Abstract
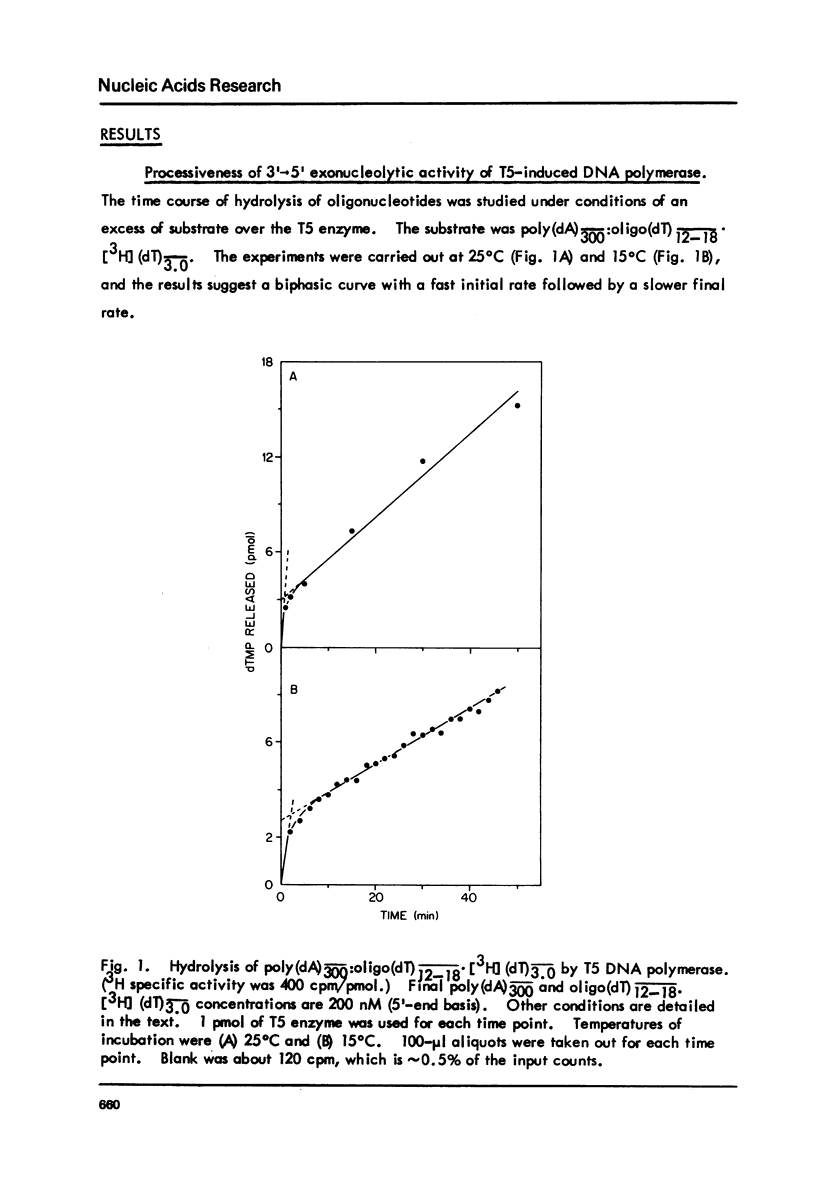
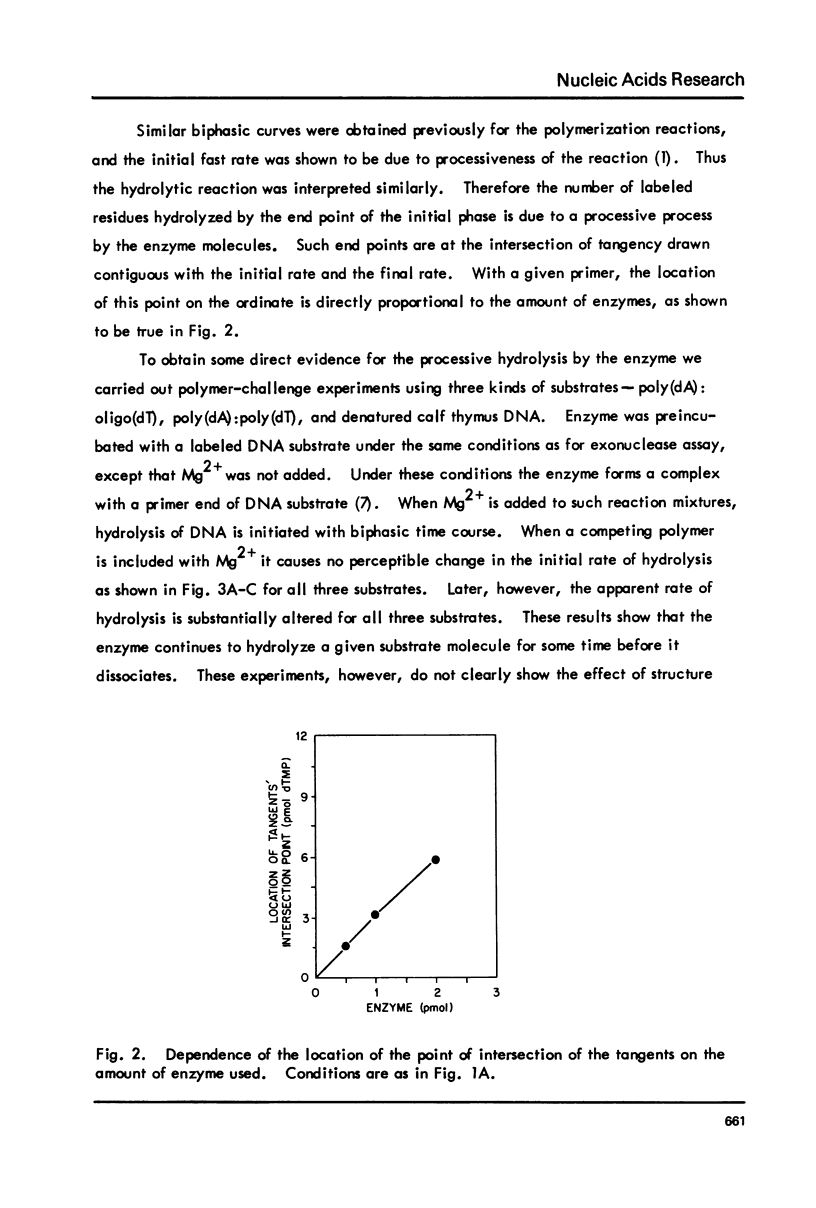
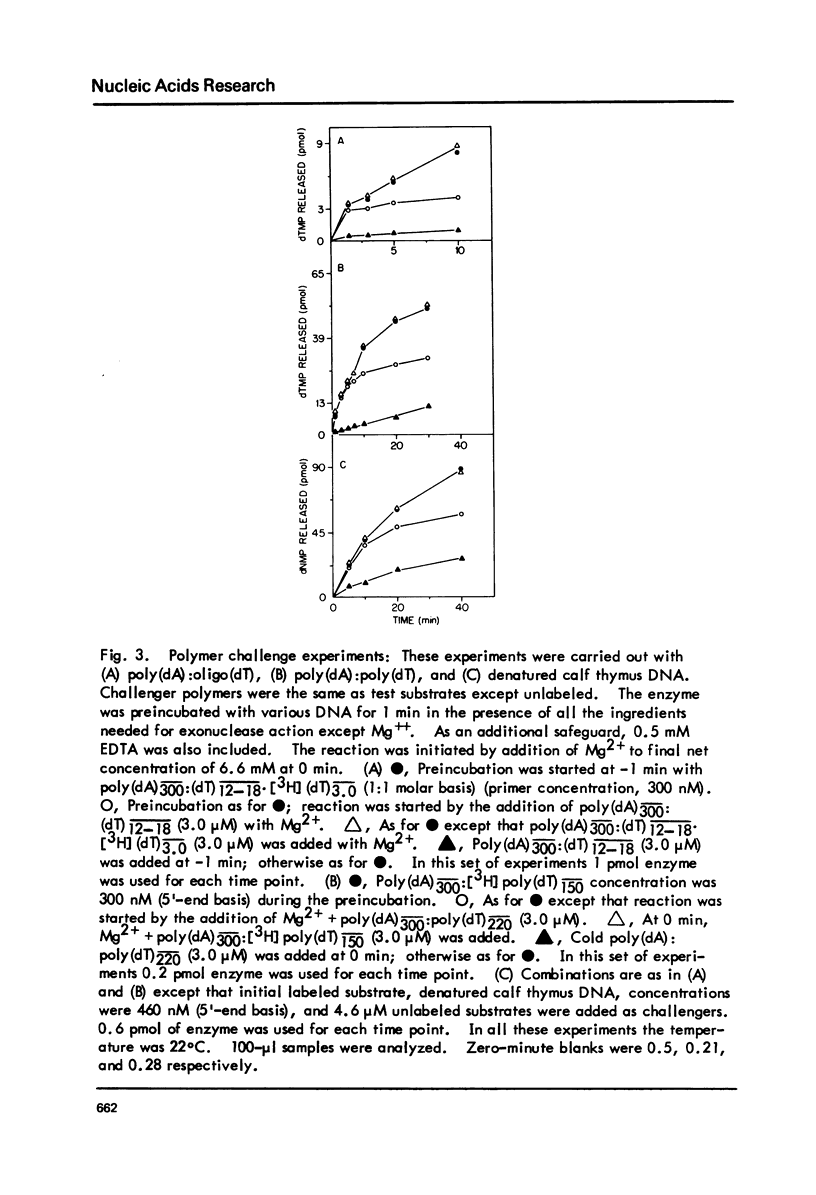
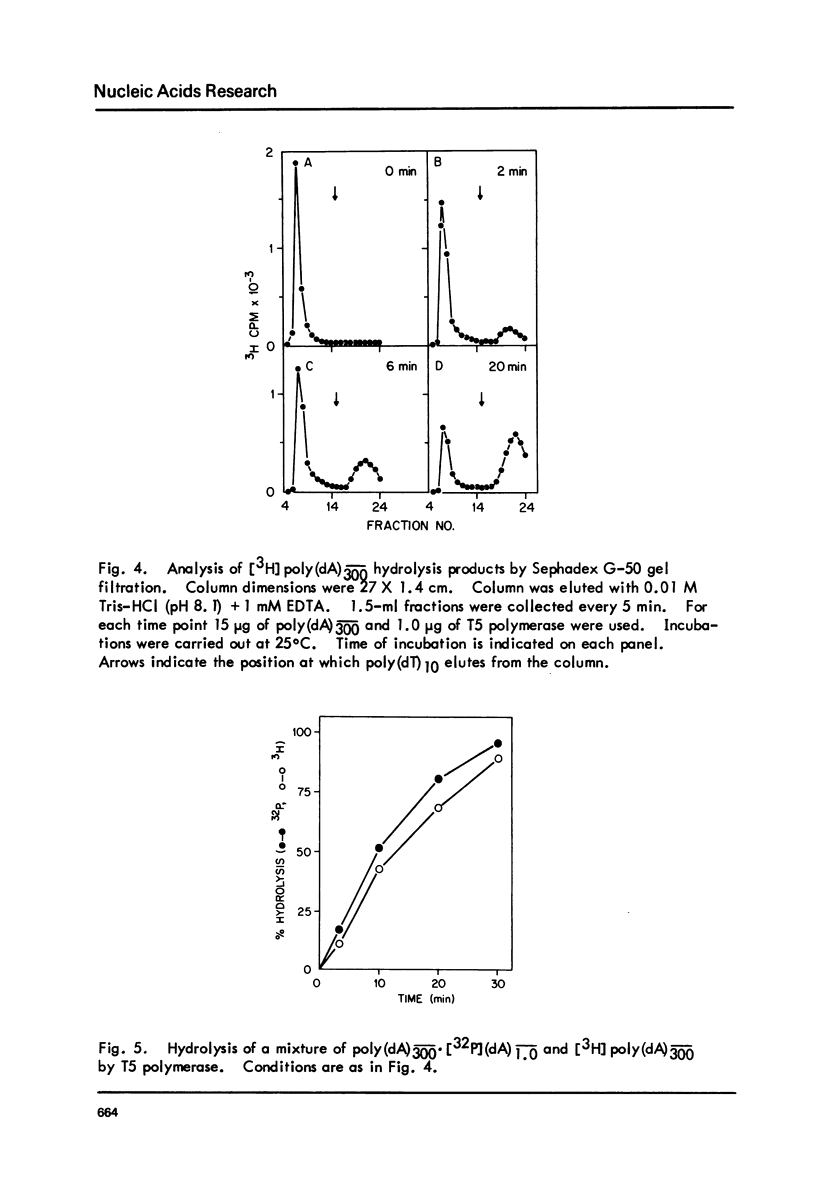
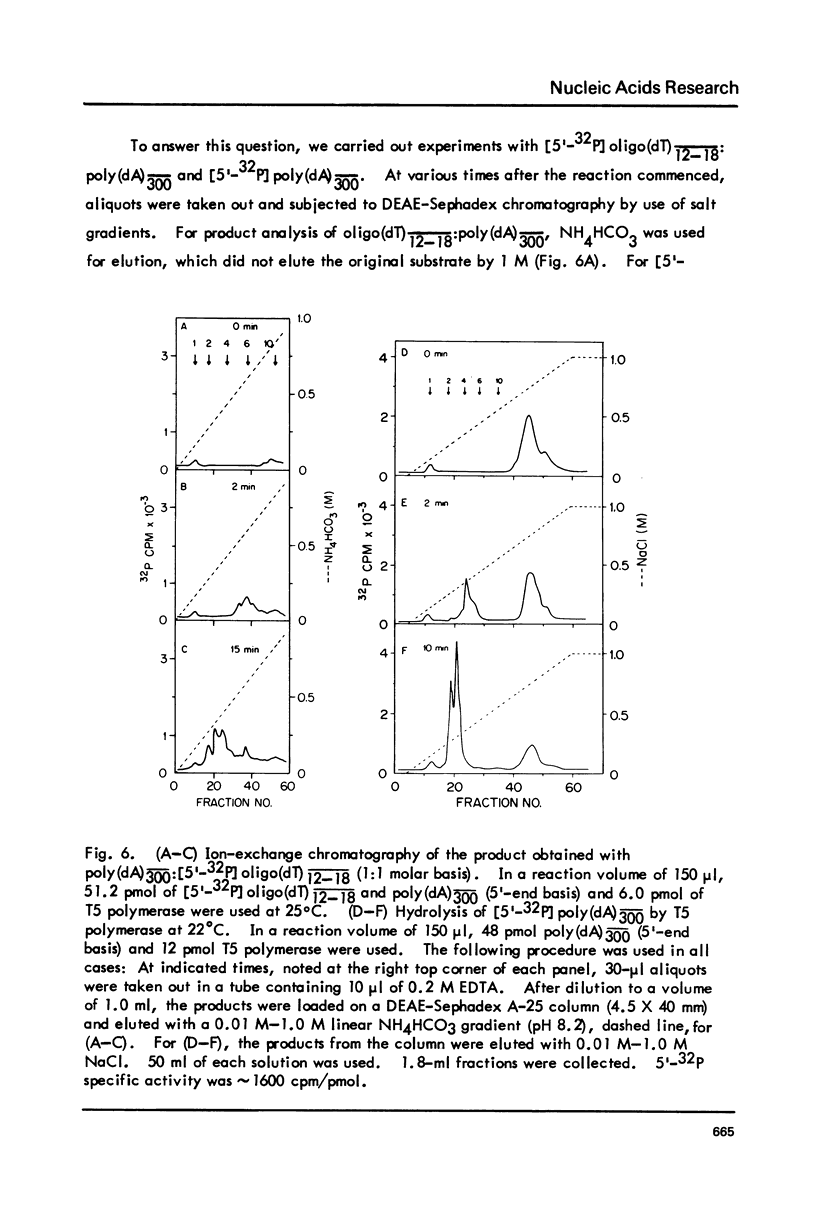
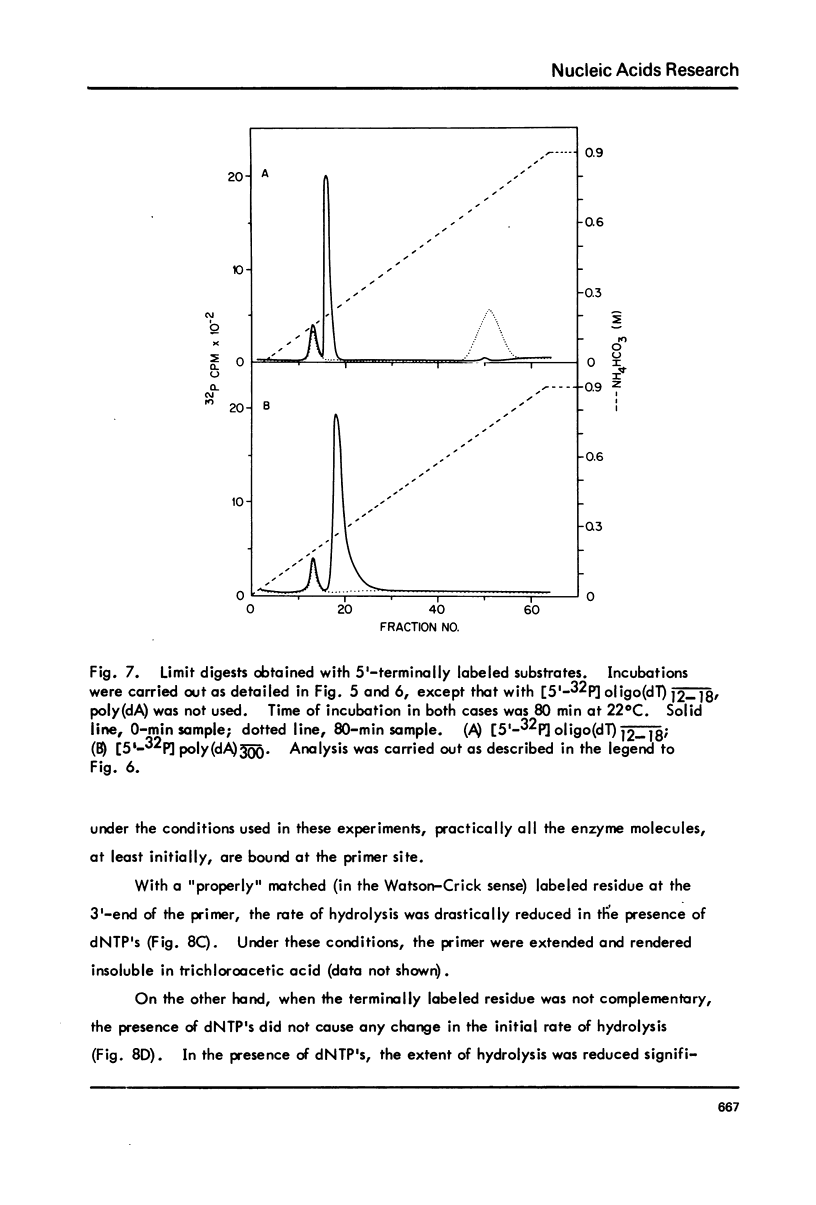
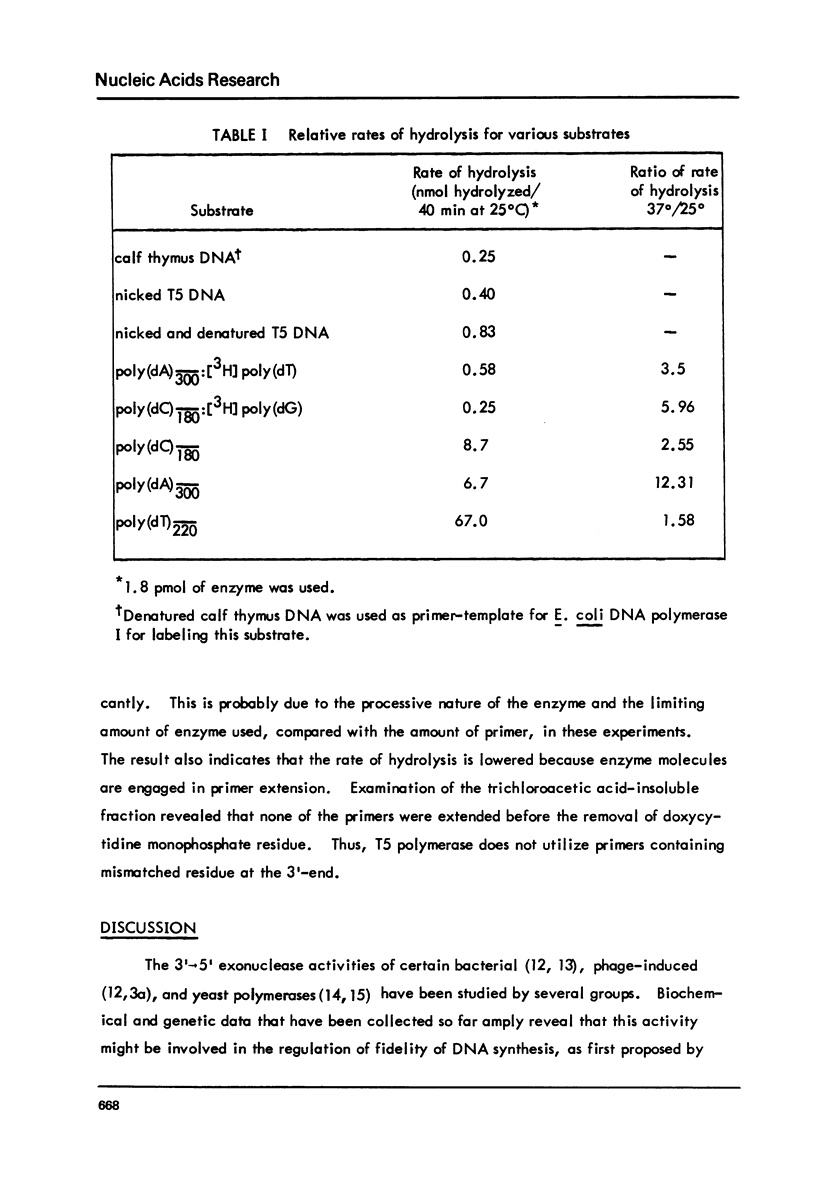
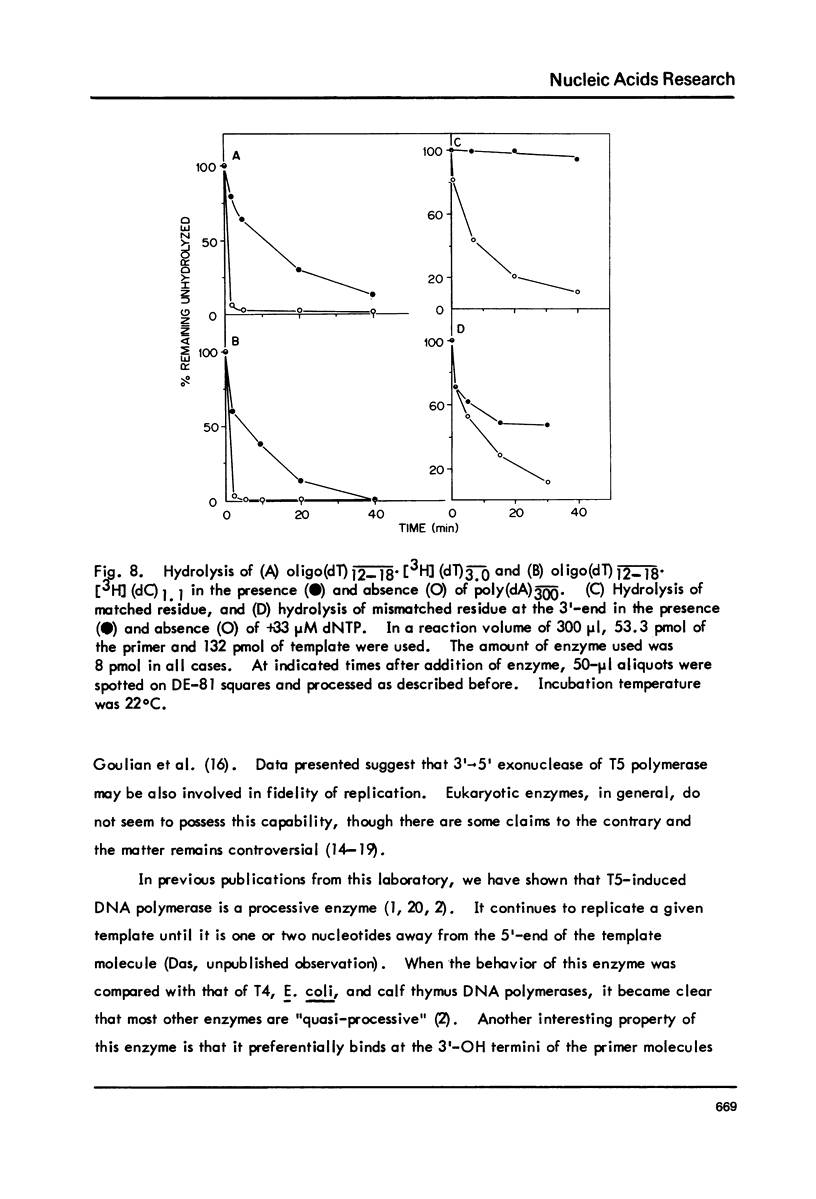
T5-induced DNA polymerase has an associated 3' to 5' exonuclease activity. Both single-stranded and duplex DNA are hydrolyzed by this enzyme in a quasi-processive manner. This is indicated by the results of polymer-challenge experiments utilizing product analysis techniques. Due to the quasi-processive mode of hydrolysis, the kinetics of label release from the 3'-terminally labeled oligonucleotide substrates, annealed to complementary homopolymers, show an initial high rate of hydrolysis. In the case of both single-stranded and duplex DNA substrates, hydrolysis seems to continue, at best, up to the point where the enzyme is five or six nucleotides away from the 5-end. The enzyme carries out mismatch repair, as evidenced by experiments with primer molecules containing improper base residues at the 3'-OH terminus. Control experiments with complementary base residues at the 3'-end indicate that extensive removal of terminal residue takes place in the presence of dNTP's only when such residues are "improper" in the Watson-Crick sense.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Holloman W. K., Kairis M. V., Spanos A., Yarranton G. T. A DNA polymerase from Ustilago maydis. 1. Purification and properties of the polymerase activity. Eur J Biochem. 1976 Feb 2;62(1):131–142. doi: 10.1111/j.1432-1033.1976.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Byrnes J. J., Black V. L. Comparison of DNA polymerase alpha and delta from bone marrow. Biochemistry. 1978 Oct 3;17(20):4226–4231. doi: 10.1021/bi00613a018. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Chang L. M. DNA polymerases from bakers' yeast. J Biol Chem. 1977 Mar 25;252(6):1873–1880. [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Exonuclease associated with bacteriophage T5-Induced DNA polymerase. J Virol. 1976 Oct;20(1):70–77. doi: 10.1128/jvi.20.1.70-77.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Mechanism of T5-induced DNA polymerase. I. Replication of short primer templates. J Biol Chem. 1977 Dec 10;252(23):8700–8707. [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Mechanism of T5-induced DNA polymerase. II. Characterization of the dead-end complex. J Biol Chem. 1977 Dec 10;252(23):8708–8712. [PubMed] [Google Scholar]
- Das S. K., Fujimura R. K. Processiveness of DNA polymerases. A comparative study using a simple procedure. J Biol Chem. 1979 Feb 25;254(4):1227–1232. [PubMed] [Google Scholar]
- Das S. K. Primer-mediated inhibition of the hydrolysis of template DNA by T5-induced DNA polymerase. Biochem Biophys Res Commun. 1977 Nov 7;79(1):247–253. doi: 10.1016/0006-291x(77)90087-0. [DOI] [PubMed] [Google Scholar]
- Fujimura R. K., Roop B. C. Characterization of DNA polymerase induced by bacteriophage T5 with DNA containing single strand breaks. J Biol Chem. 1976 Apr 10;251(7):2168–2174. [PubMed] [Google Scholar]
- Fujimura R. K., Roop B. C. Temperature-sensitive DNA polymerase induced by a bacteriophage T5 mutant: relationship between polymerase and exonuclease activities. Biochemistry. 1976 Oct 5;15(20):4403–4409. doi: 10.1021/bi00665a009. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Low R. L., Rashbaum S. A., Cozzarelli N. R. Purification and characterization of DNA polymerase III from Bacillus subtilis. J Biol Chem. 1976 Mar 10;251(5):1311–1325. [PubMed] [Google Scholar]
- Nossal N. G., Hershfield M. S. Nuclease activity in a fragment of bacteriophage T4 deoxyribonucleic acid polymerase induced by the amber mutant am B22. J Biol Chem. 1971 Sep 10;246(17):5414–5426. [PubMed] [Google Scholar]
- Nossal N. G., Singer M. F. The processive degradation of individual polyribonucleotide chains. I. Escherichia coli ribonuclease II. J Biol Chem. 1968 Mar 10;243(5):913–922. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Olivera B. M. Processivity of DNA exonucleases. J Biol Chem. 1978 Jan 25;253(2):424–429. [PubMed] [Google Scholar]


