Abstract
To investigate whether curcumin is protective against intracellular amyloid β (Aβ) toxicity, different concentrations of curcumin were applied to with intracellular Aβ in rat primary hippocampal neurons in culture. We find that at low dosages, curcumin effectively inhibits intracellular Aβ toxicity. Reactive oxidative species (ROS) is involved in mediating intracellular Aβ toxicity and possibly curcumin protection. Our results indicate that oxidative stress may mediate cell death induced by intracellular Aβ in neurons.
Keywords: Amyloid, curcumin, ROS, toxicity, Alzheimer's disease
Introduction
Alzheimer's disease (AD) features with neuronal/synaptic loss, extracellular senile plaques (SPs) and intracellular neurofibrillary tangles (NFTs). The components of senile plaques are identified as amyloid β (Aβ) peptides. Although at the late stage, Aβ depositions are observed mainly located extracellularly, the accumulation of iAβ has been observed in various systems. IAβ1-42 significantly accumulates in the pyramidal neurons of the hippocampus and the entorhinal cortex in mild cognitive impairment and AD patients at early stage [1-7]. IAβ1-42 deposition appears earlier than SP formation [1, 2, 4, 5, 8]. In addition, accumulation of iAβ1-42 is reported in several cell culture systems [9, 10]. IAβ is also observed in the APP mutant mice where synaptic loss happens before the presence of extracellular Aβ (eAβ) [11, 12]. Microinjection of intracellular Aβ1-42 into neurons induces remarkable cell death mediated by the activation of p53, Bax and caspase-6 [13, 14]. Intracellular Aβ1-42 also causes electrophysiological property changes in human primary neurons [15]. Several reagents, such as androgen [16], estrogen [16], galanin [17] and morphine [18], can protect against iAβ toxicity in human and rodent neurons.
Curcumin, the yellow pigment of turmeric, a phenolic compound, acts as a chain-breaking molecule by scavenging nitrogen oxide and superoxide anion [12-14]. It has been shown to protect against extracellular Aβ25-35 toxicity in rat primary neurons from the frontal cortex [19]. Here, we first delivered intracellular Aβ1-42 into the cultured rat primary hippocampal neurons by virus-mediated manner. We find that curcumin can protect against iAβ1-42 toxicity. Reactive oxidative species (ROS) is involved in iAβ1-42 toxicity and curcumin protection.
Methods and materials
Cell culture
Rat primary neurons were cultured from new born Sprague-Dawley rat hippocampus, following the regulations of Peking University Animal Care and Use Committee. In brief, fresh rat hippocampal tissues were dissociated with 0.25% trypsin (Invitrogen, Carlsbad, CA), which was then inactivated by 10% decomplemented fetal bovine serum (FBS, HyClone, Logan, UT). The mixture was triturated through pipette to make a homogenous mixture. After filtering the mixture through 70 μm sterilized filters, the flow-through was centrifuged. The pellet was then washed once by phosphate buffered saline (PBS) and once by Dulbecco's modified Eagle's medium (DMEM) containing 0.225% sodium bicarbonate, 1 mM sodium pyruvate, 2 mM L-glutamine, 0.1% dextrose, 1× antibiotic Pen-Strep (all from Invitrogen, Carlsbad, CA) with 5% fetal bovine serum (FBS). Cells were then plated on poly-L-lysine (Sigma, St. Louis, MO) coated plates or glass coverslips at the density of 1 × 105 cells/ml. Neurons were incubated at 37°C in DMEM without phenol red with 5% FBS and with 5% circulating CO2. Cytarabine was added to culture media 24 hours after plating at 10 μM to inhibit dividing cell growth. Medium was changed every 48 hours. Cells were treated for experiments at 7 days in culture.
Adeno-virus infection
IAβ1-42 [20] was subcloned from pEGFP-N3 into pAdTrack with BglII and XhoI digestions. Adeno-virus was packaged in HEK293 cells and the infectious particle was measured as 2 × 106 particles/ml (MOI=1.33). To infection of cell cultures, the purified virus supernatant was added to cell culture medium at the dilution of 1:500 for 24 hours.
Chemical treatments
Curcumin (Sigma, MO) was added freshly into culture medium during treatments. MitoSOX™ Red mitochondrial superoxide indicator (Molecular Probes, M36008) was used to measure ROS as described by the manufacturer.
Measurement of neuronal cell death and viability
Cells were fixed in fresh 4% paraformaldehyde, 4% sucrose in PBS for 20 minutes at room temperature and permeabilized in 0.1% Triton X-100, 0.1% sodium citrate in PBS for 2 minutes on ice. Terminal deoxynucleotidyl transferase-biotin dUTP nick-end labeling (TUNEL) staining was performed using the in situ cell death detection kit I as described by the manufacturer (Roche, Quebec, Canada). The coverslips were then washed once in distilled water for 5 minutes and mounted on glass slides to be observed under a fluorescence microscope.
The cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), which correlates cell number with mitochondrial reduction of MTT to a blue formazan precipitate [21]. In brief, cells were plated in 96-well plates beforehand. The medium was then replaced with fresh medium containing 1 mg/ml MTT. Following incubation at 37°C for 4 h, the wells were aspirated, the dye was solubilized in DMSO and the absorbency was measured at 595 nm. The viability of cells was compared with that of control cells. Lactate dehydrogenase (LDH) release was measured using the LDH Cytotoxicity Detection Kit (Roche, Quebec, Canada) according to the manufacturer's instructions.
Statistical evaluation
Statistical significance was assessed by oneway analysis of variances (ANOVA). The Sheffé's test was applied as a post hoc for the significant difference shown by ANOVAs. A p value of less than 0.05 or 0.01 was used as an indicative of statistical significance.
Results
Curcumin protected against intracellular Aβ toxicity
Rat primary hippocampal neurons in culture were confirmed by staining with β-tubulin-III, a neuronal marker (Figure 1A and 1B). The infection of by adeno-5 virus packaged with iAβ1-42 achieved around 20%-30% efficiency in these cultures (Figure 1C). When expressed intracellularly [15], Aβ1-42 induced around 50% decrease in cell viability shown by MTT assay compared with empty vector infection alone (Figure 1D). Curcumin was applied to the culture medium with iAβ1-42 infection. After 24 hours of incubation, curcumin at various concentrations had either protective or destructive effects on neurons (Figure 1E). At low dosages (1-50 μM), curcumin increased cell viability while at dosages higher than 80 μM, curcumin decreased cell viability, suggesting that curcumin at certain concentrations, can be protective against iAβ1-42 toxicity in rat primary neurons. The protection of curcumin was further confirmed by application of 30 μM of curcumin to the culture medium. Curcumin significantly increased cell viability indicated by LDH release assay (Figure 2A) and decreased cell death measured by TUNEL assay (Figure 2B).
Figure 1.
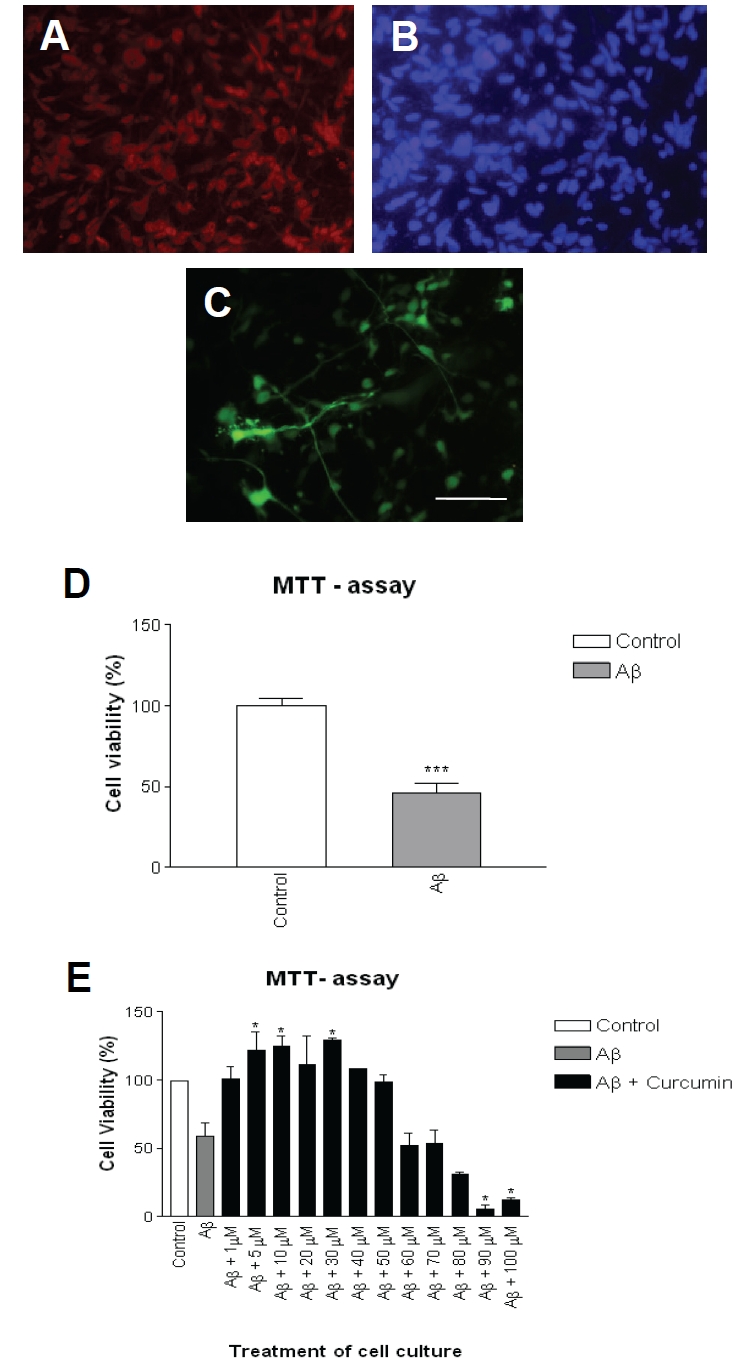
Intracellular Aβ1-42 induced toxicity in rat primary neurons. A. Rat hippocampal neurons in culture were stained with neuronal marker b-tubulin -III. B. Neurons were stained with DAPI to show the whole population. C. Around 20-30% neurons were infected indicated by EGFP positive. Scale bar: 100 μm. D. Cell viability was indicated by MTT assay at 24 hours after infection. E. Cell viability was indicated by MTT assay at 24 hours after infection and treatment. Data represented mean + SE (n=200 cells/ preparation, each experiment was repeated in 3 preparations). ***: p < 0.001 compared with control group; *: p < 0.05 compared with control group.
Figure 2.
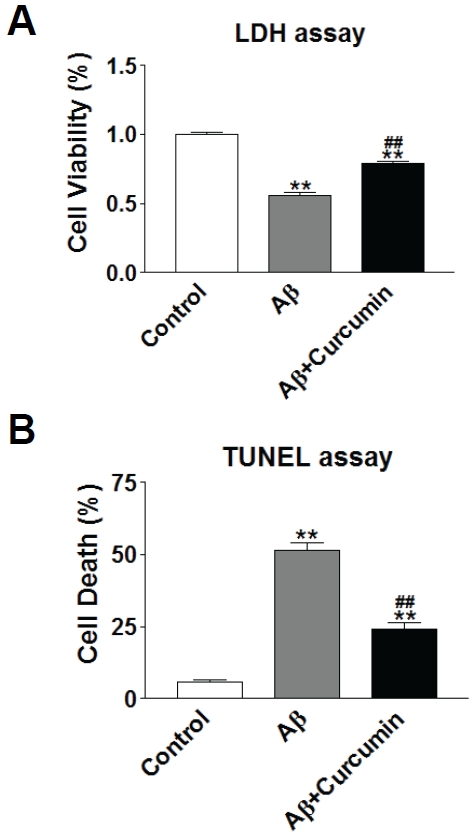
Curcumin protected against intracellular Aβ1 -42 toxicity. A. Cell viability was indicated by LDH release assay at 24 hours after infection and treatment. B. Cell death was indicated by TUNEL assay at 24 hours after infection and treatment. **: p<0.01 compared with control group. ##: p<0.01 compared with Aβ group.
ROS was involved in intracellular Aβ toxicity
To further investigate the possible mechanism of intracellular Aβ toxicity, we examined if ROS was involved since evidence showed Aβ interacted with mitochondria function [22]. Relative ROS levels were measured in the control (Figure 3A and 3B), iAβ1-42 infected (Figure 3C and 3D) and iAβ1-42 with curcumin treatment (Figure 3E and 3F) groups. ROS levels increased remarkably with iAβ1-42 infection, whereas curcumin treatment dramatically decreased ROS level (Figure 3G), suggesting that ROS played an important role mediating intracellular Aβ toxicity in rat primary neurons and curcumin may play protective role through lowering down ROS levels.
Figure 3.
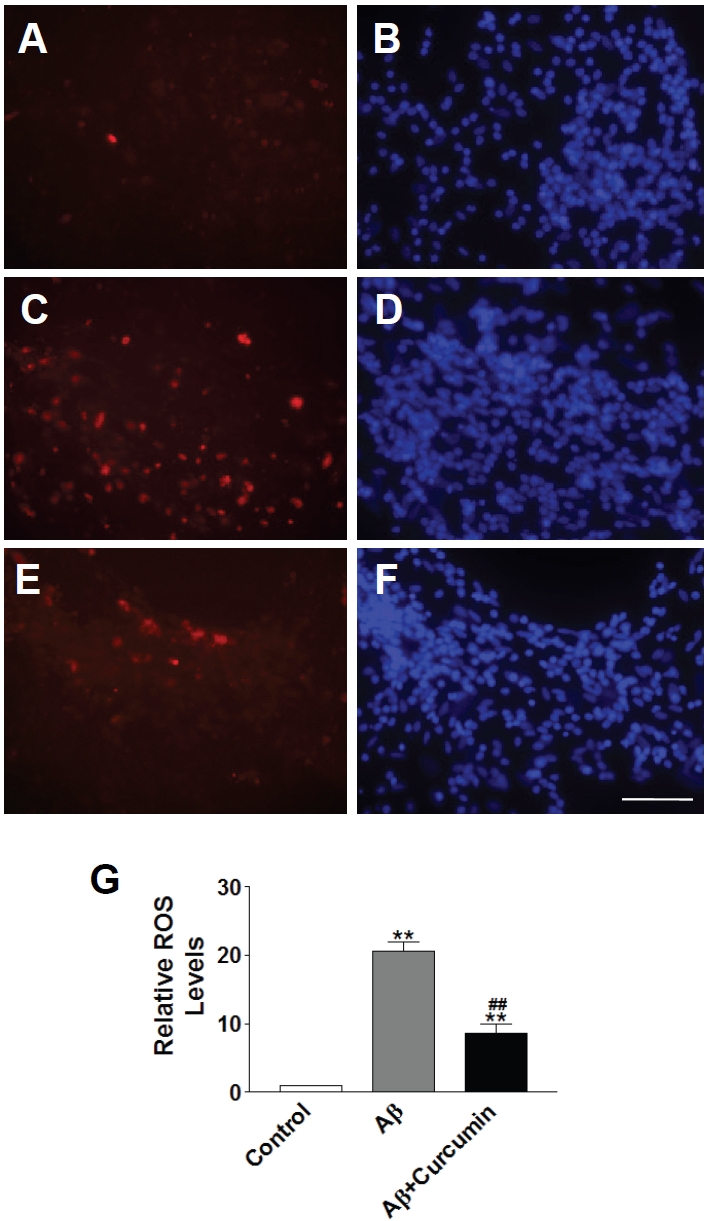
ROS was involved in intracellular Aβ1-42 toxicity. A. ROS level stained with MitoSOX™ Red mitochondrial superoxide indicator in control neurons. B. DAPI staining indicated cell population in control neurons. C. ROS level stained with MitoSOX™ Red mitochondrial superoxide indicator in Aβ-infected neurons. D. DAPI staining indicated cell population in Aβ-infected neurons. E. ROS level stained with MitoSOX™ Red mitochondrial superoxide indicator in Aβ-infected neurons in the presence of curcumin. F. DAPI staining indicated cell population in Aβ-infected neurons in the presence of curcumin. Scale bar: 100 μm. G. Quantification of relative ROS levels in neurons. Data represented mean+SE (n=500 cells/preparation, each experiment was repeated in 3 preparations). **: p<0.01 compared with control group. ##: p<0.01 compared with Aβ group.
Discussion
Our results of the present study indicate that curcumin can protect against intracellular Aβ1-42 -induced cytotoxicity in rat primary hippocampal neurons, which is consistent with our previous data that curcumin inhibits Aβ25-35-induced cytotoxicity in rat primary prefrontal cortex neurons [19]. Other studies also support the beneficial role of curcumin. Curcumin decreases the levels of soluble and insoluble Aβ in transgenic APPswe mice [23]. Similar protection of curcumin is also reported in animal model with human Aβ infused with lipoprotein chaperone into the cerebral ventricles [24]. Curcumin treatment reverses the change of synaptophysin and post-synaptic density 95 (PSD-95) as well as impaired performance in water maze test [25]. Curcumin binds to SPs in the brain tissues when fed or injected in the carotid artery in Tg2576 mice [25]. These data suggest that curcumin crosses the blood-brain barrier and plays anti-amyloid roles, which makes curcumin a potential drug candidate for amyloid hypothesis-based therapy. A phase II, double-blind, placebo -controlled study of curcumin safety and tolerability in human AD patients is undergoing [25]. Our data from this study confirm that curcumin is protective against intracellular Aβ toxicity in neuronal cultures.
Evidence supports that neurotoxicity induced by intracellular Aβ is partially caused by the formation of ROS, leading to increase of oxidative stress. It is showed that endocytosed Aβ, through binding with specific potential receptors, such as the receptor for advanced glycation end products (RAGE) and the class A scavenger-receptor, leads to increased reactive oxygen production [26-28]. In the other hand, ROS may trigger a positive feedback mechanism for intracellular Aβ toxicity. Oxidative stress can influence Aβ production by interacting with amyloid precursor protein [29]. Indirectly, oxidative stress can also influence Aβ processing by modulating the activity and levels of β-secretase and γ-secretase [26, 30, 31]. Curcumin is suggested to be a more potent anti-oxidant than vitamin E α-tocopherol [32]. Our data suggest that ROS is involved in intracellular Aβ toxicity and curcumin may play its protective role through decreasing ROS levels in neurons. Taken together, our study indicates that curcumin may be beneficial to AD pathology.
Acknowledgments
This work was supported by the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2009CB941301), Roche Research Grant, Peking University President Research Grant and Ministry of Education Recruiting Research Grant.
References
- 1.Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK. Intraneuronal Alzheimer Aβ42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol. 2002;161:1869–1879. doi: 10.1016/s0002-9440(10)64463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Andrea MR, Nagele RG, Gumula NA, Reiser PA, Polkovitch DA, Hertzog BM, Andrade-Gordon P. Lipofuscin and Aβ42 exhibit distinct distribution patterns in normal and Alzheimer's disease brains. Neurosci Lett. 2002;323:45–49. doi: 10.1016/s0304-3940(01)02444-2. [DOI] [PubMed] [Google Scholar]
- 4.D'Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DH. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer's disease. Histopathology. 2001;38:120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- 5.Tabira T, Chui DH, Kuroda S. Significance of intracellular Abeta42 accumulation in Alzheimer's disease. Front Biosci. 2002;7:a44–49. doi: 10.2741/tabira. [DOI] [PubMed] [Google Scholar]
- 6.Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirota ni K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- 7.Nagele RG, D'Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of β-amyloid(1-42) in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 8.Wang HY, D'Andrea MR, Nagele RG. Cerebellar diffuse amyloid plaques are derived from dendritic Aβ42 accumulations in Purkinje cells. Neurobiol Aging. 2002;23:213–223. doi: 10.1016/s0197-4580(01)00279-2. [DOI] [PubMed] [Google Scholar]
- 9.Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P, Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc Natl Acad Sci USA. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang AJ, Chandswangbhuvana D, Margol L, Glabe CG. Loss of endosomal/lysosomal membrane impermeability is an early event in amyloid Aβ1-42 pathogenesis. J Neurosci Res. 1998;52:691–698. doi: 10.1002/(SICI)1097-4547(19980615)52:6<691::AID-JNR8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Masliah E, Sisk A, Mallory M, Mucke L, Schenk D, Games D. Comparison of neurodegen-erative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer's disease. J Neurosci. 1996;16:5795–5811. doi: 10.1523/JNEUROSCI.16-18-05795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid (3 peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156:519–529. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M, Chen L, Lee DH, Yu LC, Zhang Y. The role of intracellular amyloid β in Alzheimer's disease. Prog Neurobiol. 2007;83:131–139. doi: 10.1016/j.pneurobio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Hou JF, Cui J, Yu LC, Zhang Y. Intracellular amyloid induces impairments on electrophysiological properties of cultured human neurons. Neurosci Lett. 2009;462:294–299. doi: 10.1016/j.neulet.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid β1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui J, Chen Q, Yue X, Jiang X, Gao GF, Yu LC, Zhang Y. Galanin protects against intracellular amyloid toxicity in human primary neurons. J Alzheimers Dis. 2010;19:529–544. doi: 10.3233/JAD-2010-1246. [DOI] [PubMed] [Google Scholar]
- 18.Cui J, Wang Y, Dong Q, Wu S, Xiao X, Hu J, Chai Z, Zhang Y. Morphine Protects against Intracellular Amyloid Toxicity by Inducing Estradiol Release and Upregulation of Hsp70. J Neurosci. 2011;31:16227–16240. doi: 10.1523/JNEUROSCI.3915-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin XY, Cheng Y, Cui J, Zhang Y, Yu LC. Potential protection of curcumin against amyloid β -induced toxicity on cultured rat prefrontal cortical neurons. Neurosci Lett. 2009;463:158–161. doi: 10.1016/j.neulet.2009.07.047. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Goodyer C, LeBlanc A. Selective and protracted apoptosis in human primary neurons microinjected with active caspase-3, -6, -7, and -8. J Neurosci. 2000;20:8384–8389. doi: 10.1523/JNEUROSCI.20-22-08384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Pagani L, Eckert A. Amyloid-β interaction with mitochondria. Int J Alzheimers Dis. 2011;2011:925050. doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 β is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 25.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pratico D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann N Y Acad Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 27.Strydom A, Dickinson MJ, Shende S, Pratico D, Walker Z. Oxidative stress and cognitive ability in adults with Down syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:76–80. doi: 10.1016/j.pnpbp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-β peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 29.Multhaup G, Scheuermann S, Schlicksupp A, Simons A, Strauss M, Kemmling A, Oehler C, Cappai R, Pipkorn R, Bayer TA. Possible mechanisms of APP-mediated oxidative stress in Alzheimer's disease. Free Radic Biol Med. 2002;33:45–51. doi: 10.1016/s0891-5849(02)00806-7. [DOI] [PubMed] [Google Scholar]
- 30.Pratico D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Fukui K, Takatsu H, Shinkai T, Suzuki S, Abe K, Urano S. Appearance of amyloid β-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J Alzheimers Dis. 2005;8:299–309. doi: 10.3233/jad-2005-8309. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Park SY, Kim JK. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from βA(1-42) insult. Neurosci Lett. 2001;303:57–61. doi: 10.1016/s0304-3940(01)01677-9. [DOI] [PubMed] [Google Scholar]


