Abstract
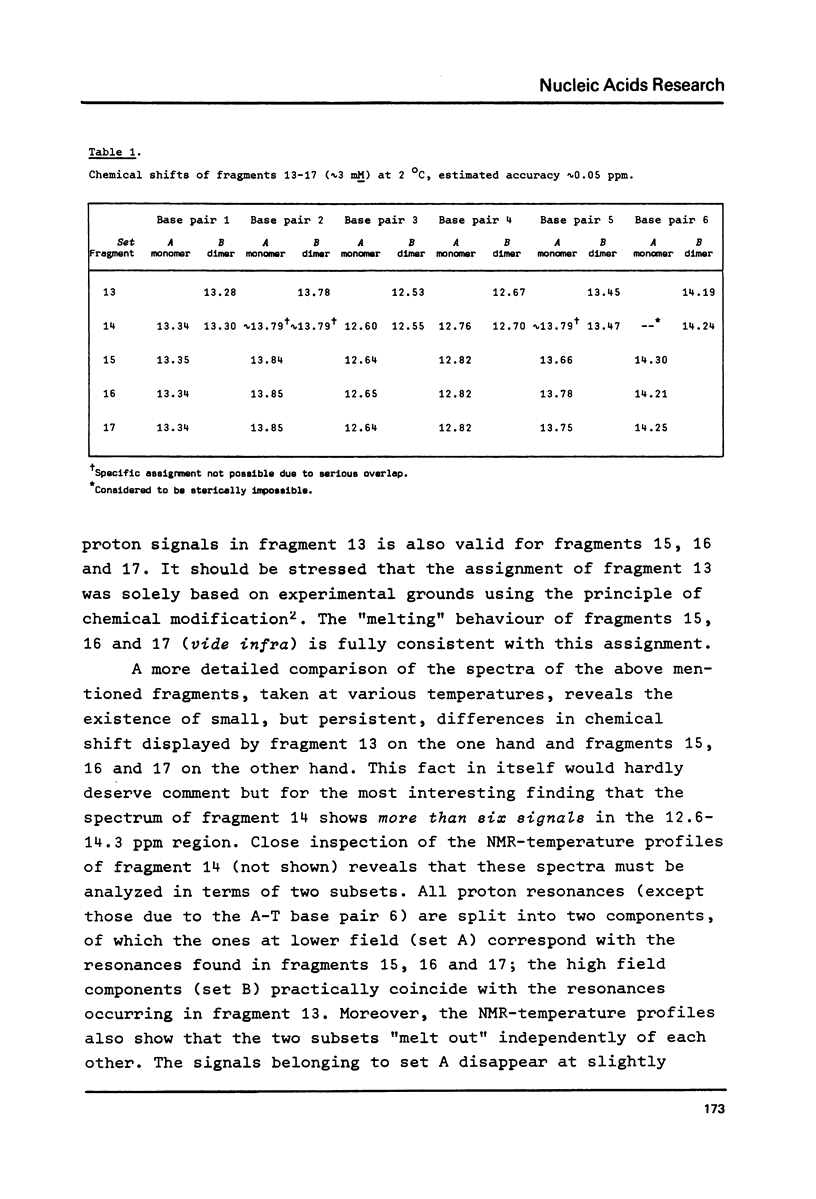
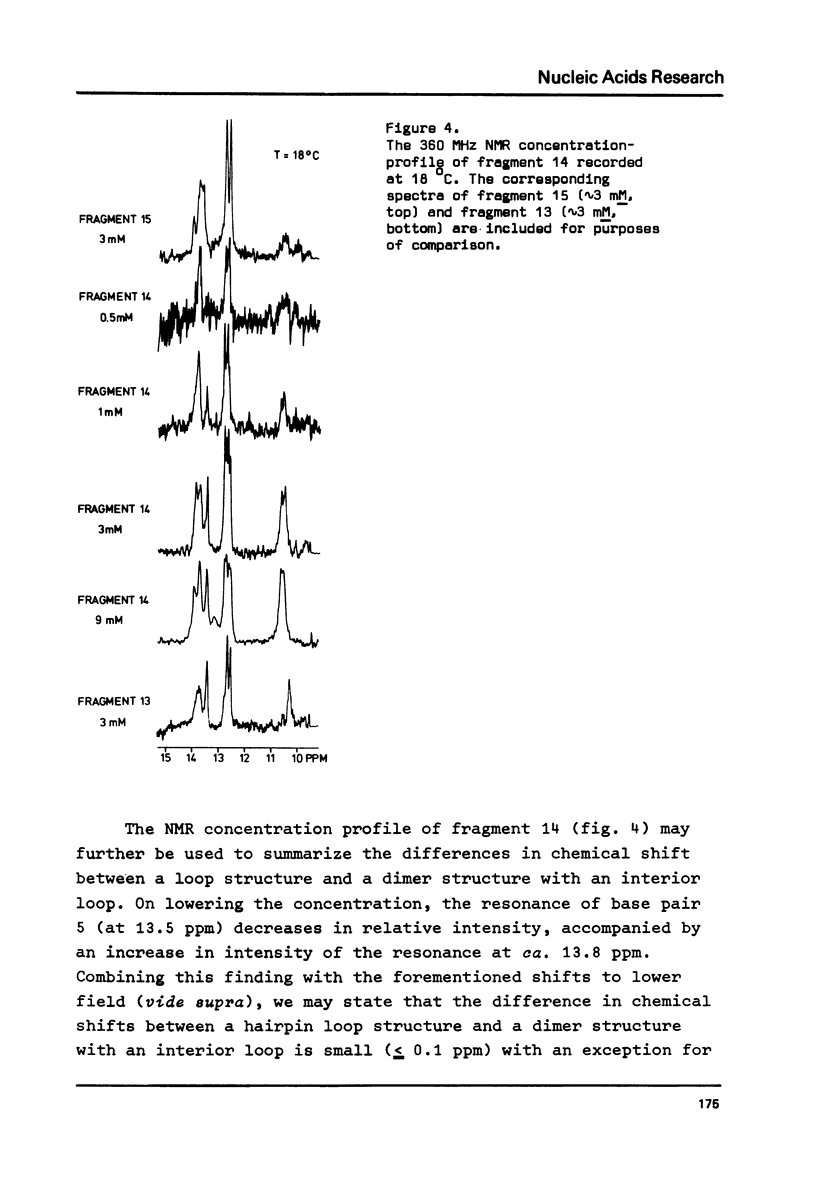
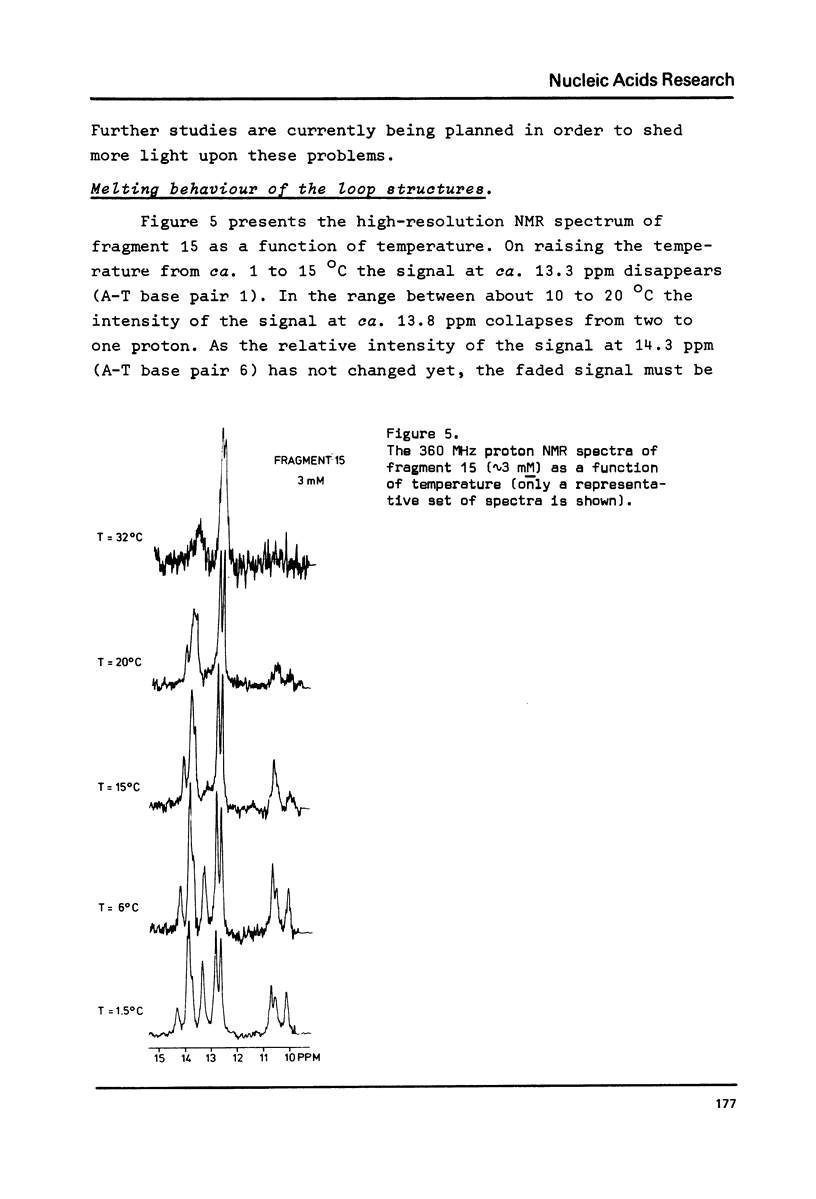
A comparative nuclear magnetic resonance study of the hydrogen-bonded imino protons in a series of synthetic DNA fragments is presented. The fragments ATCCTA(Tn)TAGGAT are in principle capable of forming either a self-complementary hairpin loop structure (monomer form) or an interior loop structure (dimeric form). It has been shown, that for n = 1 only the dimer structure is present in aqueous solution, whereas the exclusive existence of the hairpin loop structure is indicated for n = 3, 4 & 5. Surprisingly, for n = 2 two different structures appear to be present in solution. Concentration studies show that both monomers and dimers exist side by side in this case. Hairpins as well as interior loops form extra "melting sites" in addition to the wellknown fraying phenomenon at the terminus of the double helix.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Altona C., Van Boom J. H., Haasnoot C. A. Conformational analysis of a DNA triplet in aqueous solution. Thymidylyl-(3'-5')-thymidylyl-(3'-5')-2'-deoxyadenosine, d(T-T-A), studied by 1H nuclear magnetic resonance at 360 MHz. Eur J Biochem. 1976 Dec 11;71(2):557–562. doi: 10.1111/j.1432-1033.1976.tb11145.x. [DOI] [PubMed] [Google Scholar]
- Arter D. B., Schmidt P. G. Ring current shielding effects in nucleic acid double helices. Nucleic Acids Res. 1976 Jun;3(6):1437–1447. doi: 10.1093/nar/3.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Kan L. S., Ts'o P. O. Conformation and interaction of short nucleic acid double-stranded helices. I. Proton magnetic resonance studies on the nonexchangeable protons of ribosyl ApApGpCpUpU. Biochemistry. 1975 Nov 4;14(22):4847–4863. doi: 10.1021/bi00693a012. [DOI] [PubMed] [Google Scholar]
- Cornelis A. G., Haasnoot J. H., den Hartog J. F., de Rooij M., van Boom J. H., Cornelis A. Local destabilisation of a DNA double helix by a T--T wobble pair. Nature. 1979 Sep 20;281(5728):235–236. doi: 10.1038/281235a0. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the atomic or "local" contributions to proton chemical shifts due to the anisotropy of the diamagnetic susceptibility of the nucleic acid base. Biochem Biophys Res Commun. 1976 May 17;70(2):578–581. doi: 10.1016/0006-291x(76)91086-x. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Hilbers C. W. Proton nuclear magnetic resonance investigations of fraying in double-stranded d-ApTpGpCpApT in H2O solution. Biochemistry. 1975 Jun 17;14(12):2651–2656. doi: 10.1021/bi00683a014. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Uhlenbeck O. C., Levine M. D. Estimation of secondary structure in ribonucleic acids. Nature. 1971 Apr 9;230(5293):362–367. doi: 10.1038/230362a0. [DOI] [PubMed] [Google Scholar]


