-zero months were permuted (i.e. months where at least one anthrax case occurred). P-values have not been adjusted for multiple comparisons. If adjusted, the significant P-value would be 0·01.
indicates significance at the adjusted P-value of 0·01
indicates significance at the non-adjusted P-value of 0·05
indicates close to significance. NS indicates non-significance because the real test statistic was equal to the permuted test statistic (i.e. because of small numbers of occurrences). Shaded cells correspond to co-occurrences of anthrax that were significant (P < 0·05) using just presence / absence data.
Spatial heterogeneity in anthrax cases and seroprevalence were evident (Figs 1b and 3). Suspected cases in livestock occurred predominantly in pastoralist areas to the east of SNP, with some locations (i.e. Olbalbal, Oiti and Olduvai, see Fig. 3) appearing as endemic foci. Seroprevalences in domestic dogs reflected these marked regional differences, with very low seroprevalences in agropastoral western communities, and higher but variable prevalences in the eastern communities (Fig. 3a). Of the 314 sampled dogs, 77 were seropositive and three statistically significant predictors of seropositivity were found (Table 3): age, soil alkalinity and location (Loliondo and the NCA vs. the West), but there was no significant difference in risk between the NCA and Loliondo. Overall seroprevalence was lower in Ngorongoro Crater wildlife populations than in Serengeti populations (P < 0.001). Seropositivity in both buffalo and lions was associated with alkaline soil and no other environmental factors were found to be significant. Suspected buffalo carcasses located during the 2009 outbreak in Maswa tended to be localized around river basins (Fig. 1b inset) and were significantly closer to major rivers than would be expected if location of death was random (t = −3.838, P < 0.001).
Table 3.
Results of multivariate Generalized Linear Mixed Model (for domestic dogs and buffalo) and Generalized Linear Model (for lions) analysis of risk factors for seropositivity
| Species | Predictor | Unit | Estimate | SE | z-score | P-value | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Domestic dogs | Intercept | −6·303 | 0·983 | −6·411 | <0·001 | NA | |
| n = 314 | Age | Months | 0·04 | 0·01 | 3·975 | <0·001 | 1·041 (1·020, 1·062) |
| Area | West | − | − | − | − | 1 | |
| Loliondo | 2·705 | 0·951 | 2·846 | 0·004 | 14·95 (2·321, 96·33) | ||
| NCA | 4·168 | 0·866 | 4·812 | <0·001 | 64·61 (11·83, 353·0) | ||
| Soil pH | ≤7 | − | − | − | − | 1 | |
| >7 | 2·5 | 0·587 | 4·256 | <0·001 | 12·17(3·850, 38·46) | ||
| Buffalo | Intercept | −1·946 | 0·617 | −3·153 | 0·002 | NA | |
| n = 49 | Soil pH | ≤7 | − | − | − | − | 1 |
| >7 | 2·314 | 0·754 | 3·067 | 0·002 | 10·11 (2·305, 44·35) | ||
| Lion | Intercept | −1·840 | 0·678 | −2·715 | 0·007 | NA | |
| n = 245 | Age | 0·910 | 0·204 | 4·462 | <0·001 | 2·483 (1·665, 3·703) | |
| Soil pH | ≤7 | − | − | − | − | 1 | |
| >7 | 1·166 | 0·498 | 2·342 | 0·019 | 3·210 (1·209, 8·520) |
OR, odds ratio; CI, confidence intervals; NCA, Ngorongoro Conservation Area.
Human anthrax case reports were sporadic and originated exclusively from pastoralist villages in the NCA and Loliondo division (Fig. 1b) consistent with areas of high seroprevalence in domestic dogs and cases in wildlife and livestock. The only significant association detected with co-occurrence of human cases was the presence of zebra cases (Table 2).
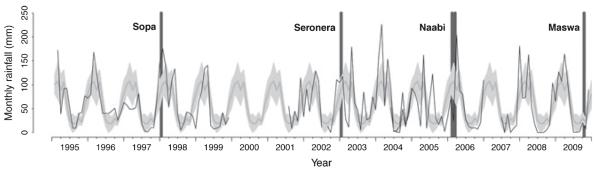
The large outbreaks in wildlife were significantly associated with cumulative extremes in weather conditions (Fig. 4): the Sopa (January 1998) outbreak followed the extreme El Niño rains in 1997 and the Seronera (January 2003) outbreak followed particularly heavy short rains, whereas the Naabi (February/March 2006) outbreak occurred after the short rains failed and the Maswa (October/November 2009) outbreak followed a prolonged dry period. Of the different models that examined deviations in rainfall, the most significant (P < 0.005) predictor of these four outbreaks was a 3-month cumulative deviation from mean monthly rainfall (i.e. deviations from the 3 months preceding the outbreak and from the outbreak month). Related predictors were also significant (deviation from mean monthly or annual rainfall during the outbreak month, as well as 1-, 2-, 3- and 4-month lags, including and excluding rainfall in the outbreak month), but were not independent. Using only the first month of an outbreak as the response variable reduced the statistical power so only 3-month and 2-month lagged cumulative deviations (together with deviations during the outbreak month) approached significance (P = 0.06 and P = 0.09, respectively). We did not find evidence for year-to-year variation in seropositivity in carnivores (P > 0.05), but annual rainfall was negatively associated with annual average antibody levels in Serengeti lions (P = 0.03, R2 = 0.21, n = 20, Fig. 5), i.e., lion antibody levels were on average higher during years of lower than average rainfall than in years of heavy rain.
Fig. 4.
Timing of anthrax outbreaks in relation to rainfall. Months in which outbreaks of anthrax occurred are indicated by dark grey bars. Outbreaks were defined as >10 cases including at least one confirmed by microscopy. The thick grey line denotes mean monthly rainfall (averaged across-outbreaks sites), and grey shading indicates the 95% confidence intervals. Monthly rainfall at each outbreak site (Sopa, Seronera, Naabi and Maswa) are shown by black lines.
Fig. 5.
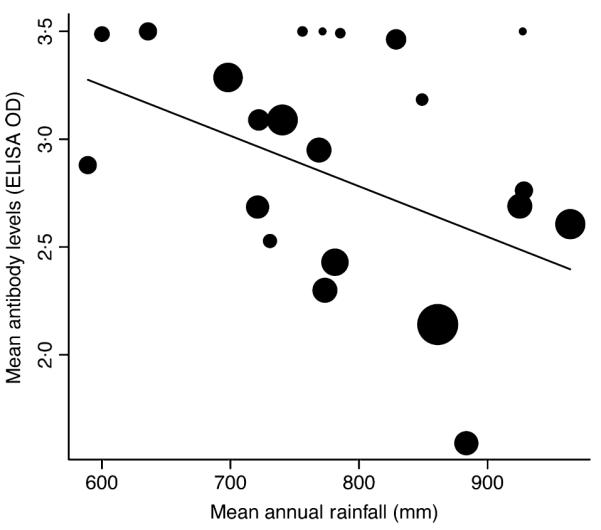
Mean annual antibody levels in Serengeti lions vs. mean annual rainfall. The symbol size reflects the number of lions sampled and the line indicates the best fitting regression (R2 = 0.21, correlation coefficient =− 0.002, n = 20, P = 0.03).
Discussion
Environmental and climatic drivers have long been recognized as important factors influencing the ecology of anthrax (Hugh-Jones & de Vos 2002); though, variable patterns in species-specific mortality and timing of outbreaks between and within ecosystems have made it difficult to understand anthrax epidemiology and to predict disease occurrence. In the Serengeti ecosystem, cases were detected regularly, but outbreaks that caused large die-offs were sporadic and spatially localized. Soil alkalinity and cumulative weather extremes were identified as useful spatial and temporal predictors of exposure and infection risk and for triggering large outbreaks. Serology combined with case reports provided further details on anthrax circulation and ecological drivers underlying species-specific patterns of exposure and mortality.
Herbivores are thought to be at most risk of contracting anthrax because of inhalation/ingestion of spores whilst grazing. Serological differences detected between herbivores and carnivores in the Serengeti confirm this and provide context for observed patterns of infection; low seroprevalence and high mortality in ungulates suggested frequently fatal exposure (with some species-specific variation, see Lembo et al. 2011), whereas high seroprevalence in carnivores (wild and domestic) and low mortality suggested a protective immune response to regular exposure, possibly through consumption of infected carcasses, which may be less lethal than direct spore inhalation/ingestion. This is further supported by age-seroprevalence in lions, indicating a high force of infection with seroconversion at a young age (Lembo et al. 2011).
Alkaline soils with high levels of calcium have been shown to be important geographical determinants of anthrax occurrence because of increased spore survival (Van Ness 1971; Dragon & Rennie 1995; Hugh-Jones & Blackburn 2009), and our results are consistent with this (Fig. 3). Spatial associations are probably maintained as anthrax carcasses contaminate the local environment with spores that can remain viable for decades (Dragon et al. 2005). Soil characteristics that restrict spore dispersal may increase the likelihood of grazing animals acquiring a lethal dose (Hugh-Jones & Blackburn 2009), as may drainage channels that concentrate spores. ‘Incubator areas’ have been hypothesized to occur when favourable soils collect water and organic matter providing a milieu for spore germination and multiplication (Durrheim et al. 2009). These factors may explain why anthrax appears to persist endemically in foci, such as in certain pastoralist villages, where low numbers of cases (in livestock and humans) were detected annually, and seroprevalences in dogs were consistently high (possibly from scavenging on infected carcasses). Seroprevalences in dogs also indicated circulation of anthrax in one agro-pastoralist village to the west of SNP with high soil alkalinity, where no human or livestock anthrax cases were reported (Fig. 3a), further emphasizing the utility of serological data from sentinel populations.
While soil characteristics can explain much about the spatial localization of cases, climate anomalies appear necessary to trigger large outbreaks. However, the mechanisms by which this occurs remain unclear and may vary by location. Associations with drought (Prins & Weyerhaeuser 1987; de Vos 1990; Turnbull et al. 1991; Lindeque & Turnbull 1994; de Vos & Bryden 1996; Bryden 1999; Pollack 1999; Smith et al. 1999; Shiferaw et al. 2002; Promed-Mail 2004a,b; Barrett 2006; Dudley 2006; Clegg et al. 2007; Muoria et al. 2007; Wafula, Patrick & Charles 2008) have been suggested to result from range degeneration and/or over-utilization leading to spore ingestion or inhalation (Beyer & Turnbull 2009), or contamination of limited water resources (Clegg et al. 2007). Alternatively, climate may indirectly affect animal health; hot, dry conditions may lower host resistance (Hugh-Jones & Blackburn 2009) and cause nutritional stress, thereby increasing infection probabilities (de Vos 1990). Outbreaks following prolonged rains are less commonly reported (Lindeque & Turnbull 1994; Mlengeya et al. 1998; Wafula, Patrick & Charles 2008; Bellan 2010) and have often been linked to increased numbers of vectors (reviewed by Hugh-Jones & Blackburn 2009), for instance, Tabanid (Davies 1983) or blow flies (de Vos 1990). Intense flooding can also unearth spores, and this has been considered the cause of outbreaks far from endemic areas that were not thought to involve vectors (Durrheim et al. 2009; Lewerin et al. 2010). Climatic conditions may affect the ability of B. anthracis to germinate and/or sporulate (Hugh-Jones & Blackburn 2009). Some rainfall occurred prior to all the outbreaks we observed (Fig. 4), and experimental work suggests that rain-induced grass growth could facilitate anthrax multiplication and saprophytic growth within the rhizosphere (Saile & Koehler 2006; Schuch & Fischetti 2009).
Aggregation of buffalo cases around rivers during the 2009 drought-associated outbreak (Maswa) is consistent with droughts forcing animals to graze in more restricted locations (close to water), where spores may have accumulated and transmission may be exacerbated (Clegg et al. 2007). This could be an artefact, as animals terminally ill with anthrax are septicaemic and often seek water (World Health Organization 2008). Nonetheless, the behaviour reinforces deposition and concentration of spores around water sources. The range of open questions arising from these reported associations highlights the need for research into the natural life cycle of B. anthracis, particularly survival and vegetative growth outside the host, to understand the mechanisms triggering such spatially localized outbreaks.
In the Serengeti, associations in anthrax infection among species largely arise from concurrent multispecies localized outbreaks, driven by mass deaths in highly susceptible species. These associations reflect functional groupings and spatial overlap at times of outbreaks. A general ecological pattern emerges, whereby grazers (zebra, wildebeest, buffalo and livestock) are worst affected after droughts and browsers (impala) following heavy rains. Serengeti ungulates tend to become protein deficient toward the end of the dry season (Sinclair 1977),explaining their migration to the nutrient-rich volcanic plains at the onset of the rains (Holdo, Holt & Fryxell 2009). The preponderance of anthrax amongst grazers during the 2006 drought-associated (Naabi) outbreak is consistent with both close grazing and drought-induced nutritional stress as the poor rains prevented access to quality forage. As hindgut fermenters, the grazing intake of zebras exceeds that of most Serengeti herbivores (Gordon & Prins 2008), which may explain their apparent high susceptibility to anthrax (Lembo et al. 2011), through greater ingestion of spores. After feeding on infected carcasses, blow-flies regurgitate or defecate onto surrounding bush, disseminating anthrax bacilli. The distribution of these droplets, generally 1-3 m above ground level, means browsers are likely to be exposed whilst feeding, where as grazers are not, but still requires an initial carcass from which to disseminate infection. This may account for why impala were differentially affected during the outbreaks associated with heavy rains (Sopa-1998 and Seronera-2003). However, rains were probably necessary to loosen soils and unearth spores or promote vegetative growth that initially triggered anthrax cases in grazing wildebeest and zebra. Buffalo only overlap with migratory grazers (wildebeest and zebra) for short periods and are rarely found on the southern plains which are endemic anthrax foci for grazing livestock and wildlife, explaining the lack of association of cases in buffalo with other grazers.
Regarding antibody responses in lions, our data show a negative relationship with rainfall. Serengeti lions feed almost exclusively on grazers, which may be why we did not detect higher antibody levels in years of heavy rainfall when browsers were more affected. Differences in susceptibility and survival among prey may explain the exposure and susceptibility in carnivores. More cross-species comparisons could allow assessment of the relative roles of exposure vs. susceptibility in explaining the variable species mortality patterns characteristic of anthrax. However, high levels of exposure in most carnivores may mean that quantitative investigations in relation to timing and location of anthrax outbreaks, including longitudinal studies of serial titres from known individuals, are necessary to shed light on immunological responses and enable greater inference from serological data.
Overall, these data point to concomitant factors that affect the environmental reservoir and increase host susceptibility and exposure, through grazing restrictions, nutritional stress or vector amplification. The small number of identified outbreaks in this study limits the statistical power for resolving these issues. To tease them apart, more detailed data are required on the environmental reservoir, the condition of hosts and their behaviour relating to exposure, and the influence of weather conditions on B. anthracis in the environment and on host and vector ecology.
The quality of case finding in our study was affected by the lack of confirmatory identification of B. anthracis. Animal cases were identified exclusively by detection of encapsulated bacteria, which may have resulted in an underestimation of cases. Examination of blood smears is considered a reliable method for obtaining a diagnosis from fresh samples (<2 days), but reliability decreases with the time between animal death and sample collection (Berg et al. 2006). Although culture of B. anthracis has been shown to be more reliable than examination of blood smears, culture results can be variable because B. anthracis does not compete well with other bacteria in the decaying carcass (Turnbull et al. 1998). PCR significantly improves the confidence with which a diagnosis can be made, particularly for decomposing animals (Berg et al. 2006), and would therefore provide a valuable confirmatory test for less-than-optimal samples obtained from remote areas such as the Serengeti. We suggest that future quantitative comparisons of the sensitivity of microscopy, culture and PCR for anthrax diagnosis under field conditions could provide a useful means of determining the degree to which cases are under-diagnosed in remote ecosystems with limited diagnostic infrastructure.
While serology provided a valuable tool to infer patterns of exposure and should be validated using control groups for multispecies comparisons (Lembo et al. 2011), culture of B. anthracis would provide material for genotyping, which could reveal additional insights into patterns of anthrax circulation. Future studies within the ecosystem should prioritize isolation of B. anthracis for case confirmation. Genetic analyses in the Serengeti and other multihost natural systems should more generally address the hypothesis that clonal genotypes predominate in anthrax epizootics (Blackburn et al. 2007; Fasanella et al. 2010) and could be used to investigate differences in host susceptibility with circulating genotypes.
Our findings have a number of practical implications for reducing the impact of anthrax. Indicators of risk could be used to guide conservation and management policy such as pri-oritizing reintroduction sites and prophylactically vaccinating anthrax-susceptible species, like black rhino Diceros bicornis (Metzger et al. 2007) and African wild dogs Lycaon pictus (Creel et al. 1995). Cattle in high-risk areas could also be vaccinated prophylactically, public health warnings appropriately timed and more antibiotics provided in clinics in pastoralist areas to mitigate risks. Although hospital reports coincided with livestock and wildlife cases, these relationships were weak. This may be because low-grade sporadic infection and infrequent cattle fatalities might not be suspected as anthrax and thus the meat/carcasses be eaten, in contrast to more obvious mass anthrax mortality. Nonetheless, household surveys following the 2006 outbreak indicated several human cases including fatalities not reported to hospital (Lembo et al. 2011), which suggests that these records fail to reflect acute fatal infections.
We conclude that countervailing seasonally driven rainfall and fertility gradients in the Serengeti, which are a consistent feature of African savannas (Holdo, Holt & Fryxell 2009), might foster ideal conditions for localized endemic anthrax foci. Herbivores converge on hotspots of accumulated spores during climate extremes, when exacerbating biotic and abiotic stressors or vector populations may be amplified. However, continued and more targeted anthrax surveillance (environmental, serological and case-detection-orientated) is necessary for more statistically rigorous investigations into mechanisms triggering outbreaks and for predicting infection.
Acknowledgements
We are indebted to the Tanzania Ministries of Livestock Development and Fisheries, and Health and Social Welfare, TANAPA, TAWIRI, NCA Authority, Tanzania Commission for Science Technology, and the National Institute for Medical Research for permissions; TANAPA and TAWIRI Veterinary Units, Viral Transmission Dynamics Project, Serengeti Lion and Cheetah Projects, Frankfurt Zoological Society, livestock field-officers and health-workers in Mara, Mwanza, Shinyanga and Arusha Regions, and TGTS/FCF personnel for assistance with sample collection; and Grant Hopcraft and Michael Anderson for support with compiling ecological data. This work was supported by Google.org, the joint National Institutes of Health (NIH)/National Science Foundation (NSF) Ecology of Infectious Diseases Program under grant no. NSF/DEB0225453, the Wellcome Trust (KH), the Department for International Development Animal Health Programme (SC) and the Messerli Foundation. The opinions expressed by authors do not necessarily reflect the opinions of the Centers for Disease Control and Prevention, the institutions with which the authors are affiliated or the funding bodies.
References
- Barrett B. Anthrax, Wildlife – Botswana (Chobe National Park) 2006 ProMed, http://www.promedmail.org:archive no. 20061011.20062910.
- Bellan S. Anthrax, Wildlife – Namibia (Etosha National Park) 2010 ProMed, http://www.promedmail.org:archive no. 20100402.20101049.
- Berg T, Suddes H, Morrice G, Hornitzky M. Comparison of PCR, culture and microscopy of blood smears for the diagnosis of anthrax in sheep and cattle. Letters in Applied Microbiology. 2006;43:181–186. doi: 10.1111/j.1472-765X.2006.01931.x. [DOI] [PubMed] [Google Scholar]
- Beyer W, Turnbull PCB. Anthrax in animals. Molecular Aspects of Medicine. 2009;30:481–489. doi: 10.1016/j.mam.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, McNyset KM, Curtis A, Hugh-Jones ME. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. American Journal of Tropical Medicine and Hygiene. 2007;77:1103–1110. [PubMed] [Google Scholar]
- Bryden H. Anthrax, Wildlife – South Africa (Kruger National Park) (02) 1999 ProMed, http://www.promedmail.org:archive no. 19990730.19991288. [PubMed]
- Clegg SB, Turnbull PCB, Foggin CM, Lindeque RM. Massive outbreak of anthrax in wildlife in the Malilangwe Wildlife Reserve, Zimbabwe. Veterinary Record. 2007;160:113–118. doi: 10.1136/vr.160.4.113. [DOI] [PubMed] [Google Scholar]
- Creel S, Creel NM, Matovelo JA, Mtambo MMA, Batamuzi EK, Cooper JE. Effects of Anthrax on Endangered African Wild Dogs (Lycaon-Pictus) Journal of Zoology. 1995;236:199–209. [Google Scholar]
- Davies JCA. A major epidemic of anthrax in Zimbabwe. Central African Journal of Medicine. 1983;29:8–12. [PubMed] [Google Scholar]
- Dragon DC, Rennie RP. The ecology of anthrax spores – tough but not invincible. Canadian Veterinary Journal-Revue Veterinaire Canadienne. 1995;36:295–301. [PMC free article] [PubMed] [Google Scholar]
- Dragon DC, Bader DE, Mitchell J, Woollen N. Natural dissemination of Bacillus anthracis spores in Northern Canada. Applied and Environmental Microbiology. 2005;71:1610–1615. doi: 10.1128/AEM.71.3.1610-1615.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. Anthrax, Wildlife – Botswana (North-West, Chobe National Park) 2006 ProMed, http://www.promedmail.org:archive no. 20060825.2006 2398.
- Durrheim DN, Freeman P, Roth I, Hornitzky M. Epidemiologic questions from anthrax outbreak, Hunter Valley, Australia. Emerging Infectious Diseases. 2009;15:840–842. doi: 10.3201/eid1505.081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp T, Waldner C, Argue CK. Case-control study investigating an anthrax outbreak in Saskatchewan, Canada – Summer 2006. Canadian Veterinary Journal-Revue Veterinaire Canadienne. 2010;51:973–978. [PMC free article] [PubMed] [Google Scholar]
- FAO/IIASA/ISRIC/ISSCAS/JRC . Harmonized World Soil Database (version 1.1) FAO; Rome: 2009. [Google Scholar]
- Fasanella A, Garofolo G, Galante D, Quaranta V, Palazzo L, Lista F, Adone R, Jones MH. Severe anthrax outbreaks in Italy in 2004: considerations on factors involved in the spread of infection. New Microbiologica. 2010;33:83–86. [PubMed] [Google Scholar]
- Garofolo G, Ciammaruconi A, Fasanella A, Scasciamacchia S, Adone R, Pittiglio V, Lista F. SNR analysis: molecular investigation of an anthrax epidemic. BMC Veterinary Research. 2010;6:11. doi: 10.1186/1746-6148-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon IJ, Prins HHT. The Ecology of Browsing and Grazing. Springer; Berlin: 2008. [Google Scholar]
- Holdo RM, Holt RD, Fryxell JM. Opposing rainfall and nutrients gradients best explain the wildebeest migration in the Serengeti. The American Naturalist. 2009;173:431–445. doi: 10.1086/597229. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones M, Blackburn J. The ecology of Bacillus anthracis. Molecular Aspects of Medicine. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones ME, de Vos V. Anthrax and wildlife. Revue Scientifique Et Technique De L Office International Des Epizooties. 2002;21:359–383. doi: 10.20506/rst.21.2.1336. [DOI] [PubMed] [Google Scholar]
- Lembo T, Hampson K, Auty H, Beesley CA, Bessell PR, Packer C, Halliday JEB, Fyumagwa R, Hoare R, Ernest E, Mentzel C, Mlengeya T, Stamey K, Wilkins PP, Cleaveland S. Serologic surveillance of anthrax in the Serengeti Ecosystem, Tanzania, 1996-2009. Emerging Infectious Diseases. 2011;17:387–394. doi: 10.3201/eid1703.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerin SS, Elvander M, Westermark T, Hartzell LN, Norström AK, Ehrs S, Knutsson R, Englund S, Andersson AC, Granberg M, Bäckman S, Wilkström P, Sandstedt K. Anthrax outbreak in a Swedish beef cattle herd – 1st case in 27 years: case report. Acta Veterinaria Scandinavica. 2010;52:1. doi: 10.1186/1751-0147-52-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeque PM, Brain C, Turnbull PCB. A review of anthrax in the Etosha National Park. Salisbury Medical Bulletin. 1996;87(Suppl):24–30. [Google Scholar]
- Lindeque PM, Turnbull PCB. Ecology and epidemiology of anthrax in Etosha National Park, Namibia. Onderstepoort Journal of Veterinary Research. 1994;61:71–83. [PubMed] [Google Scholar]
- Metzger KL, Sinclair ARE, Campbell KLI, Hilborn R, Hopcraft JGC, Mduma SAR, Reich RM. Using historical data to establish baselines for conservation: the black rhinoceros (Diceros bicornis) of the Serengeti as a case study. Biological Conservation. 2007;139:358–374. [Google Scholar]
- Mlengeya T, Mbise AN, Kilewo M, Mlengeya M, Gereta E, Moshy WE, Mtui PF, Kaare M. Anthrax epizootics in Tanzania’s national parks with special interest in a recent anthrax outbreak in Serengeti National Park. Bulletin of Animal Health and Production in Africa. 1998;46:65–73. [Google Scholar]
- Muoria PK, Muruthi P, Kariuki WK, Hassan BA, Mijele D, Oguge NO. Anthrax outbreak among Grevy’s zebra (Equus grevyi) in Samburu, Kenya. African Journal of Ecology. 2007;45:483–489. [Google Scholar]
- Parkinson R, Rajic A, Jenson C. Investigation of an anthrax outbreak in Alberta in 1999 using a geographic information system. Canadian Veterinary Journal-Revue Veterinaire Canadienne. 2003;44:315–318. [PMC free article] [PubMed] [Google Scholar]
- Pienaar U.d.V. ‘N Uitbraak van miltsiekte onder wild in die Nasionalse Kruge Wildtuin 28.9.59-20.11.59. Koedoe. 1960;3:238–251. [Google Scholar]
- Pienaar U.d.V. A second outbreak of anthrax among game animals in the Kruger National Park. 5th June to 11th October, 1960. Koedoe. 1961;4:4–17. [Google Scholar]
- Pollack MP. Anthrax, Wildlife – South Africa (Kruger National Park) 1999 ProMed, http://www.promedmail.org:archive no. 19990723.19991242.
- Prins HHT, Weyerhaeuser FJ. Epidemics in populations of wild ruminants – anthrax and impala, rinderpest and buffalo in lake Manyara National Park, Tanzania. Oikos. 1987;49:28–38. [Google Scholar]
- ProMed-Mail Anthrax, elephant – Botswana (Chobe National Park) 2000 ProMed, http://www.promedmail.org:archive no. 20000307.20000313.
- Promed-Mail Anthrax, elephants – Namibia (Caprivi) (2) 2004a ProMed, http://www.promedmail.org:archive no. 20041006.20042744.
- ProMed-Mail Anthrax, wildlife – Botswana (Chobe National Park) (02) 2004b ProMed, http://www.promedmail.org:archive no. 20040923.20042628.
- ProMed-Mail Anthrax, livestock, wildlife – Namibia (Caprivi) (02) 2006 ProMed, http://www.promedmail.org:archive no. 20061113.20063255.qo.
- Raymond B, Wyres KL, Sheppard SK, Ellis RJ, Bonsall MB. Environmental factors determining the epidemiology and population genetic structure of the Bacillus cereus group in the field. PLoS Pathogens. 2010;20:e1000905. doi: 10.1371/journal.ppat.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saile E, Koehler TM. Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Applied and Environmental Microbiology. 2006;72:3168–3174. doi: 10.1128/AEM.72.5.3168-3174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch R, Fischetti VA. The secret life of the anthrax agent Bacillus anthracis: bacteriophage-mediated ecological adaptations. PLoS ONE. 2009;4:e6532. doi: 10.1371/journal.pone.0006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiferaw F, Abditcho S, Gopilo A, Laurenson MK. Anthrax outbreak in Mago National Park, southern Ethiopia. Veterinary Record. 2002;150:318–320. doi: 10.1136/vr.150.10.318. [DOI] [PubMed] [Google Scholar]
- Siamudaala VM. Ecology and epidemiology of anthrax in cattle and humans in Zambia. Japanese Journal of Veterinary Research. 2006;56:15–23. [PubMed] [Google Scholar]
- Sinclair ARE. The African Buffalo: A Study of Resource Limitation of Populations. University of Chicago Press; Chicago: 1977. [Google Scholar]
- Sinclair ARE, Packer C, Mduma SAR, Fryxell JM. Serengeti III: Human Impacts on Ecosystem Dynamics. University of Chicago Press; Chicago: 2008. [Google Scholar]
- Sirisanthana T, Brown AE. Anthrax of the gastrointestinal tract. Emerging Infectious Diseases. 2002;8:649–651. doi: 10.3201/eid0807.020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, De Vos V, Bryden HB, Hugh-Jones ME, Klevytska A, Price LB, Keim P, Scholl DT. Meso-scale ecology of anthrax in southern Africa: a pilot study of diversity and clustering. Journal of Applied Microbiology. 1999;87:204–207. doi: 10.1046/j.1365-2672.1999.00871.x. [DOI] [PubMed] [Google Scholar]
- Smith KL, DeVos V, Bryden H, Price LB, Hugh-Jones ME, Keim P. Bacillus anthracis diversity in Kruger National Park. Journal of Clinical Microbiology. 2000;38:3780–3784. doi: 10.1128/jcm.38.10.3780-3784.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull PCB, Bell RHV, Saigawa K, Munyenyembe FEC, Mulenga CK, Makala LHC. Anthrax in Wildlife in the Luangwa Valley, Zambia. Veterinary Record. 1991;128:399–403. doi: 10.1136/vr.128.17.399. [DOI] [PubMed] [Google Scholar]
- Turnbull PCB, Boehm R, Cosivi O, Doganay M, Hugh-Jones ME, Joshi DD, Latlitha MK, de Vos V. Guidelines for the Surveillance and Control of Anthrax in Humans and Animals. 3rd edn WHO/EMC/ZDI/98.6. World Health Organization; Geneva: 1998. [Google Scholar]
- Turner AJ, Galvin JW, Rubira RJ, Miller GT. Anthrax explodes in an Australian summer. Journal of Applied Microbiology. 1999;87:196–199. doi: 10.1046/j.1365-2672.1999.00869.x. [DOI] [PubMed] [Google Scholar]
- Van Ness G. Ecology of Anthrax. Science. 1971;172:1303–1307. doi: 10.1126/science.172.3990.1303. [DOI] [PubMed] [Google Scholar]
- de Vos V. The ecology of anthrax in the Kruger National Park, South Africa. Salisbury Medical Bulletin. 1990;68(Suppl):19–23. [Google Scholar]
- de Vos V, Bryden H. Anthrax in the Kruger National Park: temporal and spatial patterns of disease occurrence. Salisbury Medical Bulletin. 1996;87:26–30. [Google Scholar]
- Wafula MM, Patrick A, Charles T. Managing the 2004/05 anthrax outbreak in Queen Elizabeth and Lake Mburo National Parks, Uganda. African Journal of Ecology. 2008;46:24–31. [Google Scholar]
- World Health Organization . Anthrax in Humans and Animals. WHO; Geneva: 2008. Available from http://www.who.int/csr/resources/publications/anthrax_webs.pdf. [PubMed] [Google Scholar]