Abstract
Transfer RNAs (tRNAs) encoded by the nuclear genome are surprisingly dynamic. Although tRNAs function in protein synthesis occurring on cytoplasmic ribosomes, tRNAs can transit from the cytoplasm to the nucleus and then again return to the cytoplasm by a process known as the tRNA retrograde process. Subsets of the cytoplasmic tRNAs are also imported into mitochondria and function in mitochondrial protein synthesis. The numbers of tRNA species that are imported into mitchondria differ among organisms, ranging from just a few to the entire set needed to decode mitochondrially encoded mRNAs. For some tRNAs, import is dependent on the mitochondrial protein import machinery, whereas the majority of tRNA mitochondrial import is independent of this machinery. Although cytoplasmic proteins and proteins located on the mitochondrial surface participating in the tRNA import process have been described for several organisms, the identity of these proteins differ among organisms. Likewise, the tRNA determinants required for mitochondrial import differ among tRNA species and organisms. Here, we present an overview and discuss the current state of knowledge regarding the mechanisms involved in the tRNA retrograde process and continue with an overview of tRNA import into mitochondria. Finally, we highlight areas of future research to understand the function and regulation of movement of tRNAs between the cytoplasm and organelles.
Keywords: nuclear tRNA import, nuclear tRNA export, mitochondrial tRNA import, nucleus, mitochondria
tRNAs encoded by nuclear genomes are transcribed in the nucleus and are then exported to the cytoplasm where they perform their essential function of delivering amino acids to growing polypeptide chains as specified by mRNA codons. Since protein synthesis occurs in the cytoplasm, it was a surprising discovery that cytoplasmic tRNAs can travel in a reverse direction from the cytoplasm to the nucleus via the tRNA retrograde process – a process that is conserved in yeast and vertebrate cells 1–4. Here we discuss the possible functions of the tRNA retrograde process, describe its regulation, and explore the mechanism(s) of tRNA nuclear import. Protein synthesis also occurs in organelles-mitochondria and chloroplasts. Mitochondrial genomes of some organisms encode all the tRNAs necessary for organellar protein synthesis; mitochondrial genomes of other organisms do not encode a complete set of tRNAs or, in some cases, completely lack tRNA genes, thereby requiring import of tRNAs encoded by the nucleus in order for protein synthesis to occur. It appears that all organisms are able to import tRNAs from the cytoplasm to mitochondria, regardless of whether a complete set of tRNAs is encoded by the organellar genome 5. In addition to the systems to import cytoplasmic tRNAs into mitochondria, in at least one organism, S. cerevisiae, removal of introns from pre-tRNAs occurs on the mitochondrial outer surface 6, 7 and thus pre-tRNAs exported from the nucleus must be located to the mitochondrial surface. Here, we also discuss the different mechanisms by which tRNAs are imported from the cytoplasm to and into mitochondria. Finally, we highlight areas of future research in tRNA subcellular dynamics.
tRNA MOVEMENT BETWEEN THE NUCLEUS AND THE CYTOPLASM
In contrast to previous dogma that tRNA movement is unidirectional, nucleus to cytoplasm, it is now known that tRNAs move bi-directionally between the nucleus and the cytoplasm and from the cytoplasm to and into organelles (Fig. 1). tRNA bi-directional movement between the nucleus and the cytoplasm occurs in both yeast and vertebrate cells 1–4. The movement of tRNA between the nucleus and the cytoplasm consist of three steps. The first step, initial tRNA export, delivers newly transcribed and partially or fully processed tRNAs to the cytoplasm. The second step, tRNA retrograde nuclear import, is the movement of cytoplasmic tRNAs into the nucleus. Finally, tRNAs that had been imported into the nucleus from the cytoplasm are able to return to the cytoplasm by the step know as tRNA re-export 8.
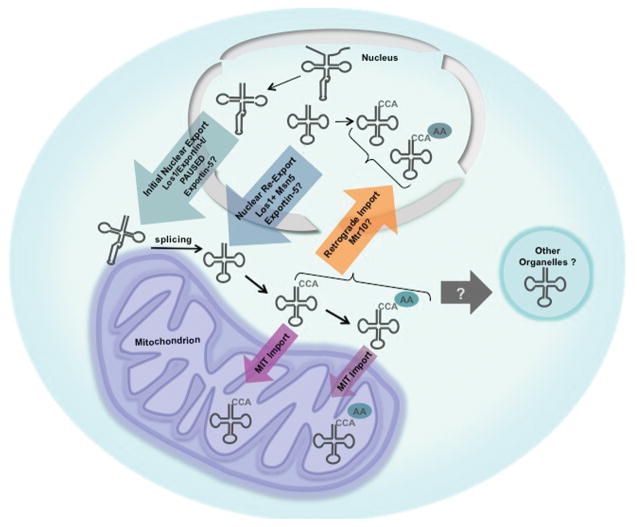
Figure 1.
Movement of tRNAs in eukaryotic cells. Nuclear encoded tRNAs are transcribed and largely processed in the nucleus. In yeast, but not in vertebrate cells, tRNAs encoded by intron-containing genes, are exported (initial tRNA export; teal arrow) to the cytoplasm prior to being spliced. Splicing occurs on the outer surface of mitochondria. tRNAs in the cytoplasm are able to return to the nucleus via retrograde import (orange arrow) and, under nutrient replete conditions, the tRNAs can be re-exported to the cytoplasm (blue arrow). A subset of tRNAs can be imported into the mitochondrial matrix, past the outer and inner mitochondrial membranes (purple arrows). It is unknown whether cytoplasmic tRNAs are imported into other organelles such as chloroplasts (grey arrow).
tRNA nuclear export
The Ran-GTP binding β-importin family which functions in most macromolecular trafficking between the nucleus and the cytoplasm plays a key role in tRNA nuclear/cytoplasmic dynamics as at least two family members participate in tRNA nuclear export: (1) yeast Los1/vertebrate Exportin-t/plant PAUSED and (2) yeast Msn5/vertebrate Exportin-5, (Figure 1). For yeast it is easy to distinguish the roles of proteins in the tRNA initial vs. re-export steps because the removal of introns from pre-tRNAs occurs in the cytoplasm on the outer mitochondrial surface 6, 7 (Figure 1). Thus, tRNAs experiencing the initial tRNA nuclear export step contain introns (if the tRNAs are encoded by intron-containing genes), whereas tRNAs re-exiting the nucleus do not contain introns as they were spliced in the cytoplasm before being imported into the nucleus. By this type of analysis Los1 was shown to participate in both the initial and the re-export steps, whereas Msn5 participates only in the tRNA re-export step, at least for tRNAs encoded by intron-containing genes 9.
Los1/Exportin-t/PAUSED is the primary tRNA exporter in yeast, vertebrate, and plant cells 10–13; however, insects lack a Los1/Exportin-t ortholog 14. Substrate binding studies demonstrated that vertebrate Exportin-t preferentially interacts with tRNAs with mature 5′ and 3′ termini with appropriate tertiary structure 15, 16. These results were recently verified by a 3.2 Å resolution structural study of S. pombe Los1/Exportin-t, Xpo-t, in complex with Ran-GTP and tRNA which documented that Xpo-t contacts the tRNA acceptor arm and the TΨC and D loops, leaving the anticodon loop exposed 17.
Msn5/Exportin-5/HASTY serves multiple functions. In addition to its role in tRNA re-export in yeast 2, 9, 18 and tRNA export in insects 14, in yeast it also serves as a nuclear exporter for several transcription regulatory proteins, dependent upon their appropriate phosphorylation 19. In plants and vertebrate cells Exportin-5/HASTY serves primarily in the nuclear export of pre-microRNAs 20–22, though it has also been implicated as a minor exporter of mature tRNAs in vertebrate cells 23, 24. Although there are structural studies for the interaction of exportin-5 with microRNAs, tRNA-exportin-5 structures have not been reported 25.
Los1 and Msn5 and their orthologues are unessential in budding yeast and the other model organisms in which they have been deleted 2, 26–29; even yeast lacking both Los1 and Msn5 are healthy 2. Thus, not all the gene products that function in tRNA nuclear export have been identified.
tRNA nuclear import
Why import tRNAs into the nucleus?
Retrograde movement of tRNA from the cytoplasm is conserved in yeast and vertebrate cells, indicating that this dynamic behavior serves an important function(s). Moreover, the Fassati group proposed that HIV usurped the tRNA retrograde pathway as one mechanism to move reverse transcribed complexes through nuclear pores to the genome of nondividing neuronal cells 3. Hints as to the cellular function of tRNA nuclear import come from the yeast studies in which nuclear accumulation of previously cytoplasmic tRNA occurs under particular conditions: (1) tRNAs missing 3′ CCA nucleotides 2, 30; (2) defects in tRNA aminoacylation 31–34; (3) nutrient deprivation of amino acids 1, glucose 8, or phosphate 35. Nuclear accumulation of previously cytoplasmic tRNA was also reported for rat hepatoma cells upon amino acid deprivation 4. The results support two different possible roles for the tRNA retrograde process – tRNA quality control and regulation of protein synthesis in response to nutrient status.
The need for tRNA quality control might result from the fact that tRNAs are long-lived and may suffer damage in the cytoplasm, such as loss of the 3′-CCA extension. Indeed tRNAs with damaged CCA termini are imported into both yeast and HeLa cell nuclei 2, 3, 30, in contrast to unstructured tRNAs which are not imported into nuclei in permeabilized HeLa cells 3. Importing such damaged tRNAs into the nucleus would remove them from the pool of proteins that interact with tRNAs for protein synthesis. However, since the CCA adding enzyme is both cytoplasmic and nuclear 36, it is difficult to understand why such damaged tRNAs would not be repaired in the cytoplasm. Another role in tRNA quality control might result because many tRNA cleavages and modifications occur solely in the nucleus 37 and there may be competition between completing tRNA processing and tRNA nuclear export, sometimes erroneously resulting in export of end-extended and/or hypomodified tRNAs. Retrograde nuclear import of such putative tRNAs would remove them from the translation machinery and could provide a 2nd opportunity for completion of processing. If the retrograde import step serves a 2nd opportunity role, it is also likely to be in competition with the two known tRNA turnover pathways - the nuclear and the cytoplasmic 5′>3′ tRNA exonucleolytic rapid tRNA decay, RTD, pathway that degrades mature tRNAs with “loose” tertiary structure due to hypomodification 29, 38 and the nuclear polyadenylation-TRAMP 3′>5′ nuclear exosome Rex1 pathway that degrades pre-tRNAMeti lacking m1A and other misfolded or unprocessed RNA 39–41. These ideas regarding roles for the tRNA retrograde pathway in tRNA quality control await testing.
Accumulation of previously cytoplasmic tRNA in the nucleus when cells are deprived of nutrients or when tRNAs are uncharged is consistent with the idea that tRNAs might accumulate in the nucleus under conditions that protein synthesis should be down-regulated, as has been proposed for the movement of mRNAs to P-bodies upon glucose deprivation 42. Because tRNAs re-enter the cytoplasm upon re-feeding and mRNAs can move back to polysomes upon addition of glucose 8, 42, translation could be quickly restored when nutrients are replete, providing an inexpensive and quick process to control the rate of protein synthesis or the constellation of proteins translated. It should be noted that tRNA charging, though necessary, is not sufficient for tRNA re-export 8. To date, tests of the hypothesis that the tRNA retrograde nuclear import serves as a novel mechanism to regulate protein synthesis in response to nutrient deprivation has not been reported.
There are other possibilities for the function of tRNA nuclear import. For example, a previous study claimed that protein synthesis can occur in the nucleus 43 which would require the presence of mature charged nuclear tRNAs; however, this study has been called into question 44. Since tRNAs serve roles in addition to their essential function in protein synthesis such as targeting proteins for degradation via the N-end rule pathway 45, signaling in the general amino acid control pathway 46, regulation of apoptosis by binding cytochrome C 47, and as reverse transcription primers and for strand transfer during retroviral replication 48, 49, it is also possible that tRNAs serve a yet to be discovered function in nuclei.
Mechanism of tRNA nuclear import
tRNA nuclear import is an active process as import is energy-requiring and temperature-dependent, in vivo in yeast 2 and in vertebrate cells 4, and in nuclear import assays using permeabilized HeLa cells 3. There is conflicting data regarding the role of the Ran pathway in the tRNA import process. Studies in yeast employed a temperature-sensitive mutation of the RanGAP, rna1-1; at the nonpermissive temperature, the rna1-1 mutation causes defects in macromolecular nuclear/cytoplasmic trafficking for all Ran-dependent trafficking 50. One group reported that cytoplasmic tRNAs could be imported into nuclei of haploid rna1-1 cells at the nonpermissive temperature 2, suggesting that the Ran pathway is not required for tRNA nuclear import. A second group employing yeast heterokaryon studies and the rna1-1 mutation reported that tRNA accumulation was markedly reduced in the nucleus not encoding the tRNA 1, suggesting that the Ran pathway is required for tRNA nuclear import. Ran-dependency is supported by the fact that retrograde tRNA nuclear accumulation is dependent upon the β-importin family member, Mtr10 (vertebrate TNPO3/TRN-SR2 or Transportin-3) 1, 35 (Figure 1). It is difficult to reconcile the different results, but perhaps there are both Ran-independent and Ran-dependent tRNA nuclear import pathways.
How Mtr10 affects nuclear accumulation of cytoplasmic tRNAs is unknown. A likely way would be for Mtr10 to serve as a tRNA importer by binding tRNA, either directly or via an adaptor. As Mtr10 is known to import other RNA binding proteins such as Npl3 51, this is an attractive idea, but it is unknown whether Mtr10 binds tRNA directly or interacts with tRNA via an adaptor. It is also feasible that in yeast Mtr10 acts as a positive effector of the retrograde import machinery or that it imports a protein that is responsible for nuclear retention of imported tRNA; this latter possibility seems unlikely because when MTR10 is deleted in combination with deletion of either of the tRNA exporters, Los1 or Msn5, there is no nuclear tRNA accumulation 9.
Although it was reported that Ran components are not required for tRNA-mediated nuclear import of HIV retrotranscribed complexes (RTC) in HeLa cells 3, TNPO3/TRN-SR2 has also been implicated in HIV RTC nuclear accumulation, but appears to act by binding tRNA in the nucleus and thereby disassembling the tRNA-RTC complex prior to viral genomic integration (A. Fassati, personal comm.).
Regulation of tRNA nuclear import and/or re-export
Cytoplasmic tRNAs accumulate in nuclei under various conditions: when tRNAs are damaged or uncharged or when cells are deprived of nutrients. One explanation as to why cytoplasmic tRNAs accumulate in nuclei under these conditions is that tRNAs might be imported into nuclei only under stress conditions. However, two types of studies showed that tRNA nuclear import is constitutive. First, in heterokaryons in fed conditions, tRNAs encoded by one nucleus accumulate in the second nucleus not encoding the tRNA when tRNA nuclear export was inhibited by deletion of LOS11. The data show that tRNAs enter the second nucleus under fed conditions, rather than only under stress conditions. Second, studies employing a nuclear tethered tRNA modification enzyme that modifies only spliced tRNAs (i.e., previously resided in the cytoplasm) showed that tRNAs enter the nucleus both in replete and nutrient deprived states 52. Constitutive tRNA nuclear import likely also occurs in vertebrate cells 3.
Since tRNAs accumulate in the nucleus when cells are nutrient deprived or when tRNAs are damaged and tRNA nuclear import is constitutive, the distribution of these tRNAs between the nucleus and the cytoplasm could be the result of up-regulation of tRNA nuclear import, down-regulation of tRNA re-export, or the combination of both, although down-regulation of re-export is favored9, 18. Redistribution of tRNA between the nucleus and cytoplasm dependent on nutrient availability is fast, occurring within ~15 min. of removal or introduction of particular nutrients, implicating a posttranscriptional process. Signaling is independent of the general amino acid control pathway as gcn2Δ cells are not blocked for starvation-induced tRNA nuclear accumulation 8. The TOR amino acid sensitive regulatory pathway has an undefined role in tRNA nuclear-cytoplasmic dynamics as rapamycin treatment failed to cause the expected tRNA nuclear accumulation for this pseudo-starvation condition 8. Cells defective in the protein kinase A (PKA) pathway or the glucose derepression pathways were blocked for starvation-induced tRNA nuclear accumulation 8. Since these strains are not only defective for PKA signaling and glucose repression, but exist in a constitutively stressed state, the lack of tRNA nuclear accumulation may be due to the inability to experience acute starvation signaling 8. Thus, at present, it is unknown which nutrient-dependent signaling pathway(s) is involved in the tRNA retrograde pathway.
Recent studies have shown that there is coordinate regulation between mRNA cytoplasmic dynamics and tRNA nuclear/cytoplasmic trafficking. When cells are deprived of nutrients, P-bodies co-assemble with mRNAs released from polysomes and the mRNAs can be recruited back to ribosomes upon re-feeding 42. Mutation or over-expression of genes involved in P-body formation - Pat1 and Dhh1 - affect the tRNA retrograde pathway in a parallel fashion53. Thus, co-deletion of PAT1 and DHH1 that prevents P-body formation and translational repression upon glucose deprivation 54 also prevents accumulation of cytoplasmic tRNAs in the nucleus upon nutrient deprivation; likewise, over-expression of PAT1 or DHH1 causes P-body formation and tRNA nuclear accumulation, even in nutrient replete conditions 53, 54. However, coordinate regulation of P-body formation/translation repression and tRNA nuclear accumulation is restricted to the early part of the P-body pathway as gene products functioning in late steps of the pathway, such as the decapping enzyme and the Xrn1 5′>3′ endonuclease, do not affect tRNA nuclear/cytoplasmic dynamics53. Coordination of early steps of P-body formation with tRNA nuclear/cytoplasmic dynamics may provide cells with effective survival in response to adverse conditions. There are other indications that tRNA nuclear/cytoplasmic dynamics are coordinated with stress responses. One of the yeast major heat shock proteins, Ssa2 binds tRNA in an ATP-sensitive manner under nutrient deprivation conditions. Deletion of SSA2 prevents tRNA nuclear accumulation upon nutrient stress but not when cells are provided with appropriate nutrients (T. Yoshihisa, person comm.). This chaperone-dependent mechanism may define another tRNA nuclear import process that functions in parallel with the Mtr10 Ran-dependent process.
Remaining questions
There are many questions regarding the movement of tRNA from the cytoplasm to the nucleus. The function of this process still must be deciphered and indeed it is quite possible that tRNA retrograde nuclear accumulation serves more than a single biological role. tRNA nuclear import is constitutive but could also be up-regulated upon nutrient deprivation and it seems quite likely that import involves both Ran-dependent and Ran-independent mechanisms. The carriers for neither mechanism have been established, although Mtr10 and Ssa2 are likely candidates. Finally, how tRNA retrograde nuclear import is connected to nutrient-sensitive signaling processes and P-body formation need to be delineated.
tRNA MOVEMENT FROM THE CYTOPLASM TO THE MITOCHONDRIAL SURFACE
Intron-containing pre-tRNAs can easily be detected in the nucleus by fluorescence in situ hybridization of tRNAs in wild-type yeast cells 6, 12. However, under normal conditions these tRNAs are not detected in the cytoplasm, even though introns are not removed until tRNAs contact the outer surface of mitochondria 6. Cytoplasmic intron-containing tRNAs accumulate if the tRNA splicing endonuclease is defective and the tRNAs appear to be diffusely distributed throughout the cytoplasm, rather than accumulated at the mitochondrial surface 6. The results document that tRNA splicing on mitochondria occurs very efficiently upon tRNA nuclear export. Whereas it is possible that tRNAs exiting the nucleus simply diffuse to the mitochondrial surface where tRNA splicing endonuclease is located, it seems more likely that a mechanism(s) exists to aid tRNA mitochondrial location (Figure 1). At present there is no information whether such a system does exist and, if so, what gene products are required for mitochondrial delivery.
tRNA IMPORT INTO MITOCHONDRIA
The eukaryotic cell has undergone amazing genetic circuitry rearrangements upon the endosymbiotic appearance of mitochondria from an α-proteobacterial ancestor 55–57. During evolution, many mitochondrial genes have been either functionally transferred to the nucleus or replaced by pre-existing nuclear genes of similar function. In maintaining mitochondrial function, a vast number of mitochondrial proteins are encoded in the nucleus, synthesized in the cytoplasm, and subsequently imported into the organelle. Together, a few mitochondria-encoded subunits along with the many nucleus-encoded mitochondria-targeted proteins assemble into fully operational respiratory complexes, generating ATP through oxidative phosphorylation58.
In early analyses of the Tetrahymena pyriformis mitochondrial genome, Suyama did not find two-thirds of the tRNA genes needed to complete mitochondrial protein synthesis. This led to his original proposal that cytosolic tRNAs must be imported into mitochondria 59 (Figure 1). To date, multiple occurrences of tRNA import have been described in diverse organisms: Tetrahymena 60–62, Trypanosoma and Leishmania 63–65, Toxoplasma 66, yeast 67, 68, Triticum69, Paramecium 70, plants 71 marsupials 72, and most recently, placental mammals 5. The number of imported tRNAs varies greatly from a few tRNAs in the case of yeast to a full set of tRNAs in some protists 73. In the kinetoplastids Leishmania and Trypanosoma, no tRNA genes are encoded in the mitochondrial genome. As a consequence, these mitochondria strictly require tRNA import for the translation of the few mitochondrial proteins still encoded in the organellar genome. In other organisms, such as yeast and humans, where most tRNAs are encoded in the mitochondrial genome, the import of some nucleus-encoded tRNAs is proposed to function in mitochondrial wobble codon decoding 68, as shown by the mitochondrial import of one nucleus-encoded lysyl tRNA to decode a lysine codon under stress conditions74. In the present review, we provide a general overview of current knowledge regarding the diversity of import systems, examine some experimental discrepancies, and accentuate future challenges.
The disappearance of a varying number of tRNA genes from mitochondrial genomes among different organisms has perhaps driven the independent evolution of systems that permit import of tRNAs from the cytoplasm 73. Common to all systems is the fate of the given tRNA in traversing an outer and an inner lipid-bilayer membrane before reaching the mitochondrial matrix, the site for mitochondrial translation. However, there are various systems residing in the cytosol commissioned to the delivery of tRNAs to the mitochondrion. The protein(s) involved in these mitochondrial delivery systems all have had prior assigned functions, such as glycolysis, translation, and amino acid activation 73. As a consequence, these proteins currently implicated in mitochondrial delivery of tRNA have now become classified as multi-functional proteins. Although there are various systems proposed to recognize a given tRNA in the cytosol and deliver it to the mitochondrion, the possibility that a given tRNA may reach the mitochondrion by the direct recognition or recruitment of a component of the outer membrane still remains, as suggested by existing in vitro import systems.
Given the integral association with components of the translation machinery, tRNAs also need to be recycled during protein synthesis. Therefore, one conundrum is how tRNAs are freed from the cytoplasmic translation machinery to reach the mitochondrion. One proposal suggests that a fraction of the nucleus-encoded tRNAs escapes the cytosolic translation machinery by interacting with protein factors that will consequently be directed to the mitochondrion. In Trypanosoma brucei, the cytosolic translation elongation factor 1a (eEF1a) is thought to play this role as a specificity determinant for a small subset of imported tRNAs75 (Figure 2). The major role of EF1a is to deliver the aminoacylated tRNA (aa-tRNA) to the ribosome in the form of an EF1a-aa-tRNA-GTP ternary complex76. However, in T. brucei, the knockdown of cytosolic eEF1a, but not of initiation factor 2, inhibits mitochondrial import of newly synthesized tRNAs before translation or growth is affected75. tRNA import requires both eEF1a and aminoacylation of the tRNA75. How this complex avoids interaction with the ribosome to enter the mitochondrial import pathway has yet to be explained.
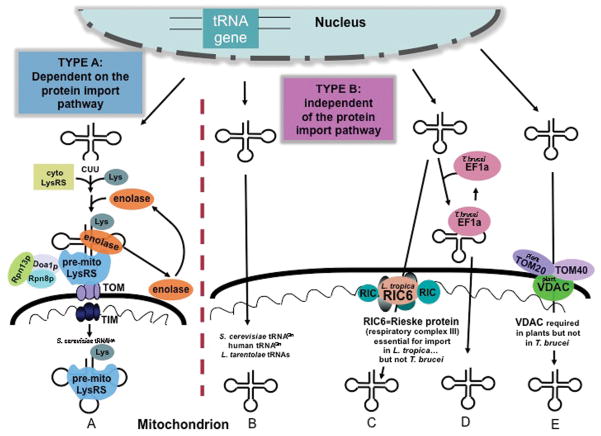
Figure 2.
Two types of tRNA import into mitochondria. Type A: import is strictly dependent on the protein import pathway A) S. cerevisiae tRNALys(CUU) (tRK1) is aminoacylated then recognized by enolase the precursor form of the mitochondrial lysyl tRNA synthetase followed by delivery to the mitochondrial surface Type B: tRNA import occurs independently from the protein import pathway. B) S. cerevisiae tRNAGln, human tRNAGln, & L. tarentolae tRNAs, D) L. tropica tRNATyr, T. brucei tRNA, E) plant tRNAGln.
Mitochondrial tRNA import systems
In refraining from the relaxed use of the term “mechanism”, we separate the mitochondria import systems into two broadly defined types (Figure 2). Type A tRNA import utilizes the known protein import pathway and has strict requirements for the presence of a membrane potential and ATP hydrolysis. In systems that fall into the type B category, tRNA import itself occurs independently of the canonical protein import pathway77, 78. Type A import is limited to the single known example from yeast where direct participation of the canonical and well-characterized protein import machinery helps drive the tRNA across the mitochondrial double membrane 79. The protein(s) that reside in the mitochondrial membranes to comprise the tRNA import machinery themselves have to be translocated from the cytoplasm and/or inserted into the mitochondrial membranes. Therefore, there exists a delicate nuance in the study of tRNA transport systems in carefully teasing the secondary effects of protein import from that of the downstream function tRNA import.
Type A mitochondrial tRNA import in yeast
In S. cerevisiae, the molecular mechanism of cytosolic tRNALys(CUU), tRK1, mitochondrial targeting involves interaction of tRNA with cytosolic factors, enabling the tRNA to escape from the cycle of cytosolic protein synthesis. A portion of aminoacylated cytosolic tRNALys(CUU) can be specifically recognized by one of the two isoforms of the glycolytic enzyme enolase, Eno2p80. Eno2p is then targeted to a large glycolytic macromolecular complex known to be tightly associated with the outer mitochondrial surface. 81 (Figure 2).
The aminoacylated cytosolic tRNALys(CUU) tRK1 is taken up by the precursor mitochondrial lysyl-tRNA synthetase, pre-mito LysRS (preMSK1p), which is responsible for the aminoacylation of its true substrate, the mitochondria-encoded tRNALys counterpart in the mitochondrial matrix (Figure 2). Therefore, pre-mito LysRS is a multi-functional cytosolic protein that also serves as the carrier for cytosolic tRNALys(CUU) translocation into the mitochondrial matrix 82. Further genetic and biochemical assays have also isolated three components of the ubiquitin/26S proteasome system, Rpn13, Rpn8, and Doa1, that interact with the tRNA and the pre-mito LysRS 81. These proteins plays a dual regulatory role, since the overall inhibition of cellular proteasome activity reduces tRNA import, while specific depletion of Rpn13 or Doa1 increases import of tRNALys(CUU)81. It is clear that unfolding is a key step in protein import into mitochondria; thus, how a structured tRNA-precursor synthetase complex enters and traverses the mitochondrial membranes remains unclear 79.
Type B tRNA import in yeast
S. cerevisiae is a unique case in which the two disparate systems, Type A and Type B import, co-exist. Type B import in yeast results in the mitochondrial delivery of two cytosolic tRNAs, tRNAGln(CUG) and tRNAGln(UUG)68. Independence from the protein import pathway for tRNAGln was demonstrated by in vitro experiments using isolated mitochondria where the membrane potential was inhibited, no cytosolic factors were present, but the tRNA was efficiently imported. The localization of both the cytoplasmic tRNAGln and the cytosolic glutaminyl sythetase (GlnRS) in highly purified mitochondria was also clearly demonstrated by in vivo immunofluorescence. The successful rescue of a mitochondrial S. cerevisiae in-frame amber stop codon mutant demonstrated the functional tie of an amber suppressor imported tRNAGln and the cytoplasmic GlnRS in the organelle in generating mitochondrial pools of Gln-tRNAGln. However, it is possible that in mitochondria the transamidation pathway also generates Gln-tRNAGln by a tRNA-dependent glutamate modification of Gln-tRNAGlu, found in bacteria, archaea, and chloroplasts 83–86. One study uncovered mitochondrial import of the cytosolic glutamate synthetase that is capable of mischarging the mitochondria-encoded tRNAGln, forming Glu-tRNAGln, that is subsequently fed into the transamidaton pathway 87. The same study was unable to detect the presence of any cytosolic tRNAGln species in yeast mitochondria 87. The The failure of detection of cytosolic tRNAGln in this mitochondrial study remains a discrepancy. There may still be the interesting possibility that two distinct events contribute to the generation of Gln-tRNAGln species in S. cerevisiae mitochondria where these events may well be subject to differences in strain and/or culture growth conditions.
Type B tRNA import into mammalian mitochondria
The Type B tRNA import system also exists in mammalian mitochondria 5. Using clean subcellular RNA fractions from rat liver and human cells, nucleus-encoded tRNAGln(CUG) and tRNAGln(UUG) species have been shown to localize to mitochondria in vivo5. Import of in vitro transcribed tRNAs, but not of heterologous RNAs, into isolated mitochondria also demonstrates that this process is tRNA-specific and does not require cytosolic factors. Although this in vitro system requires ATP, it is resistant to inhibitors of the mitochondrial electrochemical gradient, a key component of protein import. Therefore, tRNAGln import into mammalian mitochondria proceeds by a mechanism distinct from protein import. Evidence shows that both tRNAGln species and a bacterial pre-tRNAAsp can be imported in vitro into mitochondria isolated from myoclonic epilepsy with ragged-red fiber cells, given that sufficient ATP (2 mM) is provided5. This work suggests that tRNA import is more widespread than previously thought and may be a universal trait of mitochondria.
Type B tRNA import machinery in the trypanosomatids
Among the most studied tRNA import pathways are those of kinetoplastids. To date, the RNA import complex (RIC) of Leishmania tropica is by far the system with the most proteins proposed to import tRNAs into mitochondria 88–90. Mass spectrometry analyses of the multi-protein ~580 kDa L. tropica RIC revealed a total of 122 nuclear-encoded ORFs 88. Further Western blot and RNAi analyses led to the final composition of eleven major subunits, RIC1, 2, 3, 4A, 4B, 5–7, 8A, 8B and 988–90. The functional characterization of the RIC showed import of tRNAs into phospholipid vesicles in an ATP-dependent manner 90, 91.
Recent studies have explored whether the described L. tropica RIC components have functional conservation with another related kinetoplastid, Trypanosoma brucei. The involvement of the Rieske subunit of complex III (RIC6) is essential for both maintenance of membrane potential and respiration in T. brucei 92. However, down-regulation of the Rieske protein levels by RNAi had no effect on mitochondrial tRNA import in vivo and in vitro in T. brucei 92. These findings demonstrate that the Rieske protein does not play a major role in mitochondrial tRNA localization in T. brucei and corroborates previous observations 93, 94. In addition, despite relying heavily on glycolysis instead of respiration, the bloodstream stage mitochondria still import tRNAs95. Therefore, subunits of respiratory complexes are not required for both protein and tRNA import95, 96.
Remarkably, the ATP requirement and lack of a requirement for membrane potential is a conserved feature of the T. brucei and L. tarentolae pathways 93, 94, but again differs from what has been shown in L. tropica 97. Highlighting the L. tarentolae in vitro import system is the observation that the system reaches saturation at micromolar tRNA concentration, which strongly implies that the in vitro assay exhibits dynamics akin to receptor-mediated systems, is consistent with the levels of in vivo localization of these tRNAs providing a valid mimic of the in vivo situation 94, 98, 99. Currently, the true nature of the L. tropica import complex remains questionable and is under further scrutiny100. Taken together, these data highlight the numerous incongruities among the various tRNA import systems analyzed, not only between diverse organisms, but also among closely related ones. More information regarding the transporters is required before establishing commonalities among different factors involved in tRNA transport.
Type B tRNA import machinery in plant mitochondria
In plants, import of nucleus-encoded cytosolic tRNAs is essential for mitochondrial biogenesis. tRNA import is also ATP-dependent and does not require any added cytosolic factors 101. Plant mitochondrial tRNA import can be inhibited in vitro by valinomycin or oligomycin, suggesting the requirement for both a membrane potential and a functional respiratory chain, which would make it a candidate for Type A import. Indeed, two proteins from the classical protein import pathway were found to be important for tRNA import. Specifically, the translocase of the outer mitochondrial membrane complex, TOM20 and TOM40, are important in binding tRNA. However, the translocases of the inner membrane (TIMs) from the protein import pathway are apparently not involved in tRNA import to plant mitochondria 102, as they are in Type A import of yeast lysyl tRNA, where TOM and TIM protens are needed to import the precursor lysyl synthetase protein with tRNA as its cargo103. Therefore, plant mitochondrial import of tRNAs seems to follow the Type B import pathway.
The plant mitochondrial voltage-dependent anion channel (VDAC) interacts with tRNA in vitro (Figure 2) and is proposed to be a major component of the channel involved in the tRNA translocation step through the plant mitochondrial outer membrane102. Playing an essential role in metabolite transport, the VDAC provides a permeability pathway through the mitochondrial outer membrane by forming voltage-gated channels with an open pore radius of 3 nm to 1.8 nm in the closed state104. Remarkably, the VDAC is capable of selecting among small molecules, ions, and negatively charged metabolites such as succinate, malate, or ATP with a much smaller effective radius, 0.4±0.5 nm. How the VDAC performs another function in the transport of tRNAs into plant mitochondria in its open state remains a puzzle, since the tRNA tertiary L-shaped structure is large. This raises the further question of whether tRNAs must unfold prior to translocation through the VDAC.
In contrast to plants, in T. brucei there is no identifiable homolog of TOM40 in the genomic database, despite maintaining a tRNA import mechanism. Additionally, import of tRNAs occurs efficiently in T. brucei VDAC knockout cells. Thus, unlike tRNA import in plant mitochondria, the VDAC is not required for mitochondrial tRNA import in T. brucei 105.
Sequence determinants for tRNA mitochondrial import
Structural folding of a tRNA molecule is controlled by base-pairing of the primary sequence into secondary structure, the cloverleaf structure, followed by further stabilization by co-axial stacking of the helices and higher order tertiary interactions. Despite the existence of numerous tRNAs in the cell, in general, most naturally fold into one canonical L-shape tertiary structure. However, within the L-shaped structure, tRNAs have extraordinary structural versatility that fine-tunes local structure. While it seems likely that specific cis-acting sequences may act as determinants or anti-determinants for tRNA mitochondrial import, as summarized below, many studies conclude that the global tertiary fold of the tRNA molecule that seems to direct import.
In some organisms, the anticodon is an important determinant for mitochondrial tRNA import. For example, in the protozoa Tetrahymena, only one of the three isoacceptors, tRNAGln(UUG), travels into mitochondria and substitution of a single anticodon nucleotide (UUA-->UUG) is both necessary and sufficient for tRNA import and confers import on a normally non-imported glutamine tRNA 62(Figure 3A).
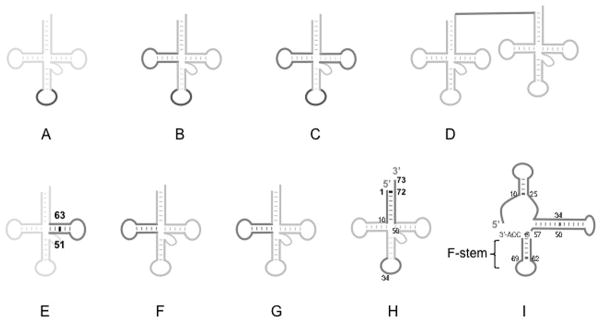
Figure 3.
Sequence determinants for tRNA import into mitochondria: A) anticodon nucleotide of T. pyriformis tRNAGln, B) anticodon and D-arm of N. tabaccum tRNAGly C) anticodon, D-arm and TΨC-arm of N. tabaccum tRNAVal, D) intergenic sequence between the precursor form of the dicistronic tRNASer and tRNALeu of T. brucei, E) T-stem base pair 51:63 of T. brucei tRNA, F) D-arm of L. tarentolae tRNAIle and tRNAGln, G) D-arm of L. tropica tRNATyr, H) anticodon, acceptor stem base pair 1:72, acceptor nucleotide, 73 of S. cerevisiae tRNALys(CUU), and I) proposed conformational rearrangement of S. cerevisiae tRNALys(CUU) into the F-structure. Dark lines highlight the sequence determinant(s).
In higher plants, import into tobacco cell mitochondria involve essential determinants in the anticodon, D-arm for tRNAGly(UCC) and the anticodon, D-arm, and TΨC-arm for tRNAVal(AAC) 101, 106,107 (Figure 3B and 3C). However, in Phaseolus vulgaris and the lower plant Marchantia polymorpha, recognition of tRNAs for import appears idiosyncratic and specific to each tRNA or isoacceptor group 108, 109.
Earlier observations suggested that in Trypanosoma brucei, dicistronic precursors containing the tRNASer and tRNALeu transcripts with a 59-nucleotide intergenic sequence were the substrate for tRNA import in vivo (Figure 3D) 110. In contrast, other experiments showed that at least in the case of some tRNA isoacceptors, import occurs regardless of the sequence context of the imported tRNA, implying that the tRNA pre-sequence does not contain sequence-specific determinants for import in T. brucei 111. For some tRNAs, internal nucleotides involved in eEF1a interactions are important. A major localization determinant of T. brucei in vivo import was found within the TΨC-stem nucleotide base-pair at nucleotides 51 and 63 within tRNAMet, tRNAIle and tRNALys 75 (Figure 3E). However, the cytosol-specific initiator tRNAMeti containing a different nucleotide pair at 51 and 63, corresponding to the main anti-determinant, was found to prevent its interaction with the cytosolic eEF1a. Being the only other cytosol-specific tRNA in T. brucei, tRNASec has its own elongation factor and does not bind eEF1a. However, upon introduction of a mutation of tRNASec to render it capable of binding to eEF1a, the mutant tRNASec was imported into mitochondria75.
In L. tarentolae, mature tRNAs are also substrates for mitochondrial import in vivo 99, 109. Localization of tRNAIle(UAU) is mostly within the mitochondrion, while tRNAGln(CUG) is primarily in the cytosol 98, 112, 113. Both in vivo and in vitro experiments further demonstrated that swapping the D-stem and loop from the mainly cytosolic tRNAGln with that from the tRNAIle produced increased mitochondrial localization of the tRNA94, 112. This D-loop exchange did not eliminate the mitochondrial localization of tRNAIle(D-Gln) in vivo and in vitro. The role of tertiary tRNA structure or additional sequence elements were proposed to contribute an essential role in import (Figure 3F) 94, 112, since proper folding of the chimeric tRNAGln(D-Ile) was confirmed by successful aminoacylation by the cytosolic glutaminyl tRNA synthetase 114.
In Leishmania tropica mitochondria, imported tRNAs have one of two signature consensus sequences: Class I tRNAs or Class II 115, 116. Class I tRNAs contain a conserved sequence motif in the D arm that positively stimulates import of type II molecules into the mitochondrial matrix. In contrast, class II tRNAs, with motifs within the variable region and the TΨC domain, are poorly imported and inhibit import of class I tRNAs 115, 116.
In contrast to the studies designed to identify tRNA determinants within the context of the L-structure, other studies have shown that structures deviating from the canonical tRNA tertiary model may be substrates for mitochondrial import. One approach addresses minimal substrates sufficient for mitochondrial import. The D-arm of tRNATyr, AUGGCAGAG, was used to isolate the L. tropica RIC88 (Figure 3G). However, in another study, more sequences were extensively surveyed, no obvious consensus sequence correlated with tRNA localization, calling into question its in vivo importance 117. In L. tarentolae mitochondria, smaller RNAs including five different 16 to 17-nucleotide mini-helix RNAs, some of non-tRNA origin, and one unstructured could be efficiently imported in vitro; however, unstructured RNAs of greater sizes (up to 33 nucleotides in length) failed to support import. 94. Therefore, while shorter RNA molecules may be efficiently imported in vitro, the import of short RNA substrates strikingly leads to the loss of discrimination by the mitochondria 94.
In yeast, an alternative tRNA structure may be imported into mitochondria. Although for tRNALys(CUU), studies mapped the acceptor stem base-pair, the discriminator nucleotide, and the wobble position C34 of the anticodon, to be important for mitochondrial import 118–120 (Figure 3H), an alternative conformation of tRNALys(CUU) into an F-structure121 (Figure 3I). In the F-structure, there is a proposed formation of a helix between base pairs C74G57 to G69C62, such that the 3′-end of the acceptor stem forms a helix with the 3′ portion of the TψC stem, therefore re-structuring the anticodon nucleotides into a long hairpin with the 5′-end of the TψC stem121. Enolase is thought to modulate the tRNA structure and play a role in mitochondrial import selectivity121. How the lysyl tRNA synthetases recognize the F-structure remains an open question.
Remaining questions
Intracellular trafficking of tRNA molecules may not be limited only to the nucleus and mitochondria, but may also expand other organelles. Inspection of chloroplast genomes shows that most tRNAs needed for translation are encoded by the organelle 122. However, the data do not eliminate the possible import of tRNAs into the chloroplast. The plastid genome of a non-photosynthetic parasitic flowering plant Epifagus virginiana lacks all genes for photosynthesis and respiration found in chloroplast genomes of green plants 123. This highly reduced, compact plastid genome of E. virginiana maintains functionality while missing six ribosomal proteins and 13 tRNA genes which are found in the chloroplast DNAs of its photosynthetic flowering plant host 123. To compensate for these losses, import of nucleus-encoded gene products including tRNAs may occur 124. tRNA import into chloroplasts and plastids remains an unexplored area.
A daunting number of mutations located within tRNA genes account for numerous human mitochondrial neuromuscular and neurodegenerative disorders 125, 126. Within the current horizon, experimental designs incorporate the transplantation of a surrogate tRNA import machinery into a human cell line to deliver a given tRNA to the defective mitochondria 127–130. Since mammalian mitochondria actively import tRNAs, Since mammalian mitochondria actively import tRNAs, the prospect of mitochondrial therapy is possible. However, the rescue of tRNA import in vitro into mitochondria from MERFF patients required the addition of excess ATP. This observation raises the possibility that unless alternative ATP sources are provided in vivo, both native and surrogate import systems may fail as potential therapies5. Use of a surrogate system has been reported to rescue some level of mitochondria function. However, it is not clear how this or any in vitro system represents the in vivo situation with whole organisms. It could well be that import systems may themselves be compromised by mutations that affect certain mitochondrial functions such as the ability to synthesize enough ATP. Secondly, the proteins involved in tRNA import may require the protein import pathway for proper assembly in the membranes. Therefore, in examining defects in tRNA import, the careful teasing apart of secondary effects due to a malfunction of mitochondrial protein import seems pertinent.
All organisms share a common goal in translocation of a given tRNA to the mitochondrial matrix for translation, yet much about the nature of the import machinery still remains to be fully understood. Although the requirement of external ATP has been shown in all systems studied to date, its precise role at the outer membrane is still unknown, except in plants where ATP is required for the tRNA binding step at the surface of mitochondria. Across the eukaryotes, a fundamental observation is that mitochondrial genomes bear a higher burden of deleterious mutations than that of nuclear genomes131. Although evolutionary forces have driven the reduction of organellar genomes, the cell also developed means of recruiting cytosolic factors encoded in the nuclear genome to maintain organellar function. Alluding to the growing prospect that tRNA import may also occur in other organelles, the variations on the theme seen among nucleus and mitochondria tRNA import systems may shed some light on how the cell meticulously coordinates the travel itinerary of tRNAs within its confines.
Acknowledgments
We thank Drs. A. Fassati and T. Yoshihisa for communication of unpublished results and Dr. J. Alfonzo for comments on the manuscript. The work from the authors’ laboratories was supported by NIH grants GM27930 (to AKH) and GM084065 to J. Alfonzo.
References
- 1.Shaheen HH, Hopper AK. Retrograde movement of tRNAs from the cytoplasm to the nucleus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2005;102:11290–11295. doi: 10.1073/pnas.0503836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takano A, Endo T, Yoshihisa T. tRNA actively shuttles between the nucleus and cytosol in yeast. Science. 2005;309:140–142. doi: 10.1126/science.1113346. [DOI] [PubMed] [Google Scholar]
- 3.Zaitseva L, Myers R, Fassati A. tRNAs promote nuclear import of HIV-1 intracellular reverse transcription complexes. PLoS Biol. 2006;4:e332. doi: 10.1371/journal.pbio.0040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen HH, Horetsky RL, Kimball SR, Murthi A, Jefferson LS, Hopper AK. Retrograde nuclear accumulation of cytoplasmic tRNA in rat hepatoma cells in response to amino acid deprivation. Proc Natl Acad Sci U S A. 2007;104:8845–8850. doi: 10.1073/pnas.0700765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubio MA, Rinehart JJ, Krett B, Duvezin-Caubet S, Reichert AS, Soll D, Alfonzo JD. Mammalian mitochondria have the innate ability to import tRNAs by a mechanism distinct fromprotein import. Proc Natl Acad Sci U S A. 2008;105:9186–9191. doi: 10.1073/pnas.0804283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshihisa T, Yunoki-Esaki K, Ohshima C, Tanaka N, Endo T. Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol Biol Cell. 2003;14:3266–3279. doi: 10.1091/mbc.E02-11-0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshihisa T, Ohshima C, Yunoki-Esaki K, Endo T. Cytoplasmic splicing of tRNA in Saccharomyces cerevisiae. Genes Cells. 2007;12:285–297. doi: 10.1111/j.1365-2443.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 8.Whitney ML, Hurto RL, Shaheen HH, Hopper AK. Rapid and reversible nuclear accumulation of cytoplasmic tRNA in response to nutrient availability. Mol Biol Cell. 2007;18:2678–2686. doi: 10.1091/mbc.E07-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthi A, Shaheen HH, Huang HY, Preston MA, Lai TP, Phizicky EM, Hopper AK. Regulation of tRNA bidirectional nuclear-cytoplasmic trafficking in Saccharomyces cerevisiae. Mol Biol Cell. 2010;21:639–649. doi: 10.1091/mbc.E09-07-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutay U, Lipowsky G, Izaurralde E, Bischoff FR, Schwarzmaier P, Hartmann E, Gorlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 11.Arts GJ, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar S, Hopper AK. tRNA nuclear export in saccharomyces cerevisiae: in situ hybridization analysis. Mol Biol Cell. 1998;9:3041–3055. doi: 10.1091/mbc.9.11.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmuth K, Lau DM, Bischoff FR, Kunzler M, Hurt E, Simos G. Yeast Los1p has properties of an exportin-like nucleocytoplasmic transport factor for tRNA. Mol Cell Biol. 1998;18:6374–6386. doi: 10.1128/mcb.18.11.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata S, Sasaki M, Miki T, Shimamoto A, Furuichi Y, Katahira J, Yoneda Y. Exportin-5 orthologues are functionally divergent among species. Nucleic Acids Res. 2006;34:4711–4721. doi: 10.1093/nar/gkl663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arts GJ, Kuersten S, Romby P, Ehresmann B, Mattaj IW. The role of exportin-t in selective nuclear export of mature tRNAs. EMBO J. 1998;17:7430–7441. doi: 10.1093/emboj/17.24.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipowsky G, Bischoff FR, Izaurralde E, Kutay U, Schafer S, Gross HJ, Beier H, Gorlich D. Coordination of tRNA nuclear export with processing of tRNA. RNA. 1999;5:539–549. doi: 10.1017/s1355838299982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook AG, Fukuhara N, Jinek M, Conti E. Structures of the tRNA export factor in the nuclear and cytosolic states. Nature. 2009;461:60–65. doi: 10.1038/nature08394. [DOI] [PubMed] [Google Scholar]
- 18.Eswara MB, McGuire AT, Pierce JB, Mangroo D. Utp9p facilitates Msn5p-mediated nuclear reexport of retrograded tRNAs in Saccharomyces cerevisiae. Mol Biol Cell. 2009;20:5007–5025. doi: 10.1091/mbc.E09-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopper AK. Nucleocytoplasmic transport: Inside out regulation. Curr Biol. 1999;9:R803–806. doi: 10.1016/s0960-9822(99)80494-1. [DOI] [PubMed] [Google Scholar]
- 20.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 21.Park MY, Wu G, Gonzalez-Sulser A, Vaucheret H, Poethig RS. Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci U S A. 2005;102:3691–3696. doi: 10.1073/pnas.0405570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calado A, Treichel N, Muller EC, Otto A, Kutay U. Exportin-5-mediated nuclear export of eukaryotic elongation factor 1A and tRNA. EMBO J. 2002;21:6216–6224. doi: 10.1093/emboj/cdf620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohnsack MT, Regener K, Schwappach B, Saffrich R, Paraskeva E, Hartmann E, Gorlich D. Exp5 exports eEF1A via tRNA from nuclei and synergizes with other transport pathways to confine translation to the cytoplasm. EMBO J. 2002;21:6205–6215. doi: 10.1093/emboj/cdf613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada C, Yamashita E, Lee SJ, Shibata S, Katahira J, Nakagawa A, Yoneda Y, Tsukihara T. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326:1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 26.Hurt DJ, Wang SS, Lin YH, Hopper AK. Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol Cell Biol. 1987;7:1208–1216. doi: 10.1128/mcb.7.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter CA, Aukerman MJ, Sun H, Fokina M, Poethig RS. PAUSED encodes the Arabidopsis exportin-t ortholog. Plant Physiol. 2003;132:2135–2143. doi: 10.1104/pp.103.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Chen X. PAUSED, a putative exportin-t, acts pleiotropically in Arabidopsis development but is dispensablefor viability. Plant Physiol. 2003;132:1913–1924. doi: 10.1104/pp.103.023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21:87–96. doi: 10.1016/j.molcel.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 30.Feng W, Hopper AK. A Los1p-independent pathway for nuclear export of intronless tRNAs in Saccharomycescerevisiae. Proc Natl Acad Sci U S A. 2002;99:5412–5417. doi: 10.1073/pnas.082682699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarkar S, Azad AK, Hopper AK. Nuclear tRNA aminoacylation and its role in nuclear export of endogenous tRNAs in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1999;96:14366–14371. doi: 10.1073/pnas.96.25.14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosshans H, Hurt E, Simos G. An aminoacylation-dependent nuclear tRNA export pathway in yeast. Genes Dev. 2000;14:830–840. [PMC free article] [PubMed] [Google Scholar]
- 33.Azad AK, Stanford DR, Sarkar S, Hopper AK. Role of nuclear pools of aminoacyl-tRNA synthetases in tRNA nuclear export. Mol Biol Cell. 2001;12:1381–1392. doi: 10.1091/mbc.12.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steiner-Mosonyi M, Mangroo D. The nuclear tRNA aminoacylation-dependent pathway may be the principal route used to export tRNA from the nucleus in Saccharomyces cerevisiae. Biochem J. 2004;378:809–816. doi: 10.1042/BJ20031306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurto RL, Tong AH, Boone C, Hopper AK. Inorganic phosphate deprivation causes tRNA nuclear accumulation via retrograde transport in Saccharomyces cerevisiae. Genetics. 2007;176:841–852. doi: 10.1534/genetics.106.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe CL, Hopper AK, Martin NC. Mechanisms leading to and the consequences of altering the normal distribution of ATP(CTP):tRNA nucleotidyltransferase in yeast. J Biol Chem. 1996;271:4679–4686. doi: 10.1074/jbc.271.9.4679. [DOI] [PubMed] [Google Scholar]
- 37.Hopper AK, Phizicky EM. tRNA transfers to the limelight. Genes Dev. 2003;17:162–180. doi: 10.1101/gad.1049103. [DOI] [PubMed] [Google Scholar]
- 38.Chernyakov I, Whipple JM, Kotelawala L, Grayhack EJ, Phizicky EM. Degradation of several hypomodified mature tRNA species in Saccharomyces cerevisiae is mediated by Met22 and the 5′-3′ exonucleases Rat1 and Xrn1. Genes Dev. 2008;22:1369–1380. doi: 10.1101/gad.1654308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadaba S, Krueger A, Trice T, Krecic AM, Hinnebusch AG, Anderson J. Nuclear surveillance and degradation of hypomodified initiator tRNAMet in S.cerevisiae. Genes Dev. 2004;18:1227–1240. doi: 10.1101/gad.1183804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kadaba S, Wang X, Anderson JT. Nuclear RNA surveillance in Saccharomyces cerevisiae: Trf4p-dependent polyadenylation of nascent hypomethylated tRNA and an aberrant form of 5S rRNA. RNA. 2006;12:508–521. doi: 10.1261/rna.2305406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Copela LA, Fernandez CF, Sherrer RL, Wolin SL. Competition between the Rex1 exonuclease and the La protein affects both Trf4p-mediated RNA quality control and pre-tRNA maturation. RNA. 2008;14:1214–1227. doi: 10.1261/rna.1050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iborra FJ, Jackson DA, Cook PR. Coupled transcription and translation within nuclei of mammalian cells. Science. 2001;293:1139–1142. doi: 10.1126/science.1061216. [DOI] [PubMed] [Google Scholar]
- 44.Dahlberg JE, Lund E, Goodwin EB. Nuclear translation: what is the evidence? RNA. 2003;9:1–8. doi: 10.1261/rna.2121703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varshavsky A. The N-end rulepathway of protein degradation. Genes Cells. 1997;2:13–28. doi: 10.1046/j.1365-2443.1997.1020301.x. [DOI] [PubMed] [Google Scholar]
- 46.Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. doi: 10.1146/annurev.micro.59.031805.133833. [DOI] [PubMed] [Google Scholar]
- 47.Mei Y, Yong J, Liu H, Shi Y, Meinkoth J, Dreyfuss G, Yang X. tRNA binds to cytochrome c and inhibits caspase activation. Mol Cell. 37:668–678. doi: 10.1016/j.molcel.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marquet R, Isel C, Ehresmann C, Ehresmann B. tRNAs as primer of reverse transcriptases. Biochimie. 1995;77:113–124. doi: 10.1016/0300-9084(96)88114-4. [DOI] [PubMed] [Google Scholar]
- 49.Piekna-Przybylska D, DiChiacchio L, Mathews DH, Bambara RA. A sequence similar to tRNA 3 Lys gene is embedded in HIV-1 U3-R and promotes minus-strand transfer. Nat Struct Mol Biol. 17:83–89. doi: 10.1038/nsmb.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbett AH, Koepp DM, Schlenstedt G, Lee MS, Hopper AK, Silver PA. Rna1p, a Ran/TC4 GTPase activating protein, is required for nuclear import. J Cell Biol. 1995;130:1017–1026. doi: 10.1083/jcb.130.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senger B, Simos G, Bischoff FR, Podtelejnikov A, Mann M, Hurt E. Mtr10p functions as a nuclear import receptor for the mRNA-binding protein Npl3p. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murthi A, Hopper AK. Genome-wide screen for inner nuclear membrane protein targeting in Saccharomyces cerevisiae: roles for N-acetylation and an integral membrane protein. Genetics. 2005;170:1553–1560. doi: 10.1534/genetics.105.043620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hurto RL, Hopper AK. P-body components, Dhh1 and Pat1, are involved in tRNA nuclear-cytoplasmic dynamics. RNA. 2011;17:912–924. doi: 10.1261/rna.2558511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coller J, Parker R. General translational repression by activators of mRNA decapping. Cell. 2005;122:875–886. doi: 10.1016/j.cell.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- 56.Gray MW. Evolution of organellar genomes. Curr Opin Genet Dev. 1999;9:678–687. doi: 10.1016/s0959-437x(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 57.Lang BF, Gray MW, Burger G. Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet. 1999;33:351–397. doi: 10.1146/annurev.genet.33.1.351. [DOI] [PubMed] [Google Scholar]
- 58.Hjort K, Goldberg AV, Tsaousis AD, Hirt RP, Embley TM. Diversity and reductive evolution of mitochondria among microbial eukaryotes. Philos Trans R Soc Lond B Biol Sci. 365:713–727. doi: 10.1098/rstb.2009.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suyama Y. The origins of mitochondrial ribonucleic acids in Tetrahymena pyriformis. Biochemistry. 1967;6:2829–2839. doi: 10.1021/bi00861a025. [DOI] [PubMed] [Google Scholar]
- 60.Okada K, Muneyoshi Y, Endo Y, Hori H. Production of yeast (m2G10) methyltransferase (Trm11 and Trm112 complex) in a wheat germ cell-free translation system. Nucleic Acids Symp Ser (Oxf) 2009:303–304. doi: 10.1093/nass/nrp152. [DOI] [PubMed] [Google Scholar]
- 61.Rusconi CP, Cech TR. Mitochondrial import of only one of three nuclear-encoded glutamine tRNAs in Tetrahymena thermophila. EMBO J. 1996;15:3286–3295. [PMC free article] [PubMed] [Google Scholar]
- 62.Rusconi CP, Cech TR. The anticodon is the signal sequence for mitochondrial import of glutamine tRNA in Tetrahymena. Genes Dev. 1996;10:2870–2880. doi: 10.1101/gad.10.22.2870. [DOI] [PubMed] [Google Scholar]
- 63.Simpson AM, Suyama Y, Dewes H, Campbell DA, Simpson L. Kinetoplastid mitochondria contain functional tRNAs which are encoded in nuclear DNA and also contain small minicircle and maxicircle transcripts of unknown function. Nucleic Acids Res. 1989;17:5427–5445. doi: 10.1093/nar/17.14.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hancock K, LeBlanc AJ, Donze D, Hajduk SL. Identification of nuclear encoded precursor tRNAs within the mitochondrion of Trypanosoma brucei. J Biol Chem. 1992;267:23963–23971. [PubMed] [Google Scholar]
- 65.Mottram JC, Bell SD, Nelson RG, Barry JD. tRNAs of Trypanosoma brucei. Unusual gene organization and mitochondrial importation. J Biol Chem. 1991;266:18313–18317. [PubMed] [Google Scholar]
- 66.Esseiva AC, Naguleswaran A, Hemphill A, Schneider A. Mitochondrial tRNA import in Toxoplasma gondii. J Biol Chem. 2004;279:42363–42368. doi: 10.1074/jbc.M404519200. [DOI] [PubMed] [Google Scholar]
- 67.Martin RP, Schneller JM, Stahl AJ, Dirheimer G. Import of nuclear deoxyribonucleic acid coded lysine-accepting transfer ribonucleic acid (anticodon C-U-U) into yeast mitochondria. Biochemistry. 1979;18:4600–4605. doi: 10.1021/bi00588a021. [DOI] [PubMed] [Google Scholar]
- 68.Rinehart J, Krett B, Rubio MA, Alfonzo JD, Soll D. Saccharomyces cerevisiae imports the cytosolic pathway for Gln-tRNA synthesis into the mitochondrion. Genes Dev. 2005;19:583–592. doi: 10.1101/gad.1269305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joyce PB, Gray MW. Nucleotide sequence of a second glutamine tRNA gene in wheat mitochondrial DNA. Nucleic Acids Res. 1989;17:4885. [PMC free article] [PubMed] [Google Scholar]
- 70.Pritchard AE, Seilhamer JJ, Mahalingam R, Sable CL, Venuti SE, Cummings DJ. Nucleotide sequence of the mitochondrial genome of Paramecium. Nucleic Acids Res. 1990;18:173–180. doi: 10.1093/nar/18.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marechal-Drouard L, Weil JH, Guillemaut P. Import of several tRNAs from the cytoplasm into the mitochondria in bean Phaseolus vulgaris. Nucleic Acids Res. 1988;16:4777–4788. doi: 10.1093/nar/16.11.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorner M, Altmann M, Paabo S, Morl M. Evidence for import of a lysyl-tRNA into marsupial mitochondria. Mol Biol Cell. 2001;12:2688–2698. doi: 10.1091/mbc.12.9.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salinas T, Duchene AM, Marechal-Drouard L. Recent advances in tRNA mitochondrial import. Trends Biochem Sci. 2008;33:320–329. doi: 10.1016/j.tibs.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Kamenski P, Kolesnikova O, Jubenot V, Entelis N, Krasheninnikov IA, Martin RP, Tarassov I. Evidence for an adaptation mechanism of mitochondrial translation via tRNA import from the cytosol. Mol Cell. 2007;26:625–637. doi: 10.1016/j.molcel.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 75.Bouzaidi-Tiali N, Aeby E, Charriere F, Pusnik M, Schneider A. Elongation factor 1a mediates the specificity of mitochondrial tRNA import in T. brucei. EMBO J. 2007;26:4302–4312. doi: 10.1038/sj.emboj.7601857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mirande M. Processivity of translation in the eukaryote cell: role of aminoacyl-tRNA synthetases. FEBS Lett. 2010;584:443–447. doi: 10.1016/j.febslet.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 77.Hartl FU, Pfanner N, Nicholson DW, Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989;988:1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- 78.Baker KP, Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991;349:205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- 79.Matouschek A, Pfanner N, Voos W. Protein unfolding by mitochondria. The Hsp70 import motor. EMBO Rep. 2000;1:404–410. doi: 10.1093/embo-reports/kvd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Entelis N, Brandina I, Kamenski P, Krasheninnikov IA, Martin RP, Tarassov I. A glycolytic enzyme, enolase, is recruited as a cofactor of tRNA targeting toward mitochondria in Saccharomyces cerevisiae. Genes Dev. 2006;20:1609–1620. doi: 10.1101/gad.385706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brandina I, Smirnov A, Kolesnikova O, Entelis N, Krasheninnikov IA, Martin RP, Tarassov I. tRNA import into yeast mitochondria is regulated by the ubiquitin-proteasome system. FEBS Lett. 2007;581:4248–4254. doi: 10.1016/j.febslet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Tarassov I, Entelis N, Martin RP. Mitochondrial import of a cytoplasmic lysine-tRNA in yeast is mediated by cooperation of cytoplasmic and mitochondrial lysyl-tRNA synthetases. EMBO J. 1995;14:3461–3471. doi: 10.1002/j.1460-2075.1995.tb07352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Curnow AW, Hong K, Yuan R, Kim S, Martins O, Winkler W, Henkin TM, Soll D. Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc Natl Acad Sci U S A. 1997;94:11819–11826. doi: 10.1073/pnas.94.22.11819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schon A, Kannangara CG, Gough S, Soll D. Protein biosynthesis in organelles requires misaminoacylation of tRNA. Nature. 1988;331:187–190. doi: 10.1038/331187a0. [DOI] [PubMed] [Google Scholar]
- 85.Charron C, Roy H, Blaise M, Giege R, Kern D. Non-discriminating and discriminating aspartyl-tRNA synthetases differ in the anticodon-binding domain. EMBO J. 2003;22:1632–1643. doi: 10.1093/emboj/cdg148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng L, Tumbula-Hansen D, Toogood H, Soll D. Expanding tRNA recognition of a tRNA synthetase by a single amino acid change. Proc Natl Acad Sci U S A. 2003;100:5676–5681. doi: 10.1073/pnas.0631525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frechin M, Senger B, Braye M, Kern D, Martin RP, Becker HD. Yeast mitochondrial Gln-tRNA(Gln) is generated by a GatFAB-mediated transamidation pathway involving Arc1p-controlled subcellular sorting of cytosolic GluRS. Genes Dev. 2009;23:1119–1130. doi: 10.1101/gad.518109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mukherjee S, Basu S, Home P, Dhar G, Adhya S. Necessary and sufficient factors for the import of transfer RNA into the kinetoplast mitochondrion. EMBO Rep. 2007;8:589–595. doi: 10.1038/sj.embor.7400979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chattopadhyay MK, Park MH, Tabor H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc Natl Acad Sci U S A. 2008;105:6554–6559. doi: 10.1073/pnas.0710970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Goswami S, Dhar G, Mukherjee S, Mahata B, Chatterjee S, Home P, Adhya S. A bifunctional tRNA import receptor from Leishmania mitochondria. Proc Natl Acad Sci U S A. 2006;103:8354–8359. doi: 10.1073/pnas.0510869103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Mahata B, Bhattacharyya SN, Mukherjee S, Adhya S. Correction of translational defects in patient-derived mutant mitochondria by complex-mediated import of a cytoplasmic tRNA. J Biol Chem. 2005;280:5141–5144. doi: 10.1074/jbc.C400572200. [DOI] [PubMed] [Google Scholar]
- 92.Paris Z, Rubio MA, Lukes J, Alfonzo JD. Mitochondrial tRNA import in Trypanosoma brucei is independent of thiolation and the Rieske protein. RNA. 2009;15:1398–1406. doi: 10.1261/rna.1589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nabholz CE, Horn EK, Schneider A. tRNAs and proteins are imported into mitochondria of Trypanosoma brucei by two distinct mechanisms. Mol Biol Cell. 1999;10:2547–2557. doi: 10.1091/mbc.10.8.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rubio MA, Liu X, Yuzawa H, Alfonzo JD, Simpson L. Selective importation of RNA into isolated mitochondria from Leishmania tarentolae. RNA. 2000;6:988–1003. doi: 10.1017/s1355838200991519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paris Z, Hashimi H, Lun S, Alfonzo JD, Lukes J. Futile import of tRNAs and proteins into the mitochondrion of Trypanosoma brucei evansi. Mol Biochem Parasitol. 2010 doi: 10.1016/j.molbiopara.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pino P, Aeby E, Foth BJ, Sheiner L, Soldati T, Schneider A, Soldati-Favre D. Mitochondrial translation in absence of local tRNA aminoacylation and methionyl tRNA Met formylation in Apicomplexa. Mol Microbiol. 2010;76:706–718. doi: 10.1111/j.1365-2958.2010.07128.x. [DOI] [PubMed] [Google Scholar]
- 97.Bhattacharyya SN, Adhya S. tRNA-triggered ATP hydrolysis and generation of membrane potential by the leishmania mitochondrial tRNA import complex. J Biol Chem. 2004;279:11259–11263. doi: 10.1074/jbc.C300540200. [DOI] [PubMed] [Google Scholar]
- 98.Lye LF, Chen DH, Suyama Y. Selective import of nuclear-encoded tRNAs into mitochondria of the protozoan Leishmania tarentolae. Mol Biochem Parasitol. 1993;58:233–245. doi: 10.1016/0166-6851(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 99.Kapushoc ST, Alfonzo JD, Rubio MA, Simpson L. End processing precedes mitochondrial importation and editing of tRNAs in Leishmania tarentolae. J Biol Chem. 2000;275:37907–37914. doi: 10.1074/jbc.M007838200. [DOI] [PubMed] [Google Scholar]
- 100.Schekman R. Editorial Expression of Concern: A bifunctional tRNA import receptor from Leishmania mitochondria. Proceedings of the National Academy of Sciences of teh United States of America. 2010;107:9476. doi: 10.1073/pnas.1004225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Delage L, Dietrich A, Cosset A, Marechal-Drouard L. In vitro import of a nuclearly encoded tRNA into mitochondria of Solanum tuberosum. Mol Cell Biol. 2003;23:4000–4012. doi: 10.1128/MCB.23.11.4000-4012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salinas T, Duchene AM, Delage L, Nilsson S, Glaser E, Zaepfel M, Marechal-Drouard L. The voltage-dependent anion channel, a major component of the tRNA import machinery in plant mitochondria. Proc Natl Acad Sci U S A. 2006;103:18362–18367. doi: 10.1073/pnas.0606449103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tarassov I, Entelis N, Martin RP. An intact protein translocating machinery is required for mitochondrial import of a yeast cytoplasmic tRNA. J Mol Biol. 1995;245:315–323. doi: 10.1006/jmbi.1994.0026. [DOI] [PubMed] [Google Scholar]
- 104.Colombini M. The published 3D structure of the VDAC channel: native or not? Trends in Biochemical Sciences. 2009;34:382–389. doi: 10.1016/j.tibs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Pusnik M, Charriere F, Maser P, Waller RF, Dagley MJ, Lithgow T, Schneider A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol Biol Evol. 2009;26:671–680. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- 106.Laforest MJ, Delage L, Marechal-Drouard L. The T-domain of cytosolic tRNAVal, an essential determinant for mitochondrial import. FEBS Lett. 2005;579:1072–1078. doi: 10.1016/j.febslet.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 107.Salinas T, Schaeffer C, Marechal-Drouard L, Duchene AM. Sequence dependence of tRNA(Gly) import into tobacco mitochondria. Biochimie. 2005;87:863–872. doi: 10.1016/j.biochi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Ramamonjisoa D, Kauffmann S, Choisne N, Marechal-Drouard L, Green G, Wintz H, Small I, Dietrich A. Structure and expression of several bean (Phaseolus vulgaris) nuclear transfer RNA genes: relevance to the process of tRNA import into plant mitochondria. Plant Mol Biol. 1998;36:613–625. doi: 10.1023/a:1005972023506. [DOI] [PubMed] [Google Scholar]
- 109.Akashi K, Hirayama J, Takenaka M, Yamaoka S, Suyama Y, Fukuzawa H, Ohyama K. Accumulation of nuclear-encoded tRNA(Thr) (AGU) in mitochondria of the liverwort Marchantia polymorpha. Biochim Biophys Acta. 1997;1350:262–266. doi: 10.1016/s0167-4781(96)00239-4. [DOI] [PubMed] [Google Scholar]
- 110.LeBlanc AJ, Yermovsky-Kammerer AE, Hajduk SL. A nuclear encoded and mitochondrial imported dicistronic tRNA precursor in Trypanosoma brucei. J Biol Chem. 1999;274:21071–21077. doi: 10.1074/jbc.274.30.21071. [DOI] [PubMed] [Google Scholar]
- 111.Tan TH, Pach R, Crausaz A, Ivens A, Schneider A. tRNAs in Trypanosoma brucei: genomic organization, expression, and mitochondrial import. Mol Cell Biol. 2002;22:3707–3717. doi: 10.1128/MCB.22.11.3707-3716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lima BD, Simpson L. Sequence-dependent in vivo importation of tRNAs into the mitochondrion of Leishmania tarentolae. RNA. 1996;2:429–440. [PMC free article] [PubMed] [Google Scholar]
- 113.Shi X, Chen DH, Suyama Y. A nuclear tRNA gene cluster in the protozoan Leishmania tarentolae and differential distribution of nuclear-encoded tRNAs between the cytosol and mitochondria. Mol Biochem Parasitol. 1994;65:23–37. doi: 10.1016/0166-6851(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 114.Nabholz CE, Hauser R, Schneider A. Leishmania tarentolae contains distinct cytosolic and mitochondrial glutaminyl-tRNA synthetase activities. Proc Natl Acad Sci U S A. 1997;94:7903–7908. doi: 10.1073/pnas.94.15.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bhattacharyya SN, Chatterjee S, Adhya S. Mitochondrial RNA import in Leishmania tropica: aptamers homologous to multiple tRNA domains that interact cooperatively or antagonistically at the inner membrane. Mol Cell Biol. 2002;22:4372–4382. doi: 10.1128/MCB.22.12.4372-4382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goswami S, Chatterjee S, Bhattacharyya SN, Basu S, Adhya S. Allosteric regulation of tRNA import: interactions between tRNA domains at the inner membrane of Leishmania mitochondria. Nucleic Acids Res. 2003;31:5552–5559. doi: 10.1093/nar/gkg773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suyama Y, Wong S, Campbell DA. Regulated tRNA import in Leishmania mitochondria. Biochim Biophys Acta. 1998;1396:138–142. doi: 10.1016/s0167-4781(97)00197-8. [DOI] [PubMed] [Google Scholar]
- 118.Kolesnikova O, Entelis N, Kazakova H, Brandina I, Martin RP, Tarassov I. Targeting of tRNA into yeast and human mitochondria: the role of anticodon nucleotides. Mitochondrion. 2002;2:95–107. doi: 10.1016/s1567-7249(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 119.Kazakova HA, Entelis NS, Martin RP, Tarassov IA. The aminoacceptor stem of the yeast tRNA(Lys) contains determinants of mitochondrial import selectivity. FEBS Lett. 1999;442:193–197. doi: 10.1016/s0014-5793(98)01653-6. [DOI] [PubMed] [Google Scholar]
- 120.Entelis NS, Kieffer S, Kolesnikova OA, Martin RP, Tarassov IA. Structural requirements of tRNALys for its import into yeast mitochondria. Proc Natl Acad Sci U S A. 1998;95:2838–2843. doi: 10.1073/pnas.95.6.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kolesnikova O, Kazakova H, Comte C, Steinberg S, Kamenski P, Martin RP, Tarassov I, Entelis N. Selection of RNA aptamers imported into yeast and human mitochondria. RNA. 2010;16:926–941. doi: 10.1261/rna.1914110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sugiura M, Hirose T, Sugita M. Evolution and mechanism of translation in chloroplasts. Annu Rev Genet. 1998;32:437–459. doi: 10.1146/annurev.genet.32.1.437. [DOI] [PubMed] [Google Scholar]
- 123.Wolfe KH, Morden CW, Palmer JD. Small single-copy region of plastid DNA in the non-photosynthetic angiosperm Epifagus virginiana contains only two genes. Differences among dicots, monocots and bryophytes in gene organization at a non-bioenergetic locus. J Mol Biol. 1992;223:95–104. doi: 10.1016/0022-2836(92)90718-y. [DOI] [PubMed] [Google Scholar]
- 124.Wolfe KH, Morden CW, Ems SC, Palmer JD. Rapid evolution of the plastid translational apparatus in a nonphotosynthetic plant: loss or accelerated sequence evolution of tRNA and ribosomal protein genes. J Mol Evol. 1992;35:304–317. doi: 10.1007/BF00161168. [DOI] [PubMed] [Google Scholar]
- 125.Scaglia F, Wong LJ. Human mitochondrial transfer RNAs: role of pathogenic mutation in disease. Muscle Nerve. 2008;37:150–171. doi: 10.1002/mus.20917. [DOI] [PubMed] [Google Scholar]
- 126.Wittenhagen LM, Kelley SO. Impact of disease-related mitochondrial mutations on tRNA structure and function. Trends Biochem Sci. 2003;28:605–611. doi: 10.1016/j.tibs.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 127.Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S. Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science. 2006;314:471–474. doi: 10.1126/science.1129754. [DOI] [PubMed] [Google Scholar]
- 128.Entelis NS, Kolesnikova OA, Martin RP, Tarassov IA. RNA delivery into mitochondria. Adv Drug Deliv Rev. 2001;49:199–215. doi: 10.1016/s0169-409x(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 129.Kolesnikova OA, Entelis NS, Mireau H, Fox TD, Martin RP, Tarassov IA. Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science. 2000;289:1931–1933. doi: 10.1126/science.289.5486.1931. [DOI] [PubMed] [Google Scholar]
- 130.Kolesnikova OA, Entelis NS, Jacquin-Becker C, Goltzene F, Chrzanowska-Lightowlers ZM, Lightowlers RN, Martin RP, Tarassov I. Nuclear DNA-encoded tRNAs targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the MERRF syndrome in cultured human cells. Hum Mol Genet. 2004;13:2519–2534. doi: 10.1093/hmg/ddh267. [DOI] [PubMed] [Google Scholar]
- 131.Neiman M, Taylor DR. The causes of mutation accumulation in mitochondrial genomes. Proc Biol Sci. 2009;276:1201–1209. doi: 10.1098/rspb.2008.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]