Abstract
Pregnancy-associated malaria, a manifestation of severe malaria, is the cause of up to 200,000 infant deaths a year, through the effects of placental insufficiency leading to growth restriction and preterm delivery. Development of a vaccine is one strategy for control. Plasmodium falciparum-infected red blood cells accumulate in the placenta through specific binding of pregnancy-associated parasite variants that express the VAR2CSA antigen to chondroitin sulphate A on the surface of syncytiotrophoblast cells. Parasite accumulation, accompanied by an inflammatory infiltrate, disrupts the cytokine balance of pregnancy with the potential to cause placental damage and compromise foetal growth. Multigravid women develop immunity towards VAR2CSA-expressing parasites in a gravidity-dependent manner which prevents unfavourable pregnancy outcomes. Although current vaccine design, targeting VAR2CSA antigens, has succeeded in inducing antibodies artificially, this candidate may not provide protection during the first trimester and may only protect those women living in areas endemic for malaria. It is concluded that while insufficient information about placental-parasite interactions is presently available to produce an effective vaccine, incremental progress is being made towards achieving this goal.
1. Introduction
Over 50 million women who live in areas of high malaria transmission become pregnant every year, and thousands of these women die [1]. Women in their first and second pregnancies are at particular risk of infection with Plasmodium falciparum, which is a major risk factor for maternal and foetal mortality and is implicated in 75,000–200,000 infant deaths per annum [2, 3]. Selective accumulation of parasites in the placental space results in maternal anaemia [4–6] and infant low birth weight (LBW) [7–13] through preterm delivery (PTD) [12, 13] and intrauterine growth restriction (IUGR) [7, 10, 12, 13]. Malaria demands up to 5% of the gross domestic product in sub-Saharan Africa [14].
Pregnancy-associated malaria (PAM) infection is one example of a severe malaria syndrome, mediated by the surface expression of variant surface antigens (VSAs) of P. falciparum parasitised red blood cells (pRBC) that allow adherence to vascular endothelium. In non-pregnant individuals, VSAs adhere to the ubiquitous endothelial surface proteins intercellular adhesion molecule-1 (ICAM-1) and CD36 or to other pRBC or form rosettes around non-infected RBC. Under high transmission settings with favourable breeding sites for the vector Anopheles mosquito, adults acquire natural immunity to VSAs, rendering them asymptomatic [15, 16]. In contrast, women who are immune to these parasites also display adverse consequences of infection when they become pregnant; this has contributed to the previous belief that pregnancy represents an immunocompromised state.
Regardless of the extent of previous exposure to P. falciparum during pregnancy, all pregnant women are at increased risk of malaria and appear to be more attractive to mosquitoes [17, 18]. Marked differences in symptoms are apparent between varying levels of transmission; PAM in areas of low transmission can result in severe infection and lead to foetal and maternal death [19, 20]. In these symptomatic women, fever can induce uterine contractions and increase the likelihood of PTD [21]. The presence of symptoms results in prompt diagnosis and management which reduces the incidence of unfavourable pregnancy outcomes [22]. In contrast, women living in areas endemic for malaria and hence possessing prior immunity tend to be asymptomatic in pregnancy but harbour high, undetected parasite levels in the placenta [16, 23]. PAM affects these women in a gravidity-dependent manner: primigravid (PG) women are more susceptible than multigravid (MG) women [24]. After correction for age-related susceptibility, this trend has been reported consistently and is more pronounced with increasing transmission [25, 26].
PAM is managed during pregnancy with intermittent preventive strategies using chemotherapeutic medications or insecticide-treated nets. The World Health Organization recommends that insecticide-treated nets and intermittent preventive treatment (IPTp) should be used during pregnancy [1, 22]. IPTp consists of two doses of sulfadoxine and pyrimethamine in the second and third trimesters [27]. A recent systematic review [28] demonstrates limited protection from PAM in some malaria-endemic regions. While sulfadoxine-pyrimethamine treatment remains effective in West Africa, and more so in three doses than two [29], there is a need for novel interventions. Current efforts to control the incidence of malaria infection are being hampered by rapidly increasing numbers of insecticide-resistant mosquitoes and treatment-resistant parasites [30]. Hence, production of a vaccine to protect women in high risk areas is an urgent public health priority. This paper aims to address our current understanding of this subject and to determine whether enough is known about the interactions between parasite and placenta to consider this a realistically attainable feat.
2. What Is Placental Malaria and Why Does It Occur?
Placental malaria (PM) is a subset of PAM which refers to the pathological process whereby pRBC and inflammatory cells accumulate within the intervillous space (IVS) of the placenta. At delivery, PM can be measured by microscopic examination of stained slides of placental blood, by histopathological evaluation of placental biopsies [31] or by semiquantitative polymerase chain reaction (PCR) [32]. Examination of blood smears is rapid, cheap, and easy but does not allow assessment of past infection [33], whereas this is possible with both histological visualisation of parasites and PCR-assisted grading of pigment deposition. Both the latter two methods have enabled recent determinations of how long parasites may survive in the placenta: Leke et al. [34] reported that the same parasites may be detected up to 98 days before delivery through PCR examination of parasite polymorphism. Histology does not provide an accurate diagnosis; absence of parasites or pigment at histology does not necessarily mean that infection has not occurred [35]. Lack of effective and reliable measures to diagnose placental pathology during pregnancy limits comparisons between studies [33].
2.1. Peripheral and Placental Parasite Dynamics
It is neither practical nor ethical to investigate placental parasite densities during pregnancy due to the risk of inducing foetal loss. However, placental parasite densities at term do not appear to correlate with densities of parasites in the peripheral blood which complicates diagnosis during gestation. Observations that densities of parasites in the placenta may be far higher than the densities in peripheral blood samples suggest that parasites accumulate selectively in the IVS [8, 33]. The IVS forms from the lacunae between foetal-derived syncytiotrophoblastic villi, which emerge following fertilisation and implantation of the blastocyst [36–38]. The placenta is complete by the end of the 16th week of gestation. Placental trophoblast invades the uterine wall, gaining blood supply from the spiral arteries that pass through the endometrium [38].
Placental parasite dynamics in the first trimester are not known. A recent cohort study that examined the effects of timing and frequency of P. falciparum infection on pregnancy outcomes in 2,462 subjects was unable to evaluate the effects in early pregnancy because only six women in their first trimester attended the antenatal clinic where recruitment was taking place [39]. This small number was excluded from the study, but this demonstrates the difficulty in gaining data during this stage of gestation. Density of peripheral parasites peaks between 13 and 16 weeks of gestation, suggesting that susceptibility to PAM is increased in the first trimester [8, 40]. In addition, poor placental outcomes are associated with earlier placental infections [41]. The peak in parasitaemia was first identified in a large group of women living in areas of high transmission [8]. Although this figure is highly cited, the reliability of this finding is limited by the two methods used for determining gestational age; calculation from last monthly period is affected by recall bias and fundal height measurement is unreliable before 24 weeks of gestation. Furthermore, malaria is known to cause IUGR and therefore a reduction in uterine size, leading to inaccuracy when utilising the latter as a determinant. The reasons for the discrepancies in foetal measurements between studies have been reviewed recently elsewhere [42], and these inaccuracies demonstrate a need for standardised methods for the provision of comparable data. The use of ultrasound to determine gestational age is more accurate, and it is hoped that increasing use of this method will provide more robust results [43]. Subsequently, a decrease in peripheral parasite density was reported after 16 weeks of gestation [8]. The authors termed this “recovery from infection”. However, it was observed that this reduction in peripheral levels coincided with completion of placental development, leading to the conclusion that parasites may sequester in the placenta at this time [44]. Of note, both PG and MG women “recovered” at the same time, supporting this hypothesis [8]. Following delivery, women appear to undergo rapid clearance of parasitaemia [45], and evidence suggests that subsequent to this they are at risk of peripheral infection [40].
2.2. Why Do Parasites Sequester in the Placenta?
In pregnancy, the dominant receptor for adhesion is thought to be the chondroitin sulphate A (CSA) component of the chondroitin sulphate proteoglycan (CSPG) [46–50]. Placental-parasite variants that adhere to CSA show an absence of binding to CD36 and ICAM-1 ligands, in contrast to parasite variants taken from infected non-pregnant women [51–53]. Selection of pRBC that adhere to CSA in vitro leads to the loss of antigens that bind to CD36 and ICAM-1; hence, it is thought that parasite variants that bind to CSA are mutually exclusive to those that bind to ICAM-1 and CD36 [47, 53–55]. In some instances placental parasites have displayed binding to both CD36 and CSA [56]. This is an infrequent observation and may result from the presence of two antigenically distinct variant molecules on a single pRBC [57]. However, mounting evidence supporting the mutually exclusive behaviour of antigen presentation suggests that this is unlikely [52, 58–60]. Placental-binding isolates are unable to form rosettes and do not agglutinate when exposed to immune serum from non-pregnant individuals [53, 61–64].
CSA is a sulphated glycosaminoglycan (GAG) present on the syncytiotrophoblast in the intervillous space of the placenta, located as a side chain on the tissue anticoagulant thrombomodulin [65]. It appears during the 16th week of pregnancy with the completion of the placenta. A plethora of functions for proteoglycans have been identified, including regulation of cell proliferation, differentiation, and adhesion, as reviewed in [66]. Binding to placental CSA may alter gene transcription or signal transduction or activate intracellular signalling mechanisms that lead to increased expression of inflammatory mediators [66–68]. CSA is expressed by nearly all cells but it is unclear why parasites bind only to CSA expressed by the placenta, although this may be explained by the specific patterns of sulphation of placental CSA and the structure it forms on the trophoblast membrane [69]. Previously, other placental molecules, such as hyaluronic acid (HA) [70], have been implicated in placental binding of pRBC [47], and some parasite lines have been shown to have affinity for three receptors; CSA, HA, and CD36. However, the strength of binding does not match that of CSA-parasite adherence alone [71].
2.3. VAR2CSA: A Novel Antigen in PAM
It is now well established that the parasite protein able to adhere to CSA in the placenta is VAR2CSA coded for by the var2csa gene [58, 72]. This protein is a member of the P. falciparum erythrocyte membrane protein 1 (PfEMP1) family [16, 73]. Encoded by the var multigene family, PfEMP1 molecules vary greatly between variants and strains, but each protein retains its C-terminal intracellular domain, an extracellular domain, and a single transmembrane helix [74]. The ectodomain, responsible for discrete receptor-binding properties, consists of multiple duffy binding-like (DBL) domains and cysteine-rich interdomain regions (CIDRs) [74–76]. Table 1 summarizes the lines of evidence that support the role of VAR2CSA. VSAs that are thought to specifically cause disease in PAM, such as VAR2CSA, are collectively referred to as VSAPAM [77].
Table 1.
Evidence providing support for the integral role of VAR2CSA in placental malaria.
| Evidence | Reference |
|---|---|
| Placental parasites selectively transcribe the var2csa gene | [58, 72, 84] |
| The var2csa gene is relatively conserved between PAM variants | [58, 85] |
| The var2csa gene is required for pRBC to adhere to CSA | [86] |
| If the var2csa gene is knocked out or deleted, adhesion to CSA no longer occurs | [84, 87] |
| VAR2CSA is selectively expressed on the surface of pRBC that are identified in PM | [88] |
| VAR2CSA binds specifically to CSA expressed on the placenta | [89, 90] |
| Men and non-pregnant women do not produce VAR2CSA-specific IgG | [62, 88] |
| Levels of VAR2CSA-specific IgG correlate with parity | [88, 91] |
| High levels of VAR2CSA-specific IgG protect from adverse outcomes | [88] |
| VAR2CSA is a target of naturally acquired IgG reactive with the surface of placental pRBC | [92] |
2.4. Infection with Other Human Malaria Species
Infection with P. vivax during pregnancy is associated with maternal anaemia and foetal LBW [78–81], but is not thought to increase the risk of PTD [79]. Pregnant women are at increased risk of infection with P. vivax, although this species does not appear to sequester in the placenta [35]. P. vivax is suppressed by co-infection with P. falciparum, although co-infection may lower the risk of severe anaemia [32, 82]. Little is known about placental infection with P. malariae, P. ovale, and P. knowlesi: both P. ovale and P. malariae can infect pregnant women, but pregnancy does not appear to increase susceptibility to infection with these species [7, 80, 83].
3. What Antibody Protection Is Acquired?
3.1. Antibodies to VSAPAM
Prior to the appearance of the placenta during pregnancy, women are not exposed to VSAPAM-expressing variants and therefore lack specific antibodies [47, 62]. Serum taken from MG women with previous exposure to these variants has been shown to inhibit adhesion of pRBC isolated from PG women [47, 62, 93–95]. Inhibition of binding was assumed to be protective because these same women proceeded to have several additional successful pregnancies [62]. In contrast, immune sera from PG women and adult men, with no previous exposure to VSAPAM-expressing variants, could not prevent binding to CSA [62]. Levels of pregnancy-specific antibodies have been correlated with the degree to which binding of pRBC to CSA is inhibited [63, 95, 96].
Levels of protective antibodies increase with gravidity [62, 63, 95, 96]. Inhibition of binding by antibodies contributes to parasite clonal variation, antigenic switching and hence immune evasion, although it has been reported recently that antigenic switching may occur due to intrinsic regulatory systems [97]. Antibodies are detected in both PG and MG women; low levels have been detected at 14 weeks of gestation in PG women [91, 98], in contrast to previous reports of detection being possible only after 20 weeks [95, 99]. Although these women appear capable of producing antibodies, they may not confer protection in PG women because the antibody repertoire is not broad enough to inhibit binding of more than one or two variants [99]. Complex infections with numerous phenotypes may disrupt the ability to combat effectively the response to a single variant. In addition, the presence of non-specific, non-protective immunoglobulin (Ig) may delay or interfere with the acquisition of memory B cells [100].
3.2. How Do Antibodies Protect against Adverse Outcomes?
Of the five classes of Ig, IgG is known to be the most important in malaria immunity, and, in humans, the subclasses IgG1 and IgG3 have been found to correlate most with protection from disease [25, 101]. IgG-mediated protection is thought to be achieved through anti-adhesion and opsonic activity [102–104]. Anti-adhesion antibodies have been shown to correlate with reduced levels of placental parasitaemia and to act by promoting splenic removal of parasites [62, 105]. The anti-adhesive properties of antibodies have been studied more extensively than their cytophilic properties [106]. Recent reports of correlations of opsonizing VAR2CSA-specific IgG and protection from PM may indicate that both means of parasite clearance need to be assessed in vitro [106]. Furthermore, opsonic antibodies may be a more specific predictor of outcome than overall IgG levels [106]. In one study, strong associations between levels of antibodies and protection from anaemia were reported at delivery in the absence of correlations with protection from LBW [106]. Anti-adhesive antibodies lower the parasite density, which is associated with an increase in birth weight and length of gestation [105]. Whether the increased birth weight is a result of decreased chronic malaria or due to increased length of gestation is not clear, but LBW is a major risk factor for infant mortality [107].
If a pregnant woman is unable to adequately clear parasites, chronic infection of the placenta may ensue [94]. Guitard et al. [102], studying women in Senegal, found that women could be divided into two groups. Pregnant women were either infected with parasites that persisted in the placenta until birth (from as early as 69 days before delivery) or were constantly being reinfected with new, antigenically distinct parasites, emerging throughout gestation [102]. In this latter group, samples clear of parasites were taken between those that contained parasites of new genotypes, indicating that this group of women was able to achieve effective parasite clearance between each infection. Parity status, birth weight, and time of delivery did not differ between the two groups, but women in the first group described had significantly lower levels of VAR2CSA-specific IgG initially, although levels were similar by the time of delivery [102]. Absence of antibodies in pregnancy may lead to long-lasting parasite genotypes sequestered in the placenta, which may interfere with further antibody production [102]. Lack of VSAPAM-specific IgG production is associated with an increased risk of developing chronic PM and anaemia [94]. Levels of VSA-specific IgG for non-placental variants do not appear to confer protection against poor pregnancy outcomes [94], but levels of these antibodies appear to remain constant during pregnancy [25, 98]. Similar levels of IgG that inhibit binding to ICAM-1 and CD36 have been found in non-pregnant and pregnant women in the same transmission settings [25]. Mice with prior immunity to P. berghei succumb to recrudescent parasites during pregnancy and suddenly become symptomatic in the second week of gestation [108]. Recrudescence poses a risk for women who become pregnant after emigrating from an area of malaria transmission.
3.3. Effect of Transmission Intensity on Antibody Acquisition
Megnekou et al. [25] compared the antibody responses of pregnant women living in two areas of Cameroon with different levels of transmission (2.4 infective bites/day versus 0.1–1.1 infective bites/month) [25]. In the low transmission setting, all women, regardless of age or parity, had significantly lower levels of IgG than women living in a high transmission setting [25]. The sample size of the latter group was smaller than the group from the low transmission setting but the antibody responses reported are in keeping with previous studies [109]. In the high transmission setting, PG women produced VSAPAM-specific IgG much more rapidly than those in the area of low transmission [25]. These findings support earlier reports that indicate transmission intensity is important for antibody acquisition [94, 105], yet although these trends are well described, comparison of results from different studies is limited due to vast variations in transmission levels.
Where transmission intensity is greatest, even women in their second pregnancy may have been exposed to enough variants in their first pregnancy to be protected from PM. MG women who live in areas of perennial transmission may be less able to control mixed placental infections in subsequent pregnancies than those in areas of seasonal transmission, where polyallelic infections have been correlated with LBW [102, 110, 111]. This indicates that the differences in antibody responses are not simply divided by geographical variations in transmission rates, which vary greatly across areas of sub-Saharan Africa [112]. Few data have been published on antibody levels in women with no previous exposure to parasites [113]. Where pregnant women do not appear to have been exposed during pregnancy, they have been shown to be at risk of recrudescent infections that may still cause PM due to previous exposure prior to conception [114, 115]. Also, in non-immune mice, infection with P. berghei is usually fatal and placental infection often leads to spontaneous abortion [116].
3.4. Effects of Treatment on Antibody Acquisition
If IPTp is used correctly, continued exposure to parasite antigens is alleviated [22]. Retrospective and prospective data have found that increasing the dose of IPTp in the third trimester reduces the production of VSAPAM-specific IgG [98, 117]. Observations from a longitudinal study suggest that levels of antibodies in women taking IPTp remain low for the duration of pregnancy, although antibody levels were seen to fluctuate throughout gestation [98]. This may be due to variation in the levels of parasite exposure, increased virulence of certain phenotypes, or measured differences following decay of IgG antibodies. These observations indicate that women who have received treatment in their first pregnancy will need continued therapy in subsequent pregnancies and demonstrate that results from previous cross-sectional studies may be inaccurate in representing antibody responses during gestation. This particular study does have its limitations; all women had favourable outcomes and there was a lack of placental histological examination at delivery [98].
Interestingly, immunity to malaria has been seen to increase efficacy of antimalarial chemotherapy [118], and this effect has been reported in PAM also [106]. This implies that production of a vaccine would have an additional benefit; if antibody levels do not reach those required for parasite clearance, they are at least likely to increase the efficacy of pharmacological interventions.
3.5. Antibodies Recognise Globally Distinct Parasites
Serum from MG women can recognise a number of strains of the VAR2CSA-expressing parasite variant. Distinct strains are recognised by monoclonal antibodies (mAbs) although maternal antibodies may cross-react with different var2csa-transcribing placental isolates from distinct geographical regions without previous exposure [71, 119]. Approximately 42% of pregnant women in a study cohort from Malawi and Papua New Guinea reacted to two or more globally isolated parasite lines [71]. The majority of these women were MG. Many samples also had high levels of antibodies to isolates from different regions. This may indicate limited parasite global diversity [71]. High levels of cross-reactivity seen in these populations may result from serum being cross-reactive, but not necessarily cross-inhibitory, although cross-inhibition by serum has been reported by Fried et al. [62]. Cross-reactivity of serum is thought to be either due to clonally conserved epitopes on individual VAR2CSA molecules or to polymorphic epitopes that are found in all isolates.
3.6. Maintenance of Protection
Levels of VSAPAM-specific IgG decline post-partum [95], but they have been detected at six months after delivery [98]. Previous studies have demonstrated that in MG women after delivery, up to one in 4,000 B cells show specificity for exposed epitopes of VAR2CSA [120, 121]. Persistence of memory B cells has not been examined, rendering a gap in our understanding of how MG women are able to maintain immune memory once the placenta has been expelled and antigenic exposure is lost.
4. What Other Immune Mechanisms Are Involved in PM?
4.1. Cytokine Balance in Pregnancy
The cytokine balance in pregnancy is shifted towards a predominantly anti-inflammatory response by the T helper (Th)2 subset of CD4+ T lymphocytes. This balance is mediated by the maternal placental decidua and provides a specific environment to allow persistence of the “semi-allograft” foetus (expressing paternal antigens) within the mother. At the blastocyst stage of pregnancy, Th1 proinflammatory cytokines, including interleukin (IL)-2, tumour necrosis factor α (TNF-α), and interferon gamma (IFN-γ) [122], are essential for implantation. IFN-γ is involved in remodelling of the spiral arteries to achieve adequate placental blood flow and TNF-α is necessary for induction of labour [123, 124]. Following implantation, a Th2 response is favoured which permits foetal development by production of the Th2 cytokines IL-4, IL-5, IL-10, and IL-13 [125, 126] and transforming growth factor β (TGF-β) [127, 128].
Levels of progesterone and oestrogen rise in the early stages of pregnancy, and both promote a Th2 response [129]. Secreted by the corpus luteum, progesterone maintains the endometrium, providing a welcome environment for the blastocyst and for subsequent embryo development [126, 130]. Progesterone is a potent inducer of IL-4 and IL-5, which oppose IFN-γ while inhibiting proliferation of CD8+ T cells [129]. Raised levels of this hormone may therefore influence malaria immunity [130]. Relaxin is thought to counterbalance Th2 activity of progesterone and promotes development of T cells that produce IFN-γ so is likely to play a more protective role in pregnancy [130].
4.2. Placental Malaria Affects Placental Cytokine Balance
In non-pregnant individuals, both Th1 and Th2 cytokine profiles are associated with protection, the former more active in the acute phase, for parasite clearance, and the latter during chronic infection [131, 132]. A “temporary state of reduced immunity” [8], now recognised as a prevailing Th2 response of second trimester pregnancy [125–128], is thought to favour parasite persistence and has been suggested to be responsible for the rise in parasite densities reported at 13–16 weeks [8]. Placental accumulation of pRBC appears to stimulate Th1 cytokine release by macrophages [133, 134] to aid parasite clearance, possibly through increased phagocytic activity and by production of reactive oxygen species and nitric oxide (NO) metabolites [133–136]. However, parasite densities may need to reach a threshold before inducing Th1 responses in the placenta. In mice, strong Th1 responses cause foetal loss, but in human pregnancies induction of these cytokines has been associated with maternal anaemia, spontaneous abortions, and PTD [137, 138].
Placental sequestration may induce local production of TNF-α [134, 139]. Concentrations of TNF-α appear to correspond to infiltration of monocytes and the degree of sequestration [133, 135]. Macrophages activated by haemozoin ingestion are thought to be the main source of both TNF-α and IL-8 [140]. Elevated TNF-α levels can enhance cytoadherence [141], promote monocyte recruitment [142], and affect hormonal regulation and structural integrity within the placenta [143, 144]. Higher concentrations of plasma TNF-α have been found in febrile, symptomatic pregnant women and teenage mothers [145], perhaps indicating an association with age-related immune acquisition. Gravidity-related differences in TNF-α have been reported inconsistently [145, 146]. The soluble TNF receptors, TNFR1 and 2, modulate the activity of TNF-α, and dysregulation of their control mechanisms may be linked to the development of severe malaria [147, 148].
High levels of IFN-γ have been found in placental and peripheral blood samples [133, 134, 149], with significantly higher levels found in infected pregnant women than non-infected pregnant women [149]. IFN-γ is produced by maternal CD8+ and CD4+ T cells and natural killer (NK) cells, but it is produced also by the foetal trophoblast [133]. Typically, elevated levels of IFN-γ are not associated with poor pregnancy outcomes [145] but have been correlated with gravidity-dependent protection [146]. Protection, without pathological consequences, is likely to be related to the maternal ability to prevent IFN-γ levels from exceeding an unknown pathological cut-off point. IFN-γ concentrations have been found to be higher in MG women in acute PM, and a subsequent drop in cytokine levels is observed, whereas in PG women this cytokine peaks at a lower level but remains there [133].
Migration inhibitory factor (MIF) is secreted from the foetal syncytiotrophoblast, which may be as a direct result of binding to CSA [68, 150]. MIF stimulates phagocytosis of pRBC, and increased levels of MIF have been seen in PM [150, 151]. MIF encourages accumulation of macrophages, which is consistent with the increased numbers of macrophages seen on histopathological examination at delivery [150, 151]. In one study of PG and MG women, substantially higher levels of MIF were found in placental sera than in peripheral sera, with greater concentrations of MIF in placental samples from PG compared to MG individuals [152]. Hence, it is possible that an increase in MIF may provide protection in the placenta. These differences may play a part in gravidity-specific immunity, yet equally the differences seen may be due to higher parasite densities experienced in PG women or to raised steroid levels in PG that are known to increase MIF concentrations. However, the small sample size in this study limits the reliability of the data, and future research is needed in this area [152].
It is currently not known how early Th1 responses may be stimulated or whether these responses can be induced independently of placental binding. However, a Th1 cytokine response to clear parasites also appears to occur in MG women who continue to have successful pregnancies. This would indicate that the alteration in cytokine balance may not be as detrimental to the foetus as might be expected. Similarly, this may demonstrate the nature of the host/parasite relationship because a rapid death of both mother and foetus would be lethal for the parasite. Although numerous studies have reported elevated levels of Th1 cytokines in PM, it is not a consistent finding [153, 154]. Raised levels of Th2 cytokines have also been found in PM which may indicate a positive feedback response to restore Th2 dominance. Local upregulation of IL-10, which acts directly on macrophages and causes a decrease in IL-12 production, has been reported in multiple studies [133, 134, 146, 155]. In one study cohort, women with PM were reported to have a predominant Th2 response which did not correlate with parity, age or sex, but parasite densities were consistently low [156]. TGF-β is thought to protect against severe PM, demonstrated by extended survival of P. berghei-infected mice when administered TGF-β [152], although raised levels have not been consistently correlated with parasite densities of women with predominantly successful outcomes [133].
Measurements of placental cytokine levels are limited to post-partum examination. Peripheral levels of cytokine concentrations do not correlate with cytokine levels in the placenta or with numbers of CD4+ T cells measured [145, 157]. However, cytokine responses can be examined using P. berghei [108, 114, 158]. Murine models are simpler, cheaper, and raise fewer ethical issues than simian models although data from these should be used with caution due to differences in the architecture of human and murine placentas [159]. In addition, mice show a similar Th1–Th2 shift during pregnancy to that seen in humans [160]. Data from cross-sectional studies which examine cytokine dynamics during pregnancy are difficult to use because of the type-one error incurred by measuring numerous levels of cytokines that do not increase or decrease independently [133, 156]. Discrepancies between studies could further be accounted for by the short-lived persistence of cytokines in the blood, indicating the need for more intense sampling or development of methods of cytokine quantification with increased sensitivity and specificity. Women may be more susceptible to malaria during pregnancy due to a reduction in T cell numbers. Reports of decreased T cell responses could be due to sequestration of T cells in the IVS [157, 161]. Parasite-specific T cell proliferative responses may be increased in MG women who exhibit protection from PM; Fievet et al. reported that MG women produced increased levels of Th1 cytokines to CSA-adhering strains of P. falciparum but not to non-CSA binding strains [162].
4.3. Involvement of Other Lymphocytes
Cytotoxic CD8+ T cells are implicated in the protective immune response to pre-erythrocytic malaria parasites [163]. Their role in PM has not been determined, primarily since in murine models their function beyond the liver stage of the Plasmodium life cycle remains controversial [164]. It has been a long held opinion that the mechanism of activation of CD8+ T cells precludes a protective role against blood stage malaria [165]. This is because CD8+ T cells are stimulated by antigens in association with major histocompatibility complex (MHC) class I molecules which are not expressed by RBC. However, reticulocytes (immature RBC) express MHC class I molecules and numbers of reticulocytes increase during pregnancy, and these can be infected by mature parasites [166]. CD8+ T cells may potentially play a protective role in PM if they are found to respond to these antigens. Recent observations of raised levels of CD8+ T cells in pregnant women with absent PM following parasite clearance may indicate that they may have an anti-parasite effect via a non-IFN-γ pathway [167].
4.4. Innate Immunity in PM
Dendritic cells, macrophages, NK cells, NK T cells, and γδ T cells interact non-specifically with foreign pathogens [168]. γδ T cells are known to be important in non-specific targeting of P. falciparum [168] although their role, in addition to that of dendritic cells and NK T cells, has not been studied in PM thus far.
NK cells rapidly produce TNF-α and IFN-γ which can inhibit replication of P. falciparum [169], and their presence is implicated in the antibody-dependent NK-mediated lysis of pRBC [170–172]. Unique subsets of NK cells are involved in the maintenance of early pregnancy, although alterations in NK cell behaviour may predispose to infection [173]. In an endemic area, a higher proportion of activated NK cells were found in the placenta, and this was associated with an absence of accompanying parasitaemia [167]. Interestingly, numbers of NK cells were not increased, but higher concentrations of IFN-γ were measured. If women could stimulate immediate IFN-γ release, they were able to control parasitaemia more effectively [167]. Unfortunately, this study only looked at women with favourable birth outcomes, and high numbers of NK cells have previously been associated with poor outcomes [174]. Whether or not this is related to the concentration of IFN-γ rather than to the number of cells remains to be seen. In contrast, decreased activity of NK cells has been linked to severe PM episodes [175], which may be due to the absence of NK recognition of parasite antigens, and therefore IFN-γ is not produced [176]. This finding is from in vitro experiments, where no other co-stimulants are present, which is unlikely to be the case in vivo [176].
4.5. Chemokines & Macrophages
The presence of mononuclear cells in the placenta has been correlated negatively with birth weight [135]. Stimulation of these cells promotes secretion of chemokines which contribute to the initiation of the inflammatory cascade [133]. Both CXC/α and CC/β chemokines may increase locally in PM, namely, IFN-γ inducible protein (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage-inflammatory protein 1α (MIP-1α), and macrophage-inflammatory protein 1β (MIP-1β) [17, 133]. These substances have been associated with angiogenesis, haematopoiesis, and protection from intracellular pathogens [133]. Local activation is supported by observation of greater densities of activated monocytes in the placenta than are found in the peripheral circulation [177].
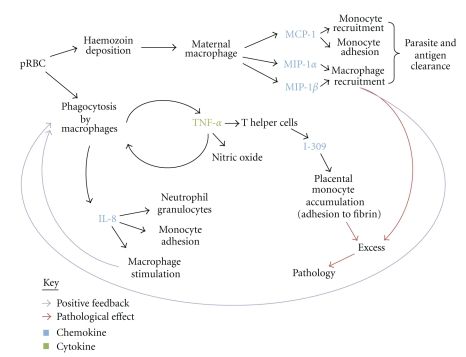
Chemokine activity aids both parasite and antigen clearance through recruitment of monocytes and macrophages. In addition to phagocytosis, macrophages present antigen to T cells [177]. Although primarily host protective, these processes have been associated with adverse effects. Figure 1 shows the probable interaction of each chemokine in PM. Subsequent recruitment of inflammatory cells creates a self-propagating and positive feedback loop.
Figure 1.
Interactions between immune cells and chemokines in placental malaria. References are found within the text. I-309 = CC/β chemokine, MCP-1: monocyte chemoattractant protein 1, MIP-1α/β: macrophage-inflammatory protein 1α/β, IL-8: interleukin 8—member of the CXC/α chemokine group.
4.6. Complement
Phagocytosis is further mediated through complement activation. In vitro observations using plasma from non-immune donors indicate that if complement deposition is prevented, 80–95% of phagocytosis of pRBC is prevented [178]. Thus, complement may play a supplementary role to uptake of antibody-opsonised pRBC in macrophage clearance observed in semi-immune women [178]. However, as seen with cytokine levels, excessive levels of complement activation may contribute to the pathogenesis associated with inflammation. In healthy pregnancies, complement component 3 (C3) is reduced by expression of CD55, CD59, and a membrane cofactor protein which are expressed by the placenta to limit local damage [129, 179]. PG women with PM have been shown to have over 50% more complement at delivery than non-infected pregnant women, although this particular study did not compare levels with MG women [180]. Nevertheless, excess complement activation may overwhelm complement regulatory proteins and contribute to placental injury.
Raised levels of complement component 5a (C5a), activated by C3a, have been found in women with PM [180]. Excessive levels of C5a have been identified as a mediator of placental and foetal injury in mice, often resulting in spontaneous abortion and IUGR [181]. C5a enhances macrophage and neutrophil activity, and excessive levels indicate that regulation of complement activation has been lost [182]. Parasite products may also increase expression of the C5a receptor on monocytes [182]. Levels of activated complement need to be examined in a larger group of women with PM and correlated with gravidity, pregnancy outcomes and degree of inflammatory infiltrate [180].
Presence of complement and TNF-α may increase expression of CSA receptors on the syncytiotrophoblast [183]. Complement and cytokine release by macrophages may occur before placental binding takes place because Toll-like receptors of the innate immune response are known to respond to the P. falciparum toxin glycosylphosphatidylinositol (PfGPI), promoting local inflammation [182]. Placental-dwelling macrophages may secrete tissue factor which initiates the clotting system; clot formation can plug the intervillous space and disrupt blood flow [184].
4.7. Cortisol Is Increased and Prolactin Is Decreased in PG Women with PAM
Raised levels of cortisol during pregnancy have been correlated with parasite load, with significantly higher levels in PG women than in MG women [185]. Cortisol is known to increase MIF-1, decrease CMI, suppress NK cell activity, and delay antibody production and may decrease IL-10 responses [175, 186, 187]. These factors could explain why PG women are more susceptible to PAM than MG women. Unfortunately, this study is confounded by the young age of PG study participants compared to the MG women (mean ages 18 and 30, respectively) [185]. In an area of unstable transmission, women of all gravidities were found to have raised levels of cortisol compared to non-infected pregnant women [188]. Prolactin is thought to be host protective in PAM, but it may be influenced by changing cortisol levels [175, 186]. A study that reported a correlation between low levels of prolactin in women with high parasite densities also found that in these women levels of IL-10 were also low [188]. High levels of cortisol in these women may have been responsible for this [188].
4.8. Co-infection with HIV Increases PAM Susceptibility
Human immunodeficiency virus (HIV) alters the typical gravidity-specific pattern of malaria risk by shifting the burden from primarily PG and secundigravid women to all pregnant women. In a retrospective analysis of 11 reports published over a15-year period, the proportional increase of P. falciparum infection during pregnancy attributable to HIV was estimated to be between 5.5 and 18.8% for populations with HIV prevalences ranging from 10 to 40% [189]. In addition, viral load has been found to increase with malaria infection in pregnant women [189, 190].
Lower levels of VSAPAM-specific IgG and decreased levels of opsonic IgG activity have been reported in women who are HIV positive [191]. HIV infection lowers numbers of CD4+ T cells, with lower levels correlating with more severe disease [192]. HIV infection further impairs cytokine responses; IL-12 may be reduced so an early Th1 shift may not occur, leading to loss of the protective rise of IFN-γ [193].
5. How Do Local and Systemic Responses Lead to Poor Outcomes?
Table 2 summarizes the key maternal and foetal outcomes of placental malaria and the immunological basis for the pathological process leading to each outcome. Absence of VSAPAM-specific IgG allows sequestration of pRBC in the placental space. Unopposed inflammation may lead to loss of microvilli and focal necrosis [33]. Placental damage stimulates cytotrophoblast proliferation resulting in a thickened cytotrophoblast membrane which may decrease the exposure of placental and foetal tissues to inflammation [194]. Maternal-foetal transfer of nutrients, oxygen, and waste products may be compromised by mechanical blockage and inflammatory cells [31].
Table 2.
Maternal and foetal outcomes of pregnancy-associated malaria.
| Effect | Possible pathogenesis | Transmission | Likely protection | References | |
|---|---|---|---|---|---|
| MOTHER | Anaemia | Severe malaria: erythrocyte destruction | BOTH | Maternal IgG | [133, 135, 162] |
| IL-10: immunosuppression, affects RBC progenitor cells, reduces available iron concentrations in plasma | [209–211] | ||||
| TNF-α: inflicts oxidative stress on RBC membranes, suppresses erythropoiesis secondary to local inflammation | BOTH | Maternal IgG? | [212, 213] | ||
| Non-PAM causes of anaemia: iron deficiency, nutrient deficiency, HIV infection, hookworm infestation | [192, 214, 215] | ||||
| Gestational hypertension | Impaired trophoblast invasion, cytokine release | BOTH? | [216] | ||
| FOETUS | Preterm delivery | Maternal anaemia | LOW | T cell memory? | [133, 212] |
| Acute parasitaemia | |||||
| TNF-α: associated with acutely high parasitaemia | |||||
| IL-10: contributes to anaemia | |||||
| Spontaneous abortion | TNF-α: necrosis of implanted foetus | LOW | [138, 196, 217] | ||
| IFN-γ increases risk of uterine contraction, activates NK cells that induce abortion | |||||
| Low birth weight, IUGR | TNF-α: chronic parasitaemia, damages local placental tissue leading to impaired maternal-foetal exchange | HIGH | Maternal IgG | [133–135, 140] | |
| Second trimester infection | |||||
| INFANT | Congenital malaria | Passage of parasites | ? | Maternal IgG | [205] |
| ↓ ability to clear parasites | T cell priming: CD4+CD25+ regulatory T cells induced, secrete IL-10, suppresses IFN-γ | ? | Maternal IgG | [205] | |
Perinatal mortality rates are higher in infants born with IUGR [195]. Risk of IUGR is increased if infections include complex multiallelic variants [98, 102], are increased in frequency, or occur during the second trimester rather than the third [39]. A major limitation of our understanding of the pathological mechanisms involved in PM surrounds the processes that lead to IUGR. The first trimester is a critical period for foetal organogenesis; the majority of growth takes place in the second trimester, and organ and cell maturation are completed during the third trimester. Prior to placental completion, early accumulation of pRBC in lacunae may induce complement activation or cytokine release which could impact negatively on embryogenesis. The majority of LBW babies born to mothers with PM are uniformly small, implying that growth restriction has been symmetrical and most likely occurred in the first trimester [109].
Raised levels of activated soluble inflammatory mediators are likely to cause pathology when unregulated during PM. TNF-α has been associated consistently with foetal LBW [145], either through IUGR [135, 139] (associated with accumulation of high concentrations of monocytes during chronic placental infection) or through PTD [133]. IFN-γ, although not associated convincingly with LBW [135, 139], can induce NK cells which may be involved in malaria-associated spontaneous foetal loss [196]. However, it is more likely that IFN-γ has a protective role in PM. PTD may be caused by a low TNF-α: IL-10 ratio (due to raised levels of both TNF-α and IL-10 in the placenta), maternal anaemia secondary to an augmentation of IL-10, or a sudden acute increase in TNF-α concentration [133]. Active inflammation with monocyte infiltration may also lead to dysregulation of the insulin-like growth factor (IGF) axis which is upregulated in the normal placenta to support growth [197].
Vascular endothelial growth factor (VEGF) and angiogenic factors, including angiopoietin 1 and 2 (ANG-1, ANG-2), are important in the control of vascular development and remodelling during placental development [180]. The soluble receptor of VEGF, sVEGFR1, opposes VEGF to inhibit trophoblastic invasion. Raised levels of sVEGFR1 have been associated with excessive levels of C5a; either C5a or the presence of parasite products that activate complement may be responsible for this [180]. Dysregulation of angiopoietin levels has been reported in women with peripheral parasitaemia, and was correlated more closely in women who had LBW babies [198]. These levels were measured at delivery, but disturbance of the balance of these growth factors could occur earlier in gestation and impact negatively on foetal growth.
These findings support the hypothesis that the pathogenesis of PM is similar to that of pre-eclampsia (PE). A case-cohort study reported an absence of relative risk between PM and PE whereas, although this type of study design does not provide a causal link, a correlation was seen between PM and gestational hypertension [199, 200]. Gestational hypertension may result from impaired placental blood flow, possibly from shallow trophoblast invasion and poor placental vascularisation, or from mechanical blockage of blood flow [201]. Reduced placental perfusion, endothelial dysfunction, and placental ischaemia are thought to contribute to IUGR [201].
Women who harbour complex infections are at increased risk of anaemia, while the infants born to these women are more likely to have infected cord blood and increased risk of malaria in the first 30 months of life [202]. Although parasite infection of cord blood is common [154, 203] and newborns may harbour an asymptomatic parasitaemia [204, 205], disease is rare [77, 205]. Protection from disease may be due to foetal haemoglobin being composed of different globin subtypes than adult haemoglobin, decreased infant exposure due to swaddling and transfer of maternal antibody [205–207]. PM may prime foetal immune T cells in utero to favour a Th2 cytokine response due to exposure to maternal cytokines [205–207]. Inflammation may disrupt IgG transporters in the syncytiotrophoblast, thereby reducing placental transfer of IgG in response to other pathogens including measles virus and Streptococcus pneumoniae [33].
6. Current Vaccine Prospects
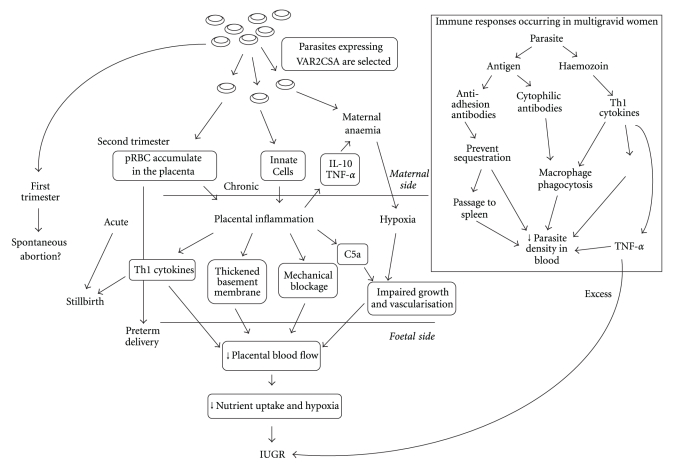
In order to produce an ideal vaccine for PAM, all the protective responses that occur in natural infections need to be stimulated. Figure 2 summarizes the potential immunological interactions in PM and the protective responses that occur in MG women. Current vaccine developments have had varied success. The ideal candidate for a PAM vaccine is the VAR2CSA molecule, the structure of which differs to that of other PfEMP1 molecules. It comprises six DBL domains and a CIDR, all of which display polymorphism between strains [69, 116]. From 32 isolates from a sample of pregnant women in Senegal, not one shared an identical sequence in the DBL5 domain [102]. The var2csa gene that codes for VAR2CSA displays significant levels of polymorphism, demonstrated by the number of possible unique antigens that have been produced. However, it is more conserved than other var genes, and VAR2CSA retains 75–83% of its amino acids between isolated strains [208]. This engenders confidence that there are a limited number of antigens possible. However, the degree of antigenic variation between globally isolated parasite lines has not been determined.
Figure 2.
Summary of immune interactions and outcomes in placental malaria. The box shows the interactions that are thought to be protective in multigravid women.
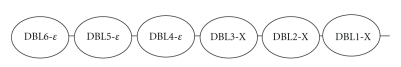
Vaccine developments with VAR2CSA have been hampered by the difficulty in producing a recombinant protein of this size. To overcome this issue initially, individual domains of VAR2CSA have been studied to determine if a particular region of the molecule is responsible for receptor binding and induction of antibodies. The individual domains are shown in Figure 3. Three of the six DBL regions (DBL2-X, DBL3-X and DBL6-ε) are known to bind to CSA, but the remaining three (DBL1-X, DBL4-ε, DBL5-ε) display limited affinity for CSA [218, 219]. It has proved difficult for study groups to adequately assess binding of different DBL regions, as interactions between recombinant proteins (expressed by Escherichia coli, baculovirus or Pichia pastoris constructs [220–222]) and CSA in vitro may not be representative of native binding [223]. Furthermore, studies that have elicited adhesion inhibition have observed it in assays of CSA on plastic mounts [103, 220, 224], which, whilst they provide an easy laboratory method of assessing adhesive properties, do not contain the same levels of sulphation as CSA on the syncytiotrophoblast. The most promising method to study adhesion behaviour is to use BeWo cells from human choriocarcinoma that provide a placental construct on which CSA is naturally found [90, 220, 224].
Figure 3.
The duffy binding-like domains of the VAR2CSA molecule. Between each DBL region is an interdomain region that appears to be important for both binding properties and antibody induction. Each region has a differing degree of affinity for the CSA molecule on the placenta and individually the domains do not appear to display as strong binding ability as the whole VAR2CSA molecule. Recent evidence suggests that the first four domains are the most important for forming a quaternary structure that forms a complex binding site [228].
Antibodies have been induced in mice and goats with recombinant DBL3, DBL4, and DBL5 domains [104, 220–222, 225, 226]. Antibodies to DBL3 and DBL5 recognised other VAR2CSA-expressing strains from diverse geographical regions, whereas cross-reactivity was not observed following immunisation with DBL1, 4, or 6 [227]. Despite displaying cross-reactivity, these antibodies did not inhibit binding, suggesting that only surface reactivity may occur with in vitro-induced antibodies. Interestingly, the most effective anti-adhesion antibodies have been induced in rats with DBL4 domains [225]. This domain is thought to display the lowest binding affinity for CSA but is highly conserved between variants [225]. It is not known if the same antibodies would be induced in vivo. The DBL5 domain displays 66–81% conservation between isolates, and strain-transcending adhesion inhibition has been seen under flow conditions using BeWo cells lines and antibodies induced in mice and goats [220].
Single domains from non-placental PfEMP1 may also bind to CSA in vitro [103] but with much weaker affinity than binding of whole VAR2CSA [104]. The full-length version induces anti-adhesion antibodies that are highly strain-specific [227]. Whole recombinant VAR2CSA has now been produced, and, although the ability to induce antibodies using this construct has been inconsistent, these findings demonstrate that all domains may be as important as each other in mediating binding and antibody stimulation [103, 104, 227]. A combination of epitopes on each domain may form a binding complex or unique binding site by forming a quaternary structure [69]. If this is the case, individual polymorphisms in DBL regions may come together to alter the binding site of VAR2CSA, which would account for the cross-reactive but not adhesion-inhibitory strain-transcending responses described [69]. Convincing evidence is emerging that supports the formation of a binding site from the first four domains and interactions with the CIDR and interdomain regions [228, 229]. These results may also indicate that finding a globally conserved epitope is unlikely. Inconsistent induction of antibodies with whole constructs could be caused by differing expression methods employed or could indicate limitations of using such a large immunogen [104, 227]. Although the biochemical interactions are beyond the scope of this review, our understanding of the true nature of parasite antigen-placenta interactions may not be far enough advanced for effective vaccine trials [69].
Induction of cytophilic activity by IgG molecules has been shown to be an effective immune mechanism [106], but this has been largely ignored in vaccine development studies to date. Perhaps a novel way forward would involve shifting the focus towards induction of these antibodies: specifically, whether they may be induced using single domains or whole antigens; and, if inducible, whether they display the same degree of specificity as anti-adhesive antibodies. The lack of identification of a conserved epitope thus far should not be considered disheartening as even antibodies that stimulate a protective response against one or two variant lines may be beneficial in PG women to help lower parasite load and increase efficacy of treatment [98].
Induction of antibodies by single domains and whole VAR2CSA has not yet been tested in humans, and it is likely that single variants will produce differing antibody responses to those found in naturally mixed allelic infections. Evidence suggests that the ability of a construct to induce antibodies depends greatly on the animal being immunised; a recent study observed that immunisation responses varied greatly and unpredictably between mice, rabbits, and rats [229] and the same is likely to be true with humans. Even if VAR2CSA could be used, the highly specific antibody repertoire gained from this would only confer protection against one parasite strain. Naturally, it takes at least one pregnancy to acquire inhibitory antibodies. It is not currently known if, or how, immunity develops to strains simultaneously, and this may further complicate trials of antibody stimulation in vivo. If an epitope is identified that allows women to develop inhibitory antibodies rapidly to a range of strains by focusing the immune response, it could provide better protection than that which is naturally acquired. Currently, findings indicate that a number of allelic variants will be needed in a vaccine due to the high levels of polymorphism within the parasite population.
A clear association has been shown between the presence of anti-adhesion antibodies and weight gain of foetuses, but precise pathological mechanisms by which antibodies reduce or inhibit associated inflammation are unknown. The inhibition of binding may decrease inflammation by preventing activation of signalling pathways on the syncytiotrophoblast that lead to cytokine production. The incomplete understanding of these mechanisms raises questions regarding how antibodies provide protection. The continued study of individual binding domains of VAR2CSA may increase our knowledge of these mechanisms even if these studies do not lead to viable vaccine possibilities.
The timing of a VAR2CSA vaccine must be considered. Judging by the appearance of placental variants in the second trimester, a vaccine would be most effective given before pregnancy but could also provide protection if given during the first trimester. This is improbable, however, because the majority of pregnancies in developing countries are not confirmed until the second trimester due to socioeconomic and cultural reasons affecting access to primary health care. Theoretically, stimulation of mAbs using individual domains may be possible in prepubescent girls and repeated over successive years, but this is unlikely to be feasible. The lack of understanding of how immunological memory is maintained hinders the validity of this option. Antibodies have been seen to persist for six months without continued antigen exposure [121], but it is uncertain if they would continue beyond this. This indicates that decay of antibodies may occur by the time a woman becomes pregnant if the vaccine is given too early, although this may depend on the specific Ig subclasses that are included in the vaccine.
A VAR2CSA vaccine would be limited in its protective target group. Women who lack naturally acquired immunity are not protected from peripheral binding variants and a VAR2CSA would not inhibit binding of these parasites, resulting in peripheral infection. Furthermore, selection pressure exerted by such a vaccine is likely to increase global antigenic diversity. Continued efforts to identify conserved epitopes are the aim of non-PAM vaccines, and proof of principle studies have led to phase III trials (reviewed in [230]). The most advanced candidate, the RTS,S vaccine against the circumsporozoite antigen, provided 25.6–59% protection in phase II trials and became the first malaria vaccine to reach phase III trials [231]. While this extensive study across seven sub-Saharan countries will not be completed before 2014, preliminary results show grounds for qualified optimism [232, 233]. It is unclear what protection this may provide during pregnancy because it is unlikely to achieve sterile immunity and despite women having antibodies against non-VSAPAM and other parasite proteins prior to pregnancy, the immunity that develops is not adequate to prevent pregnancy-associated infection.
A vaccine against P. falciparum will not protect against P. vivax which is a major problem in regions where transmission of P. falciparum is low, such as the Indian subcontinent. The protection gained from maternal anaemia due to mixed infections may be lost through a P. falciparum-specific vaccine. Further to this, the placental-binding property of P. falciparum to CSA has provided a selective advantage, but P. vivax is known to accumulate in the placenta. More recently, severe syndromes associated with P. vivax have been reported in children, which could indicate increasing virulence in this parasite [234]. Similar receptors to the VSAs have been found on the membrane of the P. vivax pRBC, but sequestration is currently not thought to occur [35].
As yet, it is not clear if vaccination with single domains or whole VAR2CSA is recognised by T cells. In the early stages of determining which epitopes stimulate protection, it is essential that T cell responses are considered in vaccine trials. Natural expression of antigens on pRBC may stimulate specific antibodies in a different way, and natural interactions of T and B cells may also determine efficacy.
If VAR2CSA is the key antigen in immune acquisition in PAM, then it may prove possible to answer the questions below:
To which part of the molecule do antibodies react?
In models, are antibodies produced to isolated domains?
Do these antibodies confer the same protection as natural antibodies?
What class of antibodies are produced using a vaccine?
How long do memory B cells persist?
Can T cell responses be induced?
If T cells are stimulated and produce a protective response that generates IFN-γ, then will a TNF-α response that harms the placenta also be induced?
Will protective innate mechanisms be stimulated by a vaccine?
What end points would we use to measure the effectiveness of a vaccine?
7. Areas for Future Research
There are a number of areas that require further research to increase our understanding of both the pathogenesis and immunology of PAM. These include the following:
pathological processes occurring in the first trimester;
pathological mechanisms leading to anaemia and LBW with P. vivax infections;
trials of new methods of diagnosing placental malaria infection;
analysis of peripheral cytokines as possible surrogate markers;
longitudinal studies in low transmission settings and longitudinal studies of cytokine dynamics;
whether the responses that lead to immunity and the responses that lead to pathology can be distinguished;
how hormones contribute directly to susceptibility in PAM;
longitudinal studies to examine how infant cohorts are affected;
further analysis of the burden of PAM outside Africa.
The majority of studies described in this review are cross-sectional or retrospective analyses of antibody and parasite dynamics in pregnant women. Levels of both parasites and antibodies fluctuate during pregnancy, and true representations of these dynamics can only be obtained through longitudinal studies [34, 98, 102]. In addition to the need for more robust studies, there is a risk that by focusing attention on a small part of the pathological processes occurring in PM, we will lose sight of what we already know. For example, although binding of CSA does not occur until the second trimester, the processes may not be as crucial to pathogenesis as currently thought: if binding to CSA increases gene expression and secretion of inflammatory mediators, such as MIF-1 that promotes macrophage aggregation, then inflammatory processes will not begin until after week 16. We cannot be certain of the development of inflammation because there is no way of assessing when this begins in pregnant women. The detection of peripheral levels of inflammatory markers, such as raised levels of TNFR1/2, sVEGR1, C5a, and IL-10, would be an ideal method of diagnosing active PM in asymptomatic women. However, no marker has been found to correlate adequately with the degree of inflammation. The pathological processes of PM are dynamic throughout gestation, but the presence of parasites in the placenta may be detrimental to embryo development before binding occurs. This could potentially be studied using var2csa-null variants in vitro using BeWo cells.
8. Conclusion
Is PAM vaccination possible? At present, the creation of a vaccine looks unlikely. Not enough is known about specific binding interactions between CSA and VAR2CSA, and globally conserved epitopes have not been identified. These hurdles, combined with the task of including all necessary parasite strains in a single vaccine and the risk that a vaccine could potentially lead to an increase in the prevalence of other malaria species not covered by the vaccine, do not favour vaccine development. If growth mechanisms are most affected in the first trimester, a VAR2CSA vaccine would be ineffective at reducing the burden of IUGR. This paper has, however, identified some of the gaps in our knowledge, and filling these may lead to ways of overcoming these obstacles. Even if a vaccine is able only to stimulate the production of a small antibody repertoire this could boost treatment effects and decrease the financial burden of PAM. In the light of growing resistance of P. falciparum to current chemotherapeutic regimens, even a vaccine that could result in lower doses and increased efficacy of these regimens has potential value, and research to that end should be welcomed.
Acknowledgments
The authors received financial support from the University of Leeds (Grant no. DEV.BIOL.319190 to A. W. Taylor-Robinson). Professors Stephen Rogerson (University of Melbourne) and Lars Hviid (University of Copenhagen) are thanked for their correspondence providing constructive comments on our suggestions and clarification of detailed queries regarding their published research.
References
- 1.WHO. A Strategic Framework for Malaria Prevention and Control during Pregnancy in the African Region. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 2.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman’s acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. The American Journal of Tropical Medicine and Hygiene. 1996;55(1, supplement):42–49. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. The American Journal of Tropical Medicine and Hygiene. 2001;64(1-2, supplement):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 4.Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Annals of Tropical Medicine and Parasitology. 1969;63(2):245–263. doi: 10.1080/00034983.1969.11686625. [DOI] [PubMed] [Google Scholar]
- 5.Shulman CE, Graham WJ, Jilo H, et al. Malaria is an important cause of anaemia in primigravidae: evidence from a district hospital in coastal Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(5):535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- 6.Cot M, Le Hesran JY, Miailhes P, et al. Effect of chloroquine prophylaxis during pregnancy on maternal haematocrit. Annals of Tropical Medicine and Parasitology. 1998;92(1):37–43. doi: 10.1080/00034989860157. [DOI] [PubMed] [Google Scholar]
- 7.McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birthweight and placental weight. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77(2):232–244. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 8.Brabin BJ. An analysis of malaria in pregnancy in Africa. Bulletin of the World Health Organization. 1983;61(6):1005–1016. [PMC free article] [PubMed] [Google Scholar]
- 9.McGregor IA. Epidemiology, malaria and pregnancy. The American Journal of Tropical Medicine and Hygiene. 1984;33(4):517–525. doi: 10.4269/ajtmh.1984.33.517. [DOI] [PubMed] [Google Scholar]
- 10.Brabin B. An assessment of low birthweight risk in primiparae as an indicator of malaria control in pregnancy. International Journal of Epidemiology. 1991;20(1):276–283. doi: 10.1093/ije/20.1.276. [DOI] [PubMed] [Google Scholar]
- 11.Nosten F, ter Kuile F, Maelankirri L, Decludt B, White NJ. Malaria during pregnancy in an area of unstable endemicity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1991;85(4):424–429. doi: 10.1016/0035-9203(91)90205-d. [DOI] [PubMed] [Google Scholar]
- 12.Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in Rural Malawi. The American Journal of Tropical Medicine and Hygiene. 1996;55(1, supplement):33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan AD, Nyirenda T, Cullinan T, et al. Malaria infection during pregnancy: intrauterine growth retardation and preterm delivery in Malawi. Journal of Infectious Diseases. 1999;179(6):1580–1583. doi: 10.1086/314752. [DOI] [PubMed] [Google Scholar]
- 14.WHO. Parasitic Diseases: Malaria. Initiative for Vaccine Research (IVR) 2011. http://www.who.int/vaccine_research/diseases/soa_parasitic/en/index4.html.
- 15.Taylor-Robinson A. Immunity to malaria increases during puberty. Trends in Parasitology. 2001;17(5):p. 213. doi: 10.1016/s1471-4922(01)01958-4. [DOI] [PubMed] [Google Scholar]
- 16.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clinical Microbiology Reviews. 2009;22(1):13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrams ET, Brown H, Chensue SW, et al. Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated β chemokine expression. Journal of Immunology. 2003;170(5):2759–2764. doi: 10.4049/jimmunol.170.5.2759. [DOI] [PubMed] [Google Scholar]
- 18.Ansell J, Hamilton KA, Pinder M, Walraven GEL, Lindsay SW. Short-range attractiveness of pregnant women to Anopheles gambiae mosquitoes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96(2):113–116. doi: 10.1016/s0035-9203(02)90271-3. [DOI] [PubMed] [Google Scholar]
- 19.Nosten F, Rogerson SJ, Beeson JG, McGready R, Mutabingwa TK, Brabin B. Malaria in pregnancy and the endemicity spectrum: what can we learn? Trends in Parasitology. 2004;20(9):425–432. doi: 10.1016/j.pt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Duffy PE, Desowitz RS. Pregnancy malaria throughout history: dangerous labors. In: Duffy PE, Fried M, editors. Malaria in Pregnancy: Deadly Parasite, Susceptible Host. London, UK: Taylor & Francis; 2001. pp. 1–25. [Google Scholar]
- 21.Looareesuwan S, Phillips RE, White NJ, et al. Quinine and severe falciparum malaria in late pregnancy. The Lancet. 1985;2(8445):4–7. doi: 10.1016/s0140-6736(85)90056-x. [DOI] [PubMed] [Google Scholar]
- 22.Nosten F, McGready R, Mutabingwa T. Case management of malaria in pregnancy. The Lancet Infectious Diseases. 2007;7(2):118–125. doi: 10.1016/S1473-3099(07)70023-3. [DOI] [PubMed] [Google Scholar]
- 23.Recker M, Bouma MJ, Bamford P, Gupta S, Dobson AP. Assessing the burden of pregnancy-associated malaria under changing transmission settings. Malaria Journal. 2009;8(1, article 245) doi: 10.1186/1475-2875-8-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brabin B, Rogerson SJ. The epidemiology and outcomes of maternal malaria. In: Duffy PE, Fried M, editors. Malaria in Pregnancy: Deadly Parasite, Susceptible Host. London, UK: Taylor & Francis; 2001. pp. 27–52. [Google Scholar]
- 25.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infection and Immunity. 2005;73(7):4112–4118. doi: 10.1128/IAI.73.7.4112-4118.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baird JK. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitology Today. 1995;11(3):105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 27.Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. The American Journal of Tropical Medicine and Hygiene. 2007;77(6, supplement):14–22. [PubMed] [Google Scholar]
- 28.Chico RM, Chandramohan D. Intermittent preventive treatment of malaria in pregnancy: at the crossroads of public health policy. Tropical Medicine and International Health. 2011;16(7):774–785. doi: 10.1111/j.1365-3156.2011.02765.x. [DOI] [PubMed] [Google Scholar]
- 29.Maiga OM, Kayentao K, Traoré BT, et al. Superiority of 3 over 2 doses of intermittent preventive treatment with sulfadoxine-pyrimethamine for the prevention of malaria during pregnancy in mali: a randomized controlled trial. Clinical Infectious Diseases. 2011;53(3):215–223. doi: 10.1093/cid/cir374. [DOI] [PubMed] [Google Scholar]
- 30.Sevene E, González R, Menéndez C. Current knowledge and challenges of antimalarial drugs for treatment and prevention in pregnancy. Expert Opinion on Pharmacotherapy. 2010;11(8):1277–1293. doi: 10.1517/14656561003733599. [DOI] [PubMed] [Google Scholar]
- 31.Ismail MR, Ordi J, Menendez C, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Human Pathology. 2000;31(1):85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 32.Muehlenbachs A, Fried M, McGready R, et al. A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. Journal of Infectious Diseases. 2010;202(10):1608–1616. doi: 10.1086/656723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brabin BJ, Romagosa C, Abdelgalil S, et al. The sick placenta—the role of malaria. Placenta. 2004;25(5):359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Leke RFG, Bioga JD, Zhou J, et al. Longitudinal studies of Plasmodium falciparum malaria in pregnant women living in a rural cameroonian village with high perennial transmission. The American Journal of Tropical Medicine and Hygiene. 2010;83(5):996–1004. doi: 10.4269/ajtmh.2010.10-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGready R, Davison BB, Stepniewska K, et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. The American Journal of Tropical Medicine and Hygiene. 2004;70(4):398–407. [PubMed] [Google Scholar]
- 36.Khong TY. Acute atherosis in pregnancies complicated by hypertension, small-for-gestational-age infants, and diabetes mellitus. Archives of Pathology and Laboratory Medicine. 1991;115(7):722–725. [PubMed] [Google Scholar]
- 37.Hamilton WJ, Boyd JD. Development of the human placenta in the first three months of gestation. Journal of Anatomy. 1960;94(7):297–328. [PMC free article] [PubMed] [Google Scholar]
- 38.Hirano H, Imai Y, Ito H. Spiral artery of placenta: development and pathology-immunohistochemical, microscopical, and electron-microscopic study. Kobe Journal of Medical Sciences. 2002;48(1-2):13–23. [PubMed] [Google Scholar]
- 39.Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Meshnick SR. The effect of timing and frequency of Plasmodium falciparum infection during pregnancy on the risk of low birth weight and maternal anemia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2010;104(6):416–422. doi: 10.1016/j.trstmh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai M, ter Kuile FO, Nosten F, et al. Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases. 2007;7(2):93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 41.Cottrell G, Mary JY, Barro D, Cot M. The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. The American Journal of Tropical Medicine and Hygiene. 2007;76(5):849–854. [PubMed] [Google Scholar]
- 42.Rijken MJ, Rijken JA, Papageorghiou AT, et al. Malaria in pregnancy: the difficulties in measuring birthweight. British Journal of Obstetrics and Gynaecology. 2011;118(6):671–678. doi: 10.1111/j.1471-0528.2010.02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landis SH, Lokomba V, Ananth CV, et al. Impact of maternal malaria and under-nutrition on intrauterine growth restriction: a prospective ultrasound study in Democratic Republic of Congo. Epidemiology and Infection. 2009;137(2):294–304. doi: 10.1017/S0950268808000915. [DOI] [PubMed] [Google Scholar]
- 44.Beeson JG, Brown GV. Pathogenesis of Plasmodium falciparum malaria: the roles of parasite adhesion and antigenic variation. Cellular and Molecular Life Sciences. 2002;59(2):258–271. doi: 10.1007/s00018-002-8421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bottero J, Briand V, Agbowai C, Doritchamou J, Massougbodji A, Cot M. Short report: spontaneous post-partum clearance of Plasmodium falciparum parasitemia in pregnant women, Benin. The American Journal of Tropical Medicine and Hygiene. 2011;84(2):267–269. doi: 10.4269/ajtmh.2011.10-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muthusamy A, Achur RN, Valiyaveettil M, et al. Chondroitin sulfate proteoglycan but not hyaluronic acid is the receptor for the adherence of Plasmodium falciparum-infected erythrocytes in human placenta, and infected red blood cell adherence up-regulates the receptor expression. The American Journal of Pathology. 2007;170(6):1989–2000. doi: 10.2353/ajpath.2007.061238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beeson JG, Rogerson SJ, Cooke BM, et al. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nature Medicine. 2000;6(1):86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flick K, Scholander C, Chen Q, et al. Role of non-immune IgG bound to PfEMP1 in placental malaria. Science. 2001;293(5537):2098–2100. doi: 10.1126/science.1062891. [DOI] [PubMed] [Google Scholar]
- 49.Rasti N, Namusoke F, Chêne A, et al. Non-immune immunoglobulin binding and multiple adhesion characterize Plasmodium falciparum-infected erythrocytes of placental origin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13795–13800. doi: 10.1073/pnas.0601519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khunrae P, Higgins MK. Structural insights into chondroitin sulfate binding in pregnancy-associated malaria. Biochemical Society Transactions. 2010;38(5):1337–1341. doi: 10.1042/BST0381337. [DOI] [PubMed] [Google Scholar]
- 51.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272(5267):1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 52.Beeson JG, Brown GV, Molyneux ME, Mhango C, Dzinjalamala F, Rogerson SJ. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. Journal of Infectious Diseases. 1999;180(2):464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beeson JG, Brown GV. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. Journal of Infectious Diseases. 2004;189(2):169–179. doi: 10.1086/380975. [DOI] [PubMed] [Google Scholar]
- 54.Beeson JG, Rogerson SJ, Brown GV. Evaluating specific adhesion of Plasmodium falciparum-infected erythrocytes to immobilised hyaluronic acid with comparison to binding of mammalian cells. International Journal for Parasitology. 2002;32(10):1245–1252. doi: 10.1016/s0020-7519(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 55.Rogerson SJ, Chaiyaroj SC, Ng K, Reeder JC, Brown GV. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. Journal of Experimental Medicine. 1995;182(1):15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noviyanti R, Brown GV, Wickham ME, Duffy MF, Cowman AF, Reeder JC. Multiple var gene transcripts are expressed in Plasmodium falciparum infected erythrocytes selected for adhesion. Molecular and Biochemical Parasitology. 2001;114(2):227–237. doi: 10.1016/s0166-6851(01)00266-3. [DOI] [PubMed] [Google Scholar]
- 57.Joergensen L, Bengtsson DC, Bengtsson A, et al. Surface co-expression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathogens. 2010;6(9) doi: 10.1371/journal.ppat.1001083. Article ID e01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salanti A, Staalsoe T, Lavstsen T, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology. 2003;49(1):179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 59.Dahlbäck M, Lavstsen T, Salanti A, et al. Changes in var gene mRNA levels during erythrocytic development in two phenotypically distinct Plasmodium falciparum parasites. Malaria Journal. 2007;6(1, article 78) doi: 10.1186/1475-2875-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schieck E, Pfahler JM, Sanchez CP, Lanzer M. Nuclear run-on analysis of var gene expression in Plasmodium falciparum. Molecular and Biochemical Parasitology. 2007;153(2):207–212. doi: 10.1016/j.molbiopara.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Udomsangpetch R, Wahlin B, Carlson J, et al. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. Journal of Experimental Medicine. 1989;169(5):1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature. 1998;395(6705):851–850. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 63.Beeson JG, Mann EJ, Elliott SR, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. Journal of Infectious Diseases. 2004;189(3):540–551. doi: 10.1086/381186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maubert B, Fievet N, Tami G, Cot M, Boudin C, Deloron P. Development of antibodies against chondroitin sulfate A-adherent Plasmodium falciparum in pregnant women. Infection and Immunity. 1999;67(10):5367–5371. doi: 10.1128/iai.67.10.5367-5371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salem HH, Maruyama I, Ishii H, Majerus PW. Isolation and characterization of thrombomodulin from human placenta. Journal of Biological Chemistry. 1984;259(19):12246–12251. [PubMed] [Google Scholar]
- 66.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell and Tissue Research. 2010;339(1):237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 67.Lucchi NW, Koopman R, Peterson DS, Moore JM. Plasmodium falciparum-infected red blood cells selected for binding to cultured syncytiotrophoblast bind to chondroitin sulfate A and induce tyrosine phosphorylation in the syncytiotrophoblast. Placenta. 2006;27(4-5):384–394. doi: 10.1016/j.placenta.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Lucchi NW, Peterson DS, Moore JM. Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malaria Journal. 2008;7(1, article 42) doi: 10.1186/1475-2875-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dahlbäck M, Nielsen MA, Salanti A. Can any lessons be learned from the ambiguous glycan binding of PfEMP1 domains? Trends in Parasitology. 2010;26(5):230–235. doi: 10.1016/j.pt.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Matejevic D, Neudeck H, Graf R, Müller T, Dietl J. Localization of hyaluronan with a hyaluronan-specific hyaluronic acid binding protein in the placenta in pre-eclampsia. Gynecologic and Obstetric Investigation. 2001;52(4):257–259. doi: 10.1159/000052986. [DOI] [PubMed] [Google Scholar]
- 71.Hommel M, Elliott SR, Soma V, et al. Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infection and Immunity. 2010;78(5):1963–1978. doi: 10.1128/IAI.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duffy MF, Byrne TJ, Elliott SR, et al. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Molecular Microbiology. 2005;56(3):774–788. doi: 10.1111/j.1365-2958.2005.04577.x. [DOI] [PubMed] [Google Scholar]
- 73.Baruch DI, Gormley JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kraemer SM, Smith JD. A family affair: var genes, PfEMP1 binding, and malaria disease. Current Opinion in Microbiology. 2006;9(4):374–380. doi: 10.1016/j.mib.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Smith JD, Kyes S, Craig AG, et al. Analysis of adhesive domains from the A4VAR Plasmodium falciparum erythrocyte membrane protein-1 identifies a CD36 binding domain. Molecular and Biochemical Parasitology. 1998;97(1-2):133–148. doi: 10.1016/s0166-6851(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 76.Smith JD, Craig AG, Kriek N, et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogerson SJ, Hviid L, Duffy PE, Leke RF, Taylor DW. Malaria in pregnancy: pathogenesis and immunity. The Lancet Infectious Diseases. 2007;7(2):105–117. doi: 10.1016/S1473-3099(07)70022-1. [DOI] [PubMed] [Google Scholar]
- 78.Singh N, Shukla MM, Srivastava R, Sharma VP. Prevalence of malaria among pregnant and non-pregnant women of district Jabalpur, Madhya Pradesh. Indian Journal of Malariology. 1995;32(1):6–13. [PubMed] [Google Scholar]
- 79.Nosten F, McGready R, Simpson JA, et al. Effects of Plasmodium vivax malaria in pregnancy. The Lancet. 1999;354(9178):546–549. doi: 10.1016/s0140-6736(98)09247-2. [DOI] [PubMed] [Google Scholar]
- 80.Diagne N, Rogier C, Cisse B, Trape JF. Incidence of clinical malaria in pregnant women exposed to intense perennial transmission. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91(2):166–170. doi: 10.1016/s0035-9203(97)90209-1. [DOI] [PubMed] [Google Scholar]
- 81.Duffy PE, Fried M. Malaria in the pregnant woman. Current Topics in Microbiology and Immunology. 2005;295(1):169–200. doi: 10.1007/3-540-29088-5_7. [DOI] [PubMed] [Google Scholar]
- 82.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1997;91(3):256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 83.Diagne N, Rogier C, Sokhna CS, et al. Increased susceptibility to malaria during the early post-partum period. The New England Journal of Medicine. 2000;343(9):598–603. doi: 10.1056/NEJM200008313430901. [DOI] [PubMed] [Google Scholar]
- 84.Tuikue Ndam NG, Salanti A, Bertin G, et al. High level of var2csa transcription by Plasmodium falciparum isolated from the placenta. Journal of Infectious Diseases. 2005;192(2):331–335. doi: 10.1086/430933. [DOI] [PubMed] [Google Scholar]
- 85.Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Molecular Microbiology. 2003;50(5):1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 86.Viebig NK, Gamain B, Scheidig C, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. The EMBO Reports. 2005;6(8):775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Duffy MF, Maier AG, Byrne TJ, et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum. Molecular and Biochemical Parasitology. 2006;148(2):117–124. doi: 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 88.Salanti A, Dahlbäck M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. Journal of Experimental Medicine. 2004;200(9):1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gamain B, Trimnell AR, Scheidig C, Schert A, Miller LH, Smith JD. Identification of multiple chondroitin sulfate A (CSA)-binding domains in the var2csa gene transcribed in CSA-binding parasites. Journal of Infectious Diseases. 2005;191(6):1010–1013. doi: 10.1086/428137. [DOI] [PubMed] [Google Scholar]
- 90.Viebig NK, Nunes MC, Scherf A, Gamain B. The human placental derived BeWo cell line: a useful model for selecting Plasmodium falciparum CSA-binding parasites. Experimental Parasitology. 2006;112(2):121–125. doi: 10.1016/j.exppara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Cox SE, Staalsoe T, Arthur P, et al. Rapid acquisition of isolate-specific antibodies to chondroitin sulfate A-adherent Plasmodium falciparum isolates in Ghanaian primigravidae. Infection and Immunity. 2005;73(5):2841–2847. doi: 10.1128/IAI.73.5.2841-2847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barfod L, Nielsen MA, Turner L, et al. Baculovirus-expressed constructs induce immunoglobulin G that recognizes VAR2CSA on Plasmodium falciparum-infected erythrocytes. Infection and Immunity. 2006;74(7):4357–4360. doi: 10.1128/IAI.01617-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hviid L, Salanti A. VAR2CSA and protective immunity against pregnancy-associated Plasmodium falciparum malaria. Parasitology. 2007;134(13):1871–1876. doi: 10.1017/S0031182007000121. [DOI] [PubMed] [Google Scholar]
- 94.Staalsoe T, Shulman CE, Bulmer JN, Kawuondo K, Marsh K, Hviid L. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. The Lancet. 2004;363(9405):283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 95.Staalsoe T, Megnekou R, Fievét N, et al. Acquisition and decay of antibodies to pregnancy-associated variant antigens on the surface of Plasmodium falciparum-infected erythrocytes that protect against placental parasitemia. Journal of Infectious Diseases. 2001;184(5):618–626. doi: 10.1086/322809. [DOI] [PubMed] [Google Scholar]
- 96.Ricke CH, Staalsoe T, Koram K, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. Journal of Immunology. 2000;165(6):3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 97.Recker M, Buckee CO, Serazin A, et al. Antigenic variation in Plasmodium falciparum malaria involves a highly structured switching pattern. PLoS Pathogens. 2011;7(3) doi: 10.1371/journal.ppat.1001306. Article ID e1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aitken EH, Mbewe B, Luntamo M, et al. Antibodies to chondroitin sulfate A-binding infected erythrocytes: dynamics and protection during pregnancy in women receiving intermittent preventive treatment. Journal of Infectious Diseases. 2010;201(9):1316–1325. doi: 10.1086/651578. [DOI] [PubMed] [Google Scholar]
- 99.O’Neil-Dunne I, Achur RN, Agbor-Enoh ST, et al. Gravidity-dependent production of antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to placental chondroitin sulfate proteoglycan during pregnancy. Infection and Immunity. 2001;69(12):7487–7492. doi: 10.1128/IAI.69.12.7487-7492.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Achtman AH, Bull PC, Stephens R, Langhorne J. Longevity of the immune response and memory to blood-stage malaria infection. Current Topics in Microbiology and Immunology. 2005;297(1):71–102. doi: 10.1007/3-540-29967-x_3. [DOI] [PubMed] [Google Scholar]
- 101.Elliott SR, Brennan AK, Beeson JG, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infection and Immunity. 2005;73(9):5903–5907. doi: 10.1128/IAI.73.9.5903-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guitard J, Andersen P, Ermont C, et al. Plasmodium falciparum population dynamics in a cohort of pregnant women in Senegal. Malaria Journal. 2010;9(6, article 165) doi: 10.1186/1475-2875-9-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salanti A, Resende M, Ditlev SB, et al. Several domains from VAR2CSA can induce Plasmodium falciparum adhesion-blocking antibodies. Malaria Journal. 2010;9(1, article 11) doi: 10.1186/1475-2875-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khunrae P, Dahlbäck M, Nielsen MA, et al. Full-length recombinant Plasmodium falciparum VAR2CSA binds specifically to CSPG and induces potent parasite adhesion-blocking antibodies. Journal of Molecular Biology. 2010;397(3):826–834. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infection and Immunity. 2003;71(11):6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng G, Aitken E, Yosaatmadja F, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. Journal of Infectious Diseases. 2009;200(2):299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McElroy PD, ter Kuile FO, Hightower AW, et al. All-cause mortality among young children in western Kenya. VI: the Asembo Bay Cohort Project. The American Journal of Tropical Medicine and Hygiene. 2001;64(1-2, supplement):18–27. doi: 10.4269/ajtmh.2001.64.18. [DOI] [PubMed] [Google Scholar]
- 108.Marinho CRF, Neres R, Epiphanio S, Gonçalves LA, Catarino MB, Penha-Gonçalves C. Recrudescent Plasmodium berghei from pregnant mice displays enhanced binding to the placenta and induces protection in multigravida. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005630. Article ID e5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiology and Infection. 2006;134(3):659–666. doi: 10.1017/S0950268805005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Walker-Abbey A, Djokam RRT, Eno A, et al. Malaria in pregnant Cameroonian women: the effect of age and gravidity on submicroscopic and mixed-species infections and multiple parasite genotypes. The American Journal of Tropical Medicine and Hygiene. 2005;72(3):229–235. [PubMed] [Google Scholar]
- 111.Schleiermacher D, Rogier C, Spiegel A, Tall A, Trape JF, Mercereau-Puijalon O. Increased multiplicity of Plasmodium falciparum infections and skewed distribution of individual MSP1 and MSP2 alleles during pregnancy in Ndiop, a senegalese village with seasonal, mesoendemic malaria. The American Journal of Tropical Medicine and Hygiene. 2001;64(5-6):303–309. doi: 10.4269/ajtmh.2001.64.303. [DOI] [PubMed] [Google Scholar]
- 112.Hay SI, Guerra CA, Gething PW, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Medicine. 2009;6(3) doi: 10.1371/journal.pmed.1000048. Article ID e1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. Journal of Infectious Diseases. 2005;191(11):1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Giobbia M, Tonon E, Zanatta A, Cesaris L, Bisoffi Z, Vaglia A. Late recrudescence of Plasmodium falciparum malaria in a pregnant woman: a case report. International Journal of Infectious Diseases. 2005;9(4):234–235. doi: 10.1016/j.ijid.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 115.Hviid L, Staalsoe T. Late recrudescence of Plasmodium falciparum malaria in pregnancy. International Journal of Infectious Diseases. 2006;10(5):p. 412. doi: 10.1016/j.ijid.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 116.Hviid L, Marinho CRF, Staalsoe T, Penha-Gonçalves C. Of mice and women: rodent models of placental malaria. Trends in Parasitology. 2010;26(8):412–419. doi: 10.1016/j.pt.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Staalsoe T, Shulman CE, Dorman EK, Kawuondo K, Marsh K, Hviid L. Intermittent preventive sulfadoxine-pyrimethamine treatment of primigravidae reduces levels of plasma immunoglobulin G, which protects against pregnancy-associated Plasmodium falciparum malaria. Infection and Immunity. 2004;72(9):5027–5030. doi: 10.1128/IAI.72.9.5027-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rogerson SJ, Wijesinghe RS, Meshnick SR. Host immunity as a determinant of treatment outcome in Plasmodium falciparum malaria. The Lancet Infectious Diseases. 2010;10(1):51–59. doi: 10.1016/S1473-3099(09)70322-6. [DOI] [PubMed] [Google Scholar]
- 119.Beeson JG, Mann EJ, Byrne TJ, et al. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. Journal of Infectious Diseases. 2006;193(5):721–730. doi: 10.1086/500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dahlbäck M, Rask TS, Andersen PH, et al. Epitope mapping and topographic analysis of VAR2CSA DBL3X involved in P. falciparum placental sequestration. PLoS Pathogens. 2006;2(11, article e124) doi: 10.1371/journal.ppat.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barfod L, Bernasconi NL, Dahlbäck M, et al. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Molecular Microbiology. 2007;63(2):335–347. doi: 10.1111/j.1365-2958.2006.05503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Piccinni M-P. T cell tolerance towards the fetal allograft. Journal of Reproductive Immunology. 2010;85(1):71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 123.Ashkar AA, Black GP, Wei Q, et al. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. Journal of Immunology. 2003;171(6):2937–2944. doi: 10.4049/jimmunol.171.6.2937. [DOI] [PubMed] [Google Scholar]
- 124.Argiĺs JM, Carbo N, Lpez-Soriano FJ. TNF and pregnancy: the paradigm of a complex interaction. Cytokine and Growth Factor Reviews. 1997;8(3):181–188. doi: 10.1016/s1359-6101(97)00012-9. [DOI] [PubMed] [Google Scholar]
- 125.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. Journal of Immunology. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 126.Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. Journal of Neuroimmunology. 2000;109(1):30–33. doi: 10.1016/s0165-5728(00)00299-x. [DOI] [PubMed] [Google Scholar]
- 127.Lea RG, Flanders KC, Harley CB, Manuel J, Banwatt D, Clark DA. Release of a transforming growth factor (TGF)-β2-related suppressor factor from postimplantation murine decidual tissue can be correlated with the detection of a subpopulation of cells containing RNA for TGF-β2. Journal of Immunology. 1992;148(3):778–787. [PubMed] [Google Scholar]
- 128.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunology Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 129.Thellin O, Coumans B, Zorzi W, Igout A, Heinen E. Tolerance to the foeto-placental ’graft’: ten ways to support a child for nine months. Current Opinion in Immunology. 2000;12(6):731–737. doi: 10.1016/s0952-7915(00)00170-9. [DOI] [PubMed] [Google Scholar]
- 130.Barrier BF, Gargiulo AR, Schust DJ. Reproductive immunology and its disorders. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology. Physiology, Pathophysiology and Clinical Management. Philadelphia, Pa, USA: Saunders Elsevier; 2009. pp. 299–309. [Google Scholar]
- 131.Taylor-Robinson AW. Regulation of immunity to Plasmodium: implications from mouse models for blood stage malaria vaccine design. Experimental Parasitology. 2010;126(3):406–414. doi: 10.1016/j.exppara.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 132.Beeson JG, Osier FHA, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends in Parasitology. 2008;24(12):578–584. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 133.Suguitan AL, Jr., Leke RGF, Fouda G, et al. Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. Journal of Infectious Diseases. 2003;188(7):1074–1082. doi: 10.1086/378500. [DOI] [PubMed] [Google Scholar]
- 134.Fievet N, Moussa M, Tami G, et al. Plasmodium falciparum induces a Th1/Th2 disequilibrium, favoring the Th1-type pathway, in the human placenta. Journal of Infectious Diseases. 2001;183(10):1530–1534. doi: 10.1086/320201. [DOI] [PubMed] [Google Scholar]
- 135.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. The American Journal of Tropical Medicine and Hygiene. 2003;68(1):115–119. [PubMed] [Google Scholar]
- 136.Taylor-Robinson AW, Smith EC. A dichotomous role for nitric oxide in protection against blood stage malaria infection. Immunology Letters. 1999;67(1):1–9. doi: 10.1016/s0165-2478(98)00148-5. [DOI] [PubMed] [Google Scholar]
- 137.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nature Medicine. 1998;4(9):1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 138.Kwak-Kim JYH, Gilman-Sachs A, Kim CE. T helper 1 and 2 immune responses in relationship to pregnancy, nonpregnancy, recurrent spontaneous abortions and infertility of repeated implantation failures. Chemical Immunology and Allergy. 2005;88(1):64–79. doi: 10.1159/000087821. [DOI] [PubMed] [Google Scholar]
- 139.Fried M, Muga RO, Misore AO, Duffy PE. Malaria elicits type 1 cytokines in the human placenta: IFN-γ and TNF-α associated with pregnancy outcomes. Journal of Immunology. 1998;160(5):2523–2530. [PubMed] [Google Scholar]
- 140.Moormann AM, Sullivan AD, Rochford RA, et al. Malaria and pregnancy: placental cytokine expression and its relationship to intrauterine growth retardation. Journal of Infectious Diseases. 1999;180(6):1987–1993. doi: 10.1086/315135. [DOI] [PubMed] [Google Scholar]
- 141.Maubert B, Guilbert LJ, Deloron P. Cytoadherence of Plasmodium falciparum to intercellular adhesion molecule 1 and chondroitin-4-sulfate expressed by the syncytiotrophoblast in the human placenta. Infection and Immunity. 1997;65(4):1251–1257. doi: 10.1128/iai.65.4.1251-1257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009;30(4):313–319. doi: 10.1016/j.placenta.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 143.Monzón-Bordonaba F, Vadillo-Ortega F, Feinberg RF. Modulation of trophoblast function by tumor necrosis factor-α: a role in pregnancy establishment and maintenance? American Journal of Obstetrics and Gynecology. 2002;187(6):1574–1580. doi: 10.1067/mob.2002.128028. [DOI] [PubMed] [Google Scholar]
- 144.Arechavaleta-Velasco F, Mayon-Gonzalez J, Gonzalez-Jimenez M, Hernandez-Guerrero C, Vadillo-Ortega F. Association of type II apoptosis and 92-kDa type IV collagenase expression in human amniochorion in prematurely ruptured membranes with tumor necrosis factor receptor-1 expression. Journal of the Society for Gynecologic Investigation. 2002;9(2):60–67. doi: 10.1016/s1071-5576(01)00159-9. [DOI] [PubMed] [Google Scholar]
- 145.Rogerson SJ, Brown HC, Pollina E, et al. Placental tumor necrosis factor alpha but not gamma interferon is associated with placental malaria and low birth weight in Malawian women. Infection and Immunity. 2003;71(1):267–270. doi: 10.1128/IAI.71.1.267-270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moore JM, Nahlen BL, Misore A, Lal AA, Udhayakumar V. Immunity to placental malaria. I. Elevated production of interferon-γ by placental blood mononuclear cells is associated with protection in an area with high transmission of malaria. Journal of Infectious Diseases. 1999;179(5):1218–1225. doi: 10.1086/314737. [DOI] [PubMed] [Google Scholar]
- 147.Kabyemela ER, Muehlenbachs A, Fried M, Kurtis JD, Mutabingwa TK, Duffy PE. Maternal peripheral blood level of IL-10 as a marker for inflammatory placental malaria. Malaria Journal. 2008;7(1, article 26) doi: 10.1186/1475-2875-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Davison BB, Kaack MB, Rogers LB, et al. The role of soluble tumor necrosis factor receptor types I and II and tumor necrosis factor-α in malaria during pregnancy. Journal of Infectious Diseases. 2006;194(1):123–132. doi: 10.1086/504694. [DOI] [PubMed] [Google Scholar]
- 149.Nmorsi OPG, Isaac C, Ohaneme BA, Obiazi HAK. Pro-inflammatory cytokines profiles in Nigerian pregnant women infected with Plasmodium falciparum malaria. Asian Pacific Journal of Tropical Medicine. 2010;3(9):731–733. [Google Scholar]
- 150.Chaisavaneeyakorn S, Lucchi N, Abramowsky C, et al. Immunohistological characterization of macrophage migration inhibitory factor expression in Plasmodium falciparum-infected placentas. Infection and Immunity. 2005;73(6):3287–3293. doi: 10.1128/IAI.73.6.3287-3293.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chaisavaneeyakorn S, Moore JM, Othoro C, et al. Immunity to placental malaria. IV. Placental malaria is associated with up-regulation of macrophage migration inhibitory factor in intervillous blood. Journal of Infectious Diseases. 2002;186(9):1371–1375. doi: 10.1086/344322. [DOI] [PubMed] [Google Scholar]
- 152.Chaiyaroj SC, Rutta ASM, Muenthaisong K, Watkins P, Na Ubol M, Looareesuwan S. Reduced levels of transforming growth factor-β1, interleukin-12 and increased migration inhibitory factor are associated with severe malaria. Acta Tropica. 2004;89(3):319–327. doi: 10.1016/j.actatropica.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 153.Diouf I, Fievet N, Doucouré S, et al. IL-12 producing monocytes and IFN-γ and TNF-α producing T-lymphocytes are increased in placentas infected by Plasmodium falciparum. Journal of Reproductive Immunology. 2007;74(1-2):152–162. doi: 10.1016/j.jri.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 154.Ismaili J, van der Sande M, Holland MJ, et al. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clinical and Experimental Immunology. 2003;133(3):414–421. doi: 10.1046/j.1365-2249.2003.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. Journal of Immunology. 1991;147(11):3815–3822. [PubMed] [Google Scholar]
- 156.Achidi EA, Apinjoh TO, Titanji VPK. Malaria parasitemia and systemic cytokine bias in pregnancy. International Journal of Gynecology and Obstetrics. 2007;97(1):15–20. doi: 10.1016/j.ijgo.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 157.Hviid L, Theander TG, Abdulhadi NH, Abu-Zeid YA, Bayoumi RA, Jensen JB. Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. European Journal of Immunology. 1991;21(5):1249–1253. doi: 10.1002/eji.1830210523. [DOI] [PubMed] [Google Scholar]
- 158.Megnekou R, Hviid L, Staalsoe T. Variant-specific immunity to Plasmodium berghei in pregnant mice. Infection and Immunity. 2009;77(5):1827–1834. doi: 10.1128/IAI.01321-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carter AM. Animal models of human placentation—a review. Placenta. 2007;28, supplement A:S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 160.Raghupathy R. Th1-type immunity is incompatible with successful pregnancy. Immunology Today. 1997;18(10):478–482. doi: 10.1016/s0167-5699(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 161.Riley EM, Schneider G, Sambou I, Greenwood BM. Suppression of cell-mediated immune responses to malaria antigens in pregnant Gambian women. The American Journal of Tropical Medicine and Hygiene. 1989;40(2):141–144. doi: 10.4269/ajtmh.1989.40.141. [DOI] [PubMed] [Google Scholar]
- 162.Fievet N, Tami G, Maubert B, et al. Cellular immune response to Plasmodium falciparum after pregnancy is related to previous placental infection and parity. Malaria Journal. 2002;1(1, article 16 ) doi: 10.1186/1475-2875-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Frevert U, Nardin E. Cellular effector mechanisms against Plasmodium liver stages. Cellular Microbiology. 2008;10(10):1956–1967. doi: 10.1111/j.1462-5822.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 164.Chandele A, Mukerjee P, Das G, Ahmed R, Chauhan VS. Phenotypic and functional profiling of malaria-induced CD8 and CD4 T cells during blood-stage infection with Plasmodium yoelii. Immunology. 2011;132(2):273–286. doi: 10.1111/j.1365-2567.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vinetz JM, Kumar S, Good MF, Fowlkes BJ, Berzofsky JA, Miller LH. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. Journal of Immunology. 1990;144(3):1069–1074. [PubMed] [Google Scholar]
- 166.Lurie S. Changes in age distribution of erythrocytes during pregnancy: a longitudinal study. Gynecologic and Obstetric Investigation. 1993;36(3):141–144. doi: 10.1159/000292613. [DOI] [PubMed] [Google Scholar]
- 167.Othoro C, Moore JM, Wannemuehler KA, et al. Elevated gamma interferon-producing NK cells, CD45RO memory-like T cells, and CD4 T cells are associated with protection against malaria infection in pregnancy. Infection and Immunity. 2008;76(4):1678–1685. doi: 10.1128/IAI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Parham P. The Immune System. 3rd edition. Abingdon, UK: Garland Science; 2009. [Google Scholar]
- 169.Kremsner PG, Winkler S, Wildling E, et al. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(1):44–47. doi: 10.1016/s0035-9203(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 170.Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. Journal of Immunology. 2003;171(10):5396–5405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- 171.Artavanis-Tsakonas K, Tongren JE, Riley EM. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clinical and Experimental Immunology. 2003;133(2):145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mavoungou E, Luty AJF, Kremsner PG. Natural killer (NK) cell-mediated cytolysis of Plasmodium falciparum-infected human red blood cells in vitro. European Cytokine Network. 2003;14(3):134–142. [PubMed] [Google Scholar]
- 173.Mavoungou E, Poaty-Mavoungou V, Touré FS, et al. Impairment of natural killer cell activity in Chlamydia trachomatis infected individuals. Tropical Medicine and International Health. 1999;4(11):719–727. doi: 10.1046/j.1365-3156.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 174.Sartelet H, Schleiermacher D, Le-Hesran JY, et al. Less HLA-G expression in Plasmodium falciparum-infected third trimester placentas is associated with more natural killer cells. Placenta. 2005;26(6):505–511. doi: 10.1016/j.placenta.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 175.Bouyou-Akotet MK, Issifou S, Meye JF, et al. Depressed natural killer cell cytotoxicity against Plasmodium falciparum-infected erythrocytes during first pregnancies. Clinical Infectious Diseases. 2004;38(3):342–347. doi: 10.1086/380646. [DOI] [PubMed] [Google Scholar]
- 176.Bouyou-Akotet MK, Mavoungou E. Natural killer cell IFN-γ activity is associated with Plasmodium falciparum infection during pregnancy. Experimental Parasitology. 2009;123(3):265–268. doi: 10.1016/j.exppara.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 177.Diouf I, Fievet N, Doucouré S, et al. Monocyte activation and T cell inhibition in Plasmodium falciparum-infected placenta. Journal of Infectious Diseases. 2004;189(12):2235–2242. doi: 10.1086/420791. [DOI] [PubMed] [Google Scholar]
- 178.Turrini F, Ginsburg H, Bussolino F, Pescarmona GP, Serra MV, Arese P. Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes: involvement of immune and non-immune determinants and dependence on parasite developmental stage. Blood. 1992;80(3):801–808. [PubMed] [Google Scholar]
- 179.Holmes CH, Simpson KL, Okada H, et al. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55) European Journal of Immunology. 1992;22(6):1579–1585. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- 180.Conroy A, Serghides L, Finney C, et al. C5a enhances dysregulated inflammatory and angiogenic responses to malaria in vitro: potential implications for placental malaria. PLoS ONE. 2009;4(3) doi: 10.1371/journal.pone.0004953. Article ID e4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunological Investigations. 2008;37(5-6):645–659. doi: 10.1080/08820130802191615. [DOI] [PubMed] [Google Scholar]
- 182.Silver KL, Higgins SJ, McDonald CR, Kain KC. Complement driven innate immune response to malaria: fuelling severe malarial diseases. Cellular Microbiology. 2010;12(8):1036–1045. doi: 10.1111/j.1462-5822.2010.01492.x. [DOI] [PubMed] [Google Scholar]
- 183.Albrecht EA, Chinnaiyan AM, Varambally S, et al. C5a-induced gene expression in human umbilical vein endothelial cells. The American Journal of Pathology. 2004;164(3):849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Imamura T, Sugiyama T, Cuevas LE, Makunde R, Nakamura S. Expression of tissue factor, the clotting initiator, on macrophages in Plasmodium falciparum-infected placentas. Journal of Infectious Diseases. 2002;186(3):436–440. doi: 10.1086/341507. [DOI] [PubMed] [Google Scholar]
- 185.Bouyou-Akotet MK, Adegnika AA, Agnandji ST, et al. Cortisol and susceptibility to malaria during pregnancy. Microbes and Infection. 2005;7(11-12):1217–1223. doi: 10.1016/j.micinf.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 186.Mavoungou E, Bouyou-Akotet MK, Kremsner PG. Effects of prolactin and cortisol on natural killer (NK) cell surface expression and function of human natural cytotoxicity receptors (NKp46, NKp44 and NKp30) Clinical and Experimental Immunology. 2005;139(2):287–296. doi: 10.1111/j.1365-2249.2004.02686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moffett-King A. Natural killer cells and pregnancy. Nature Reviews Immunology. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 188.Bayoumi NK, Elhassan EM, Elbashir MI, Adam I. Cortisol, prolactin, cytokines and the susceptibility of pregnant Sudanese women to Plasmodium falciparum malaria. Annals of Tropical Medicine and Parasitology. 2009;103(2):111–117. doi: 10.1179/136485909X385045. [DOI] [PubMed] [Google Scholar]
- 189.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. The American Journal of Tropical Medicine and Hygiene. 2004;71(2, supplement):41–54. [PubMed] [Google Scholar]
- 190.Mwapasa V, Rogerson SJ, Molyneux ME, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18(7):1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 191.Mount AM, Mwapasa V, Elliott SR, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. The Lancet. 2004;363(9424):1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 192.van Eijk AM, Ayisi JG, ter Kuile FO, et al. Human immunodeficiency virus seropositivity and malaria as risk factors for third-trimester anemia in asymptomatic pregnant women in Western Kenya. The American Journal of Tropical Medicine and Hygiene. 2001;65(5):623–630. doi: 10.4269/ajtmh.2001.65.623. [DOI] [PubMed] [Google Scholar]
- 193.Keen J, Serghides L, Ayi K, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. PLoS Medicine. 2007;4(5) doi: 10.1371/journal.pmed.0040181. Article ID e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mens PF, Bojtor EC, Schallig HDFH. Molecular interactions in the placenta during malaria infection. European Journal of Obstetrics Gynecology and Reproductive Biology. 2010;152(2):126–132. doi: 10.1016/j.ejogrb.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 195.Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clinical Obstetrics and Gynecology. 2006;49(2):257–269. doi: 10.1097/00003081-200606000-00008. [DOI] [PubMed] [Google Scholar]
- 196.Okoko BJ, Enwere G, Ota MOC. The epidemiology and consequences of maternal malaria: a review of immunological basis. Acta Tropica. 2003;87(2):193–205. doi: 10.1016/s0001-706x(03)00097-4. [DOI] [PubMed] [Google Scholar]
- 197.Umbers AJ, Boeuf P, Clapham C, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. Journal of Infectious Diseases. 2011;203(4):561–569. doi: 10.1093/infdis/jiq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Silver KL, Zhong K, Leke RGF, Taylor DW, Kain KC. Dysregulation of angiopoietins is associated with placental malaria and low birth weight. PLoS ONE. 2010;5(3) doi: 10.1371/journal.pone.0009481. Article ID e9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Etard JF, Kodio B, Ronsmans C. Seasonal variation in direct obstetric mortality in rural Senegal: role of malaria? The American Journal of Tropical Medicine and Hygiene. 2003;68(4):503–504. [PubMed] [Google Scholar]
- 200.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia through the looking glass backwards? Journal of Reproductive Immunology. 2005;65(1):1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 201.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS Medicine. 2006;3(11) doi: 10.1371/journal.pmed.0030446. Article ID e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schwarz NG, Adegnika AA, Breitling LP, et al. Placental malaria increases malaria risk in the first 30 months of life. Clinical Infectious Diseases. 2008;47(8):1017–1025. doi: 10.1086/591968. [DOI] [PubMed] [Google Scholar]
- 203.Bouyou-Akotet MK, Kombila M, Kremsner PG, Mavoungou E. Cytokine profiles in peripheral, placental and cord blood in pregnant women from an area endemic for Plasmodium falciparum. European Cytokine Network. 2004;15(2):120–125. [PubMed] [Google Scholar]
- 204.Hartman TK, Rogerson SJ, Fischer PR. The impact of maternal malaria on newborns. Annals of Tropical Paediatrics. 2010;30(4):271–282. doi: 10.1179/146532810X12858955921032. [DOI] [PubMed] [Google Scholar]
- 205.Hviid L, Staalsoe T. Malaria immunity in infants: a special case of a general phenomenon? Trends in Parasitology. 2004;20(2):66–72. doi: 10.1016/j.pt.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 206.Brabin B. Fetal anaemia in malarious areas: its causes and significance. Annals of Tropical Paediatrics. 1992;12(3):303–310. doi: 10.1080/02724936.1992.11747589. [DOI] [PubMed] [Google Scholar]
- 207.Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunology. 2001;23(2):51–59. doi: 10.1046/j.1365-3024.2001.00364.x. [DOI] [PubMed] [Google Scholar]
- 208.Bockhorst J, Lu F, Janes JH, et al. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Molecular and Biochemical Parasitology. 2007;155(2):103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 209.Oehler L, Kollars M, Bohle B, et al. Interleukin-10 inhibits burst-forming unit-erythroid growth by suppression of endogenous granulocyte-macrophage colony-stimulating factor production from T cells. Experimental Hematology. 1999;27(2):217–223. doi: 10.1016/s0301-472x(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 210.Tilg H, Ulmer H, Kaser A, Weiss G. Role of IL-10 for induction of anemia during inflammation. Journal of Immunology. 2002;169(4):2204–2209. doi: 10.4049/jimmunol.169.4.2204. [DOI] [PubMed] [Google Scholar]
- 211.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101(10):4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 212.Clark IA, Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. British Journal of Haematology. 1988;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 213.Leopardi O, Naughten W, Salvia L, et al. Malaric placentas: a quantitative study and clinico-pathological correlations. Pathology Research and Practice. 1996;192(9):892–900. doi: 10.1016/S0344-0338(96)80068-9. discussion 899–900. [DOI] [PubMed] [Google Scholar]
- 214.Guidotti RJ. Anaemia in pregnancy in developing countries. British Journal of Obstetrics and Gynaecology. 2000;107(4):437–438. doi: 10.1111/j.1471-0528.2000.tb13258.x. [DOI] [PubMed] [Google Scholar]
- 215.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. American Journal of Clinical Nutrition. 2000;72(1, supplement):247–256. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 216.Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. American Journal of Epidemiology. 2009;170(7):847–853. doi: 10.1093/aje/kwp207. [DOI] [PubMed] [Google Scholar]
- 217.Calleja-Agius J, Brincat MP. Recurrent miscarriages: what is the role of cytokines? Gynecological Endocrinology. 2008;24(12):663–668. doi: 10.1080/09513590802288275. [DOI] [PubMed] [Google Scholar]
- 218.Higgins MK. The structure of a chondroitin sulfate-binding domain important in placental malaria. Journal of Biological Chemistry. 2008;283(32):21842–21846. doi: 10.1074/jbc.C800086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Singh K, Gittis AG, Nguyen P, Gowda DC, Miller LH, Garboczi DN. Structure of the DBL3x domain of pregnancy-associated malaria protein VAR2CSA complexed with chondroitin sulfate A. Nature Structural and Molecular Biology. 2008;15(9):932–938. doi: 10.1038/nsmb.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fernandez P, Petres S, Mécheri S, Gysin J, Scherf A. Strain-transcendent immune response to recombinant VAR2CSA DBL5-ε domain block P. falciparum adhesion to placenta-derived BeWo cells under flow conditions. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012558. Article ID e12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Nielsen MA, Pinto VV, Resende M, et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infection and Immunity. 2009;77(6):2482–2487. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Avril M, Cartwright MM, Hathaway MJ, et al. Immunization with VAR2CSA-DBL5 recombinant protein elicits broadly cross-reactive antibodies to placental Plasmodium falciparum-infected erythrocytes. Infection and Immunity. 2010;78(5):2248–2256. doi: 10.1128/IAI.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fernandez P, KViebig N, Dechavanne S, et al. Var2CSA DBL6-ε domain expressed in HEK293 induces limited cross-reactive and blocking antibodies to CSA binding parasites. Malaria Journal. 2008;7(9, article 170) doi: 10.1186/1475-2875-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yosaatmadja F, Andrews KT, Duffy MF, Brown GV, Beeson JG, Rogerson SJ. Characterization of VAR2CSA-deficient Plasmodium falciparum-infected erythrocytes selected for adhesion to the BeWo placental cell line. Malaria Journal. 2008;7(3, article 51) doi: 10.1186/1475-2875-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Magistrado PA, Minja D, Doritchamou J, et al. High efficacy of anti DBL4ε-VAR2CSA antibodies in inhibition of CSA-binding Plasmodium falciparum-infected erythrocytes from pregnant women. Vaccine. 2011;29(3):437–443. doi: 10.1016/j.vaccine.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 226.Avril M, Cartwright MM, Hathaway MJ, Smith JD. Induction of strain-transcendent antibodies to placental-type isolates with VAR2CSA DBL3 or DBL5 recombinant proteins. Malaria Journal. 2011;10(1, article 36) doi: 10.1186/1475-2875-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Avril M, Hathaway MJ, Srivastava A, et al. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0016622. Article ID e16622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Dahlbäck M, Jørgensen LM, Nielsen MA, et al. The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains. Journal of Biological Chemistry. 2011;286(18):15908–15917. doi: 10.1074/jbc.M110.191510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pinto VV, Ditlev SB, Jensen KE, et al. Differential induction of functional IgG using the Plasmodium falciparum placental malaria vaccine candidate VAR2CSA. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017942. Article ID e17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. Journal of Clinical Investigation. 2010;120(12):4168–4178. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Casares S, Brumeanu TD, Richie TL. The RTS,S malaria vaccine. Vaccine. 2010;28(31):4880–4894. doi: 10.1016/j.vaccine.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 232.The RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. The New England Journal of Medicine. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 233.Butler D. Malaria vaccine results face scrutiny. Nature. 2011;478(7370):439–440. doi: 10.1038/478439a. [DOI] [PubMed] [Google Scholar]
- 234.Parakh A, Agarwal N, Aggarwal A, Aneja A. Plasmodium vivax malaria in children: uncommon manifestations. Annals of Tropical Paediatrics. 2009;29(4):253–256. doi: 10.1179/027249309X12547917868844. [DOI] [PubMed] [Google Scholar]