Abstract
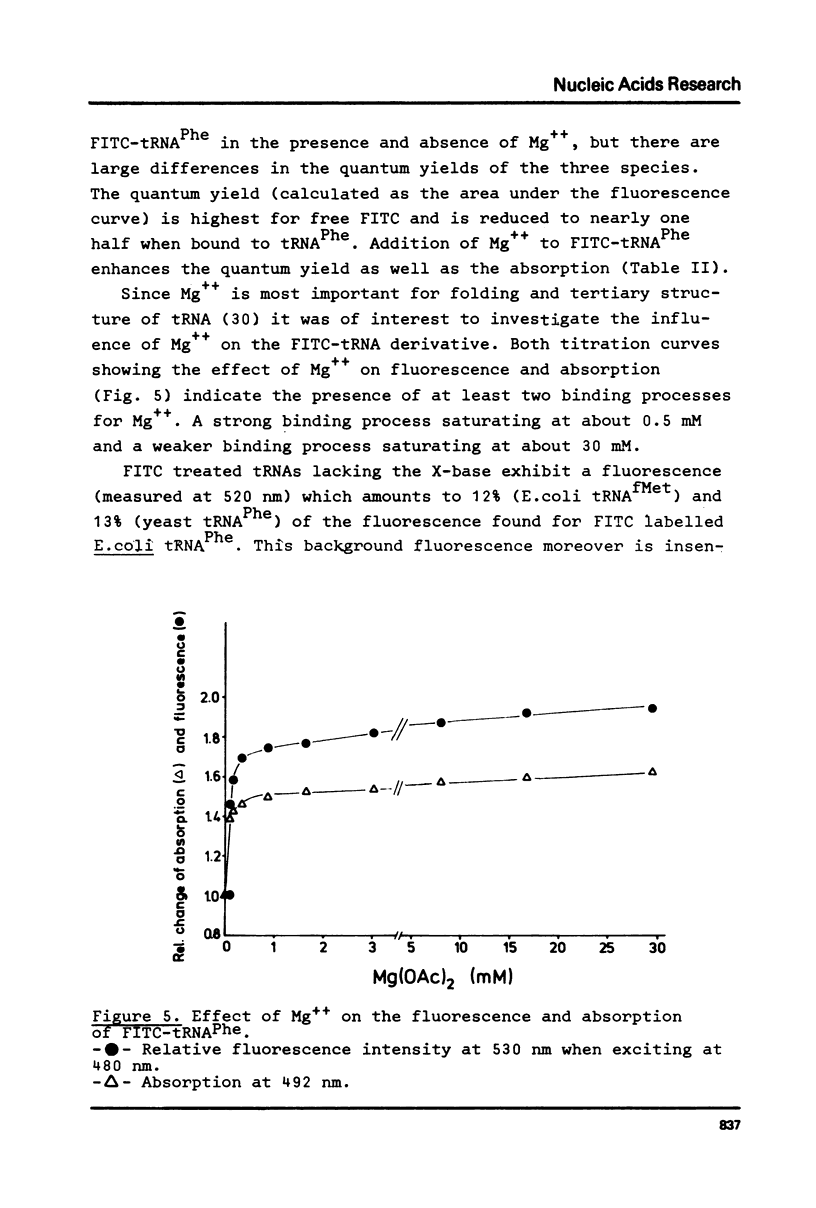
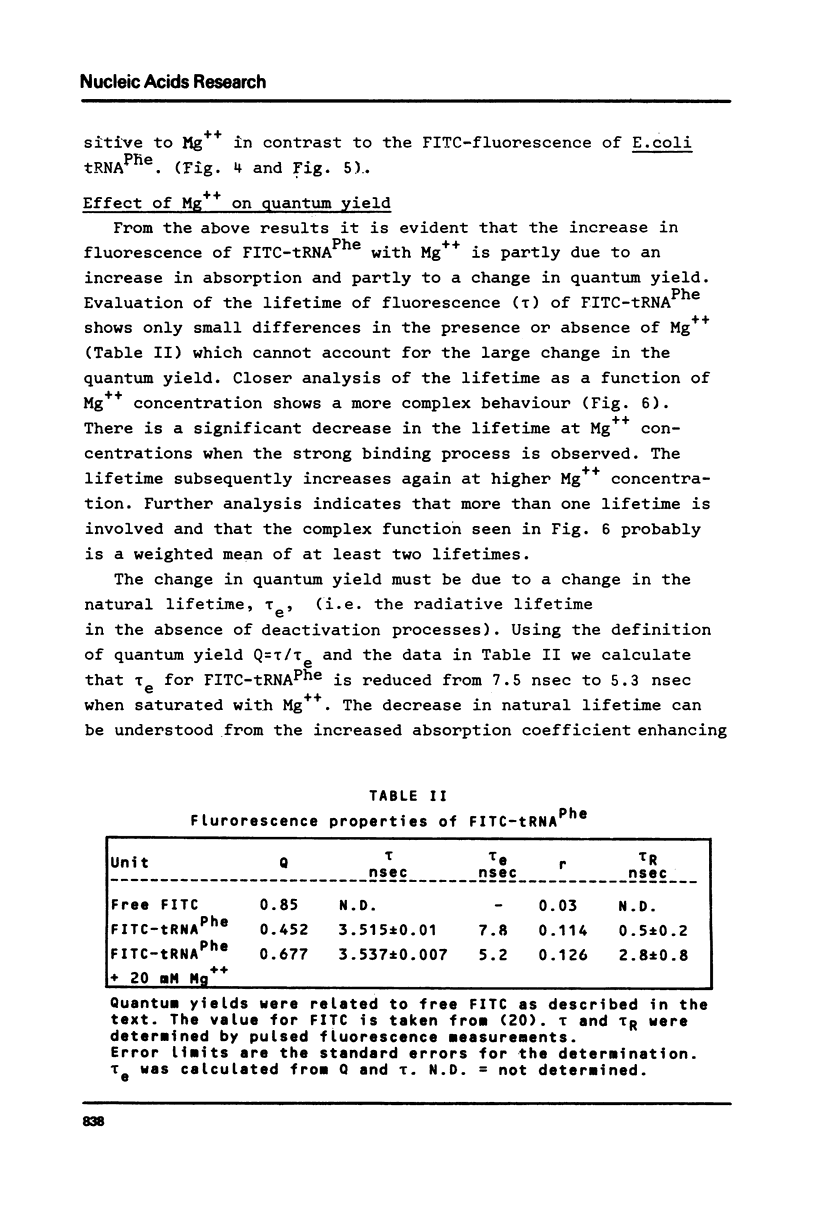
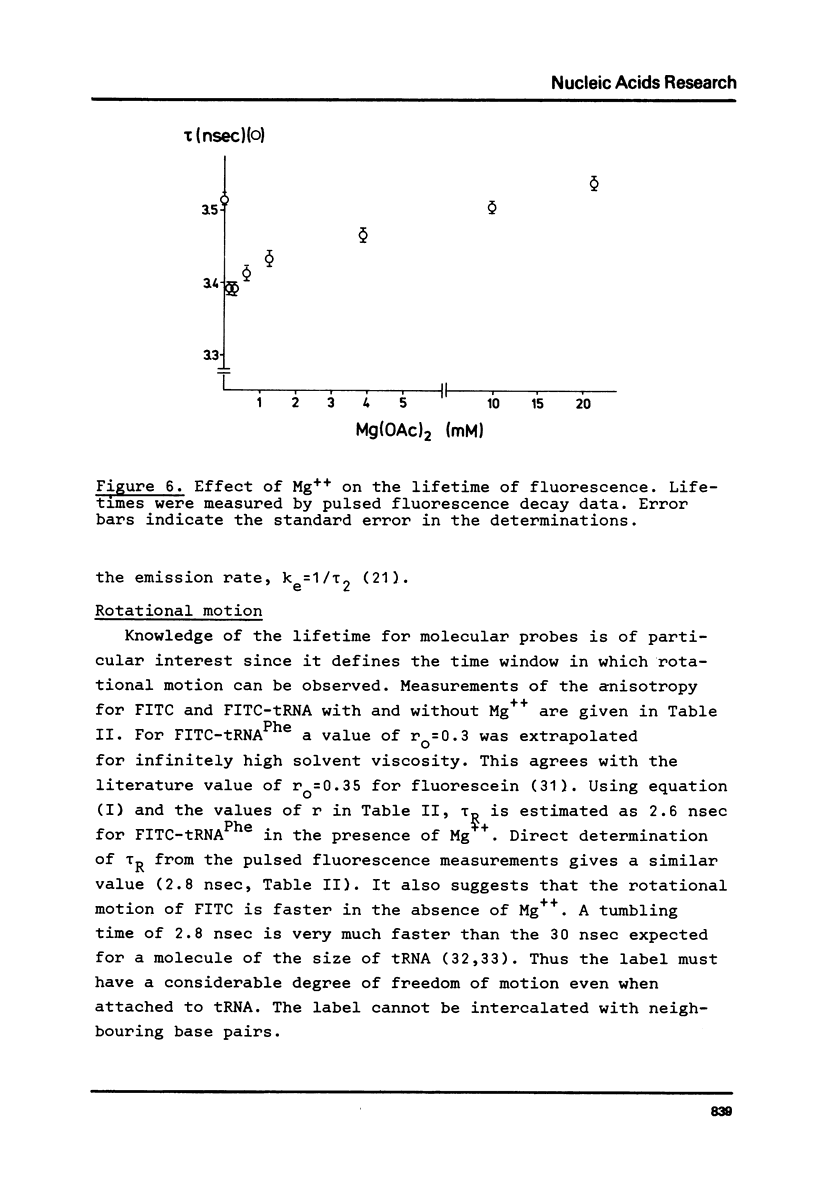
E. coli tRNAPhe has been labelled with fluorescein isothiocyanate taking advantage of the reactivity of this compound for primary aliphatic amino groups as exist in this tRNA as the modified base X(3-(3-amino-3-carboxypropyl)uracil). The extent of labelling was calculated as 1.6 nmole/A260 unit suggesting one dye molecule per tRNA. The FITC-tRNA showed full activity in aminoacylation and polypeptide synthesis. The absorption and fluorescence of the label respond markedly on addition of Mg++ to the tRNA. The label appears to be a sensitive probe of tRNAPhe tertiary structure.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Arai K., Kawakita M., Kaziro Y. Studies on the polypeptide elongation factors from E. coli. IV. Crystalline Tu-GTP, Tu-Gpp(CH2)p, and phenylalanyl-tRNA-Tu-GTP complex. J Biochem. 1974 Aug;76(2):283–292. doi: 10.1093/oxfordjournals.jbchem.a130570. [DOI] [PubMed] [Google Scholar]
- Beardsley K., Tao T., Cantor C. R. Studies on the conformation of the anticodon loop of phenylalanine transfer ribonucleic acid. Effect of environment on the fluorescence of the Y base. Biochemistry. 1970 Sep 1;9(18):3524–3532. doi: 10.1021/bi00820a005. [DOI] [PubMed] [Google Scholar]
- Brot N., Redfield B., Weissbach H. Studies on the reaction of the aminoacyl-tRNA-Tu-GTP complex with ribosomal subunits. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1388–1395. doi: 10.1016/0006-291x(70)90541-3. [DOI] [PubMed] [Google Scholar]
- Ehrenberg M., Rigler R., Wintermeyer W. On the structure and conformational dynamics of yeast phenylalanine-accepting transfer ribonucleic acid in solution. Biochemistry. 1979 Oct 16;18(21):4588–4599. doi: 10.1021/bi00588a020. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Feuer B., Yamane T. Luminescence and binding studies on tRNA-Phe. Proc Natl Acad Sci U S A. 1970 Mar;65(3):638–644. doi: 10.1073/pnas.65.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Rhodes D., Brown R. S., Klug A. A crystallographic study of metal-binding to yeast phenylalanine transfer RNA. J Mol Biol. 1977 Apr 15;111(3):315–328. doi: 10.1016/s0022-2836(77)80054-5. [DOI] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Pachmann U., Cronvall E., Rigler R., Hirsch R., Wintermeyer W., Zachau H. G. On the specificity of interactions between transfer ribonucleic acids and aminoacyl-tRNA synthetases. Eur J Biochem. 1973 Nov 1;39(1):265–273. doi: 10.1111/j.1432-1033.1973.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Kownatzki R., Maass G. Fluoresceinylthiocarbamyl-tRNATyr: a useful derivative of tRNATyr (E.coli) for physicochemical studies. Nucleic Acids Res. 1977 Feb;4(2):327–338. doi: 10.1093/nar/4.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigler R., Ehrenberg M. Fluorescence relaxation spectroscopy in the analysis of macromolecular structure and motion. Q Rev Biophys. 1976 Feb;9(1):1–19. doi: 10.1017/s0033583500002122. [DOI] [PubMed] [Google Scholar]
- Rigler R., Ehrenberg M. Molecular interactions and structure as analysed by fluorescence relaxation spectroscopy. Q Rev Biophys. 1973 May;6(2):139–199. doi: 10.1017/s003358350000113x. [DOI] [PubMed] [Google Scholar]
- Robison B., Zimmerman T. P. A conformational study of yeast phenylalanine transfer ribonucleic acid. J Biol Chem. 1971 Jan 10;246(1):110–117. [PubMed] [Google Scholar]
- Römer R., Hach R. tRNA conformation and magnesium binding. A study of a yeast phenylalanine-specific tRNA by a fluorescent indicator and differential melting curves. Eur J Biochem. 1975 Jun 16;55(1):271–284. doi: 10.1111/j.1432-1033.1975.tb02160.x. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Schechter A. N. Covalent attachment of fluorescent probes to the X-base of Escherichia coli phenylalanine transfer ribonucleic acid. Nucleic Acids Res. 1977 Jul;4(7):2161–2167. doi: 10.1093/nar/4.7.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Faulhammer H. G. Participation of X47-fluorescamine modified E. coli tRNAs in in vitro protein biosynthesis. Nucleic Acids Res. 1978 Dec;5(12):4837–4853. doi: 10.1093/nar/5.12.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Crothers D. M. Equilibrium binding of magnesium(II) by Escherichia coli tRNAfMet. Biochemistry. 1976 Jan 13;15(1):157–160. doi: 10.1021/bi00646a024. [DOI] [PubMed] [Google Scholar]
- Tao T., Nelson J. H., Cantor C. R. Conformational studies on transfer ribonucleic acid. Fluorescence lifetime and nanosecond depolarization measurements on bound ethidium bromidee. Biochemistry. 1970 Sep 1;9(18):3514–3524. doi: 10.1021/bi00820a004. [DOI] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. Replacement of Y base, dihydrouracil, and 7-methylguanine in tRNA by artificial odd bases. FEBS Lett. 1971 Nov 1;18(2):214–218. doi: 10.1016/0014-5793(71)80447-7. [DOI] [PubMed] [Google Scholar]


