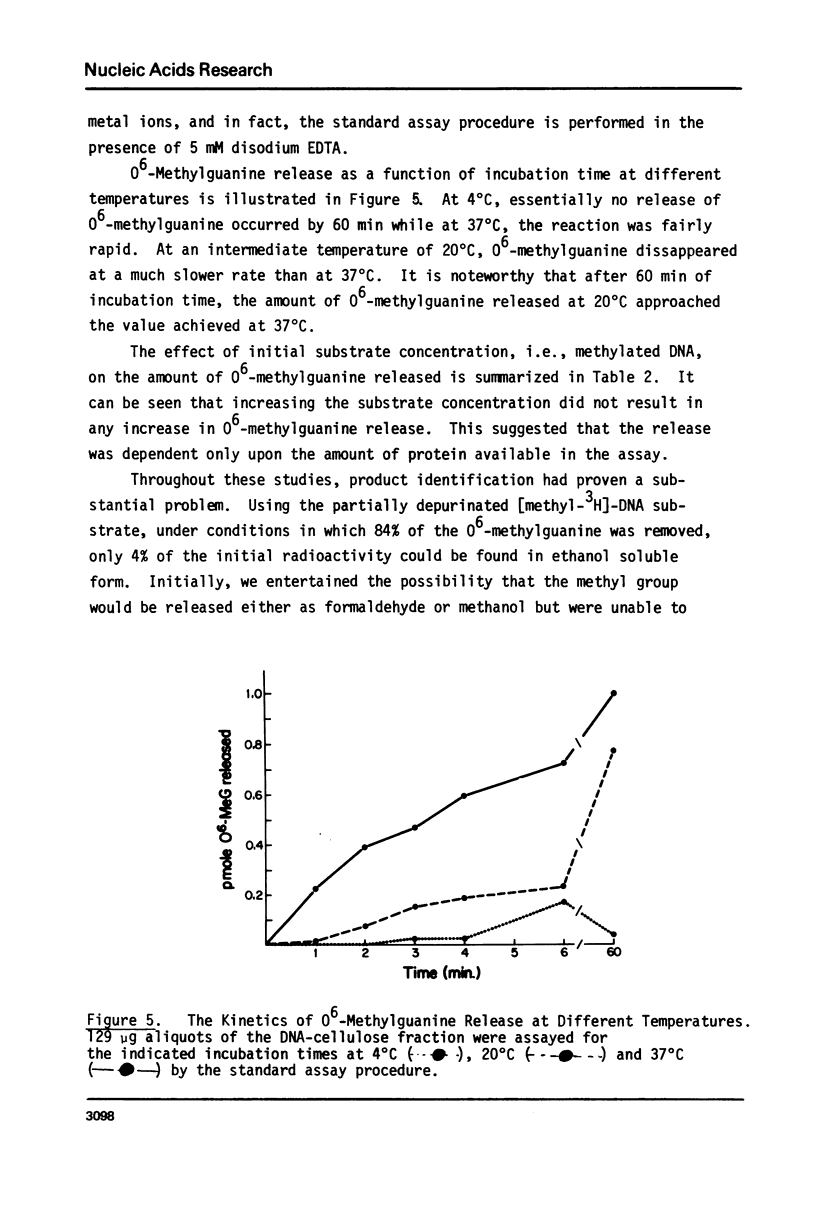
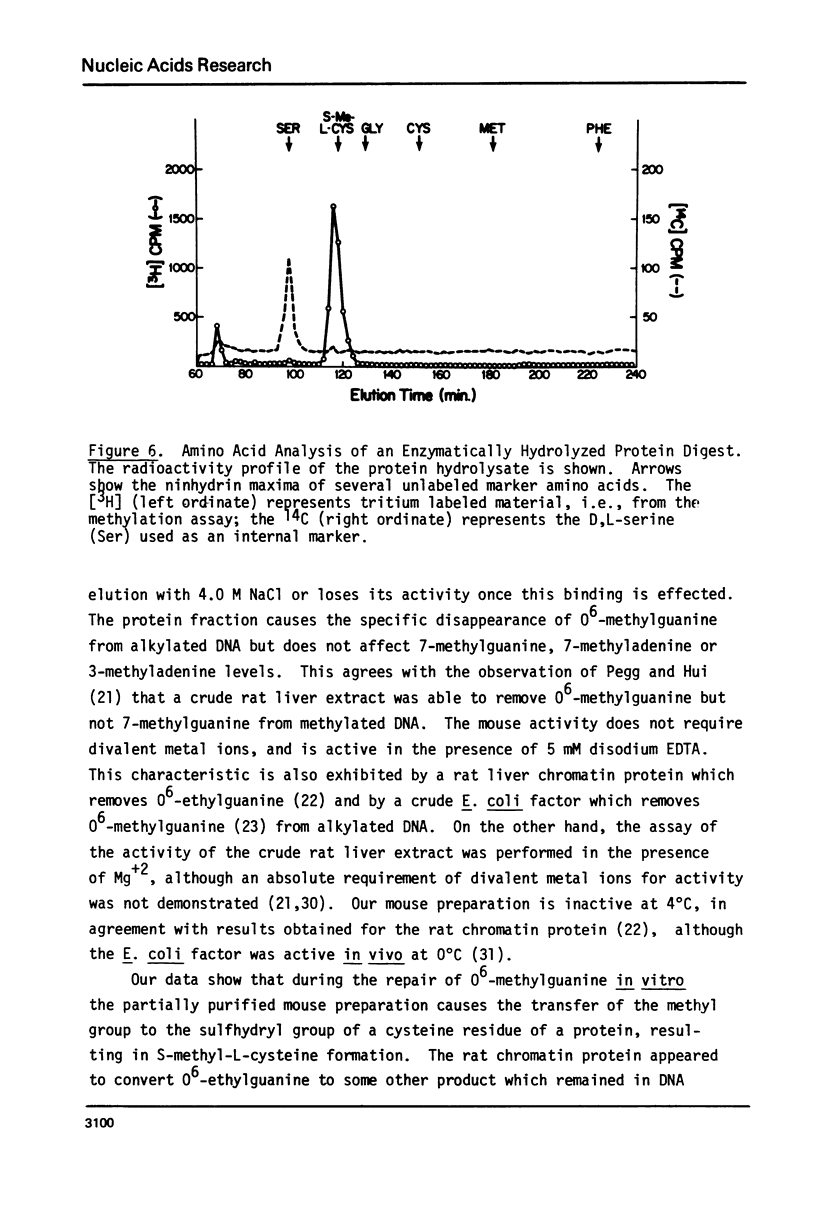
Abstract
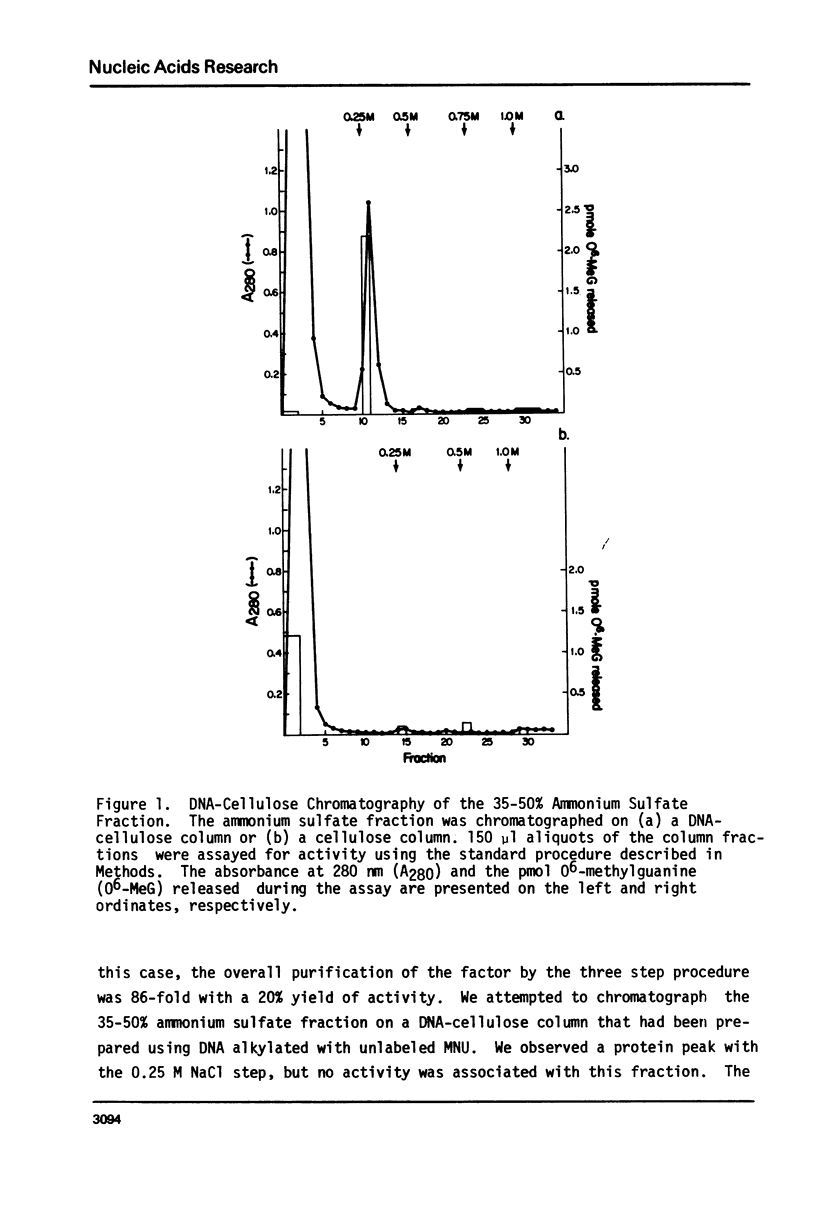
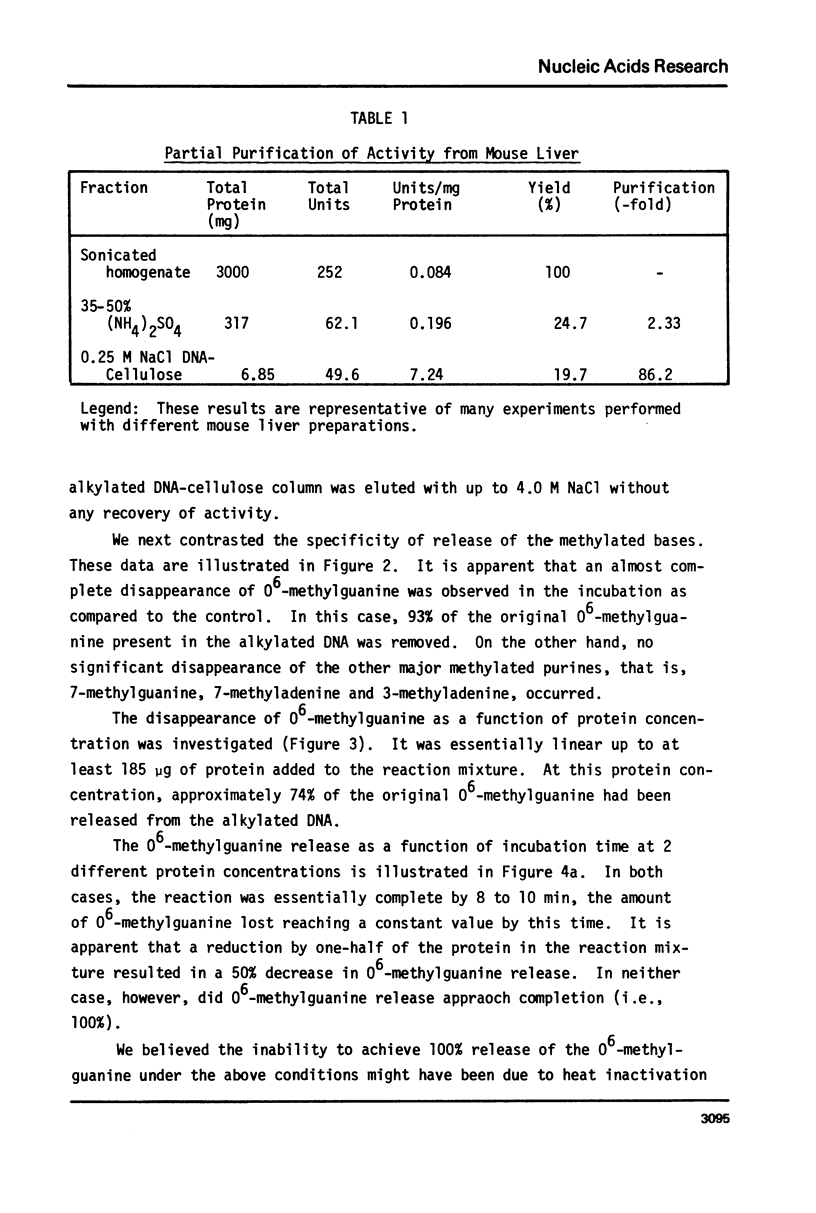
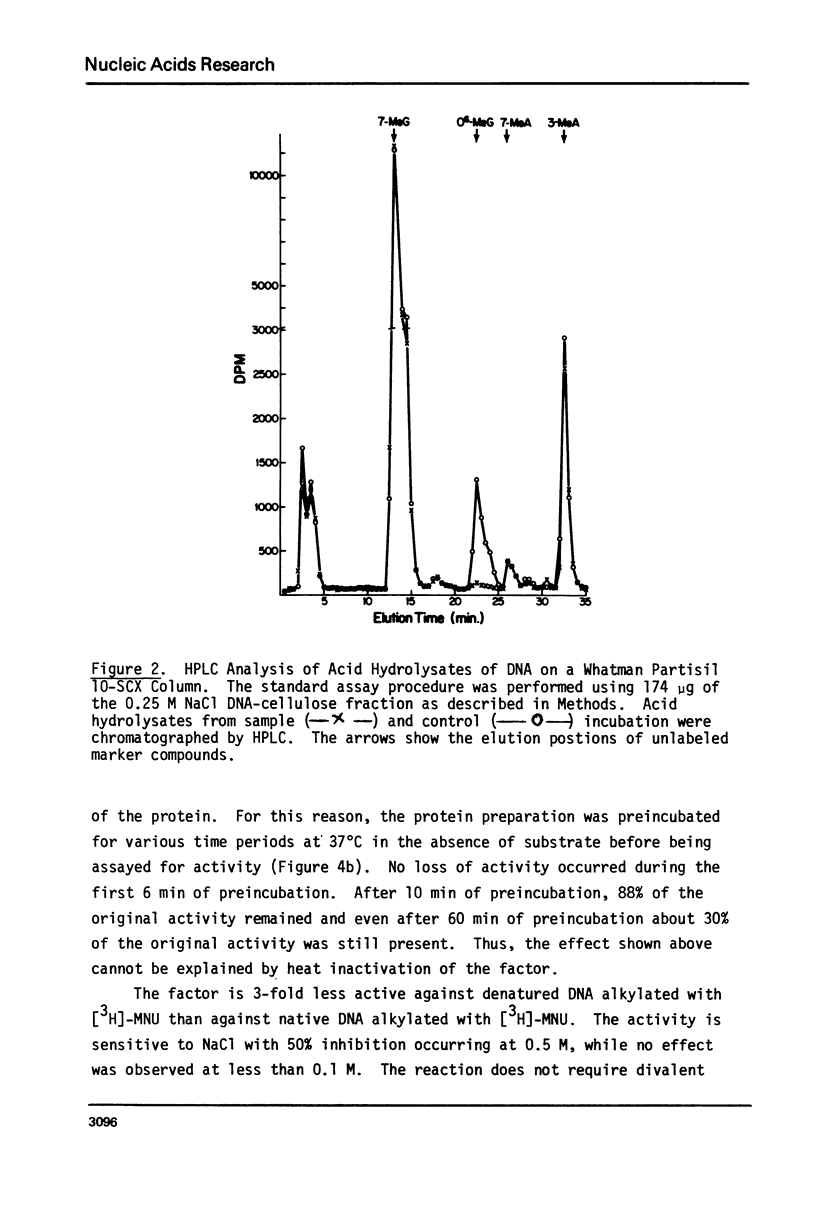
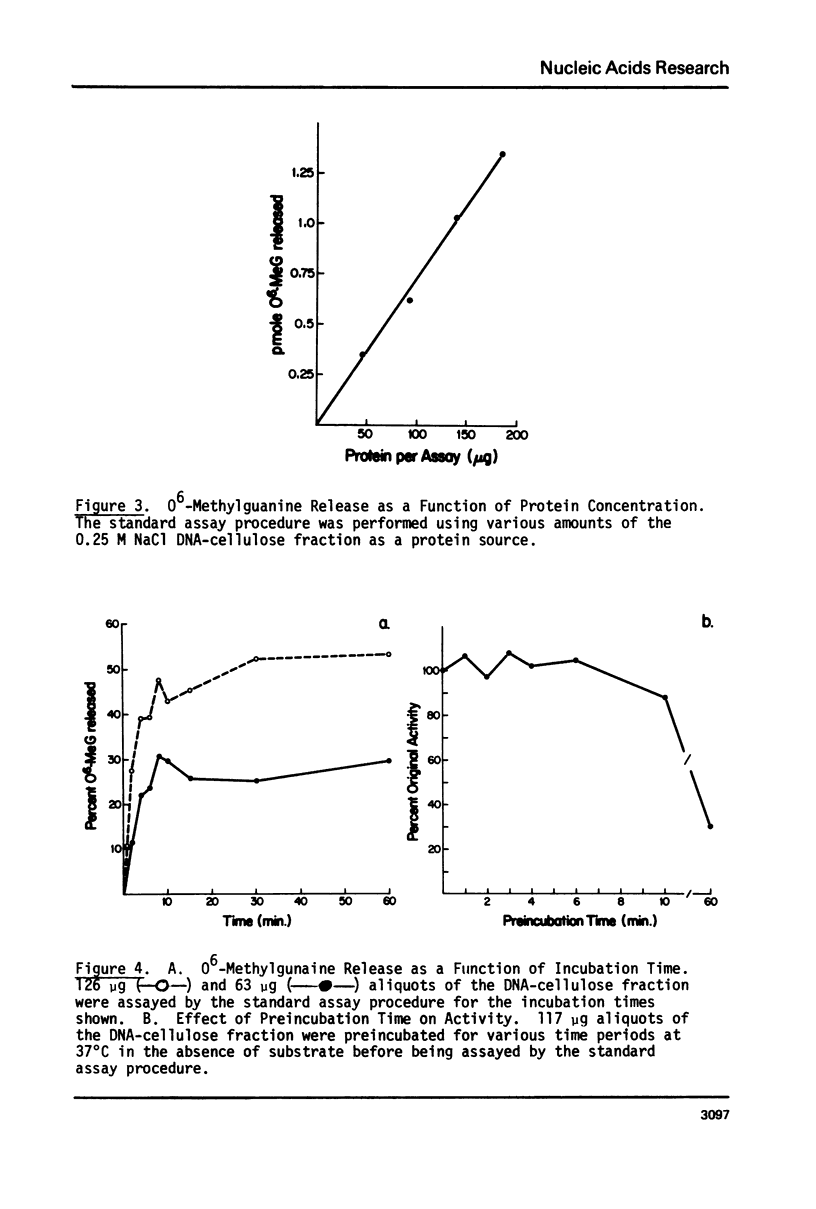
An activity from mouse liver with catalyzes the disappearance of O6-methylguanine from DNA methylated with methylnitrosourea has been partially purified by ammonium sulfate fractionation and DNA-cellulose chromatography. The activity does not require divalent metal ions and is not affected by EDTA. It is specific for the repair of O6-methylguanine lesions and does not affect the removal of 7-methylguanine, 7-methyladenine or 3-methyladenine. The disappearance of O6-methylguanine is linear with respect to the concentration of protein and is dependent on incubation temperature. The kinetics and substrate dependence experiments suggest that the protein factor is product-inactivated. Amino acid analysis of hydrolysates of protein obtained after incubation of methylated DNA with the protein factor indicates the presence of radiolabeled S-methyl-L-cysteine, suggesting that during the repair of O6-methylguanine from methylated DNA, the methyl group is transferred to a sulfhydryl of a cysteine residue of a protein. This represents the first such demonstration in a mammalian system.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abanobi S. E., Columbano A., Mulivor R. A., Rajalakshmi S., Sarma D. S. In vivo replication of hepatic deoxyribonucleic acid of rats treated with dimethylnitrosamine: presence of dimethylnitrosamine-induced O6-methylguanine, N7-methylguanine, and N3-methyladenine in the replicated hybrid deoxyribonucleic acid. Biochemistry. 1980 Apr 1;19(7):1382–1387. doi: 10.1021/bi00548a018. [DOI] [PubMed] [Google Scholar]
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Beranek D. T., Weis C. C., Swenson D. H. A comprehensive quantitative analysis of methylated and ethylated DNA using high pressure liquid chromatography. Carcinogenesis. 1980 Jul;1(7):595–606. doi: 10.1093/carcin/1.7.595. [DOI] [PubMed] [Google Scholar]
- Cox R., Irving C. C. O6-methylguanine accumulates in DNA of mammary glands after administration of N-methyl-N-nitrosourea to rats. Cancer Lett. 1979 Apr;6(4-5):273–278. doi: 10.1016/s0304-3835(79)80045-2. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Lawley P. D. Methylation of DNA in various organs of C57B1 mice by a carcinogenic dose of N-methyl-N-nitrosourea and stabiltty of some methylation products up to 18 hours. Chem Biol Interact. 1975 Jun;10(6):413–427. doi: 10.1016/0009-2797(75)90072-1. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Molecular and cellular mechanisms associated with pulse-carcinogenesis in the rat nerbous system by ethyinitrosourea: ethylation of nucleic acids and elimination rates of ethylated bases from the DNA of different tissues. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;82(1):37–64. doi: 10.1007/BF00304382. [DOI] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. Evaluation of microparticle chemically bonded reversed-phase packings in the high-pressure liquid chromatographic analysis of nucleosides and their bases. J Chromatogr. 1976 Nov 3;126:679–691. doi: 10.1016/s0021-9673(01)84111-x. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T., Griffin B. Adaptive response to alkylating agents involves alteration in situ of O6-methylguanine residues in DNA. Nature. 1979 Jul 5;280(5717):76–77. doi: 10.1038/280076a0. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Doerjer G., Keefer L. K., Rice J. M., Roller P. P., Hodgson R. M. Correlation of DNA methylation by methyl(acetoxymethyl)nitrosamine with organ-specific carcinogenicity in rats. Cancer Res. 1979 Dec;39(12):5136–5140. [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Carcinogenicity of N-methyl-N-nitrosourea: possible role of excision repair of O6-methylguanine from DNA. J Natl Cancer Inst. 1974 Dec;53(6):1839–1841. [PubMed] [Google Scholar]
- Kleihues P., Margison G. P. Exhaustion and recovery of repair excision of O6-methylguanine from rat liver DNA. Nature. 1976 Jan 15;259(5539):153–155. doi: 10.1038/259153a0. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Orr D. J. Specific excision of methylation products from DNA of Escherichia coli treated with N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1970 Aug;2(2):154–157. doi: 10.1016/0009-2797(70)90047-5. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Shah S. A. Methylation of DNA by 3H-14C-methyl-labelled N-methyl-N-nitrosourea--evidence for transfer of the intact methyl group. Chem Biol Interact. 1973 Aug;7(2):115–120. doi: 10.1016/0009-2797(73)90020-3. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Some chemical aspects of dose-response relationships in alkylation mutagenesis. Mutat Res. 1974 Jun;23(3):283–295. doi: 10.1016/0027-5107(74)90102-x. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Warren W. Specific excision of ethylated purines from DNA of Escherichia coli treated with N-ethyl-N-nitrosourea. Chem Biol Interact. 1975 Jul;11(1):55–57. doi: 10.1016/0009-2797(75)90066-6. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Planche-Martel G., Margison G. P., Pegg A. E. Effect of chronic treatment of rats with dimethylnitrosamine on the removal of O6-methylguanine from DNA. Cancer Res. 1980 Feb;40(2):452–458. [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Balog B. Formation and subsequent excision of O6-ethylguanine from DNA of rat liver following administration of diethylnitrosamine. Cancer Res. 1979 Dec;39(12):5003–5009. [PubMed] [Google Scholar]
- Pegg A. E. Dimethylnitrosamine inhibits enzymatic removal of O6-methylguanine from DNA. Nature. 1978 Jul 13;274(5667):182–184. doi: 10.1038/274182a0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Enzymatic removal of O6-methylguanine from DNA by mammalian cell extracts. Biochem Biophys Res Commun. 1978 Sep 14;84(1):166–173. doi: 10.1016/0006-291x(78)90278-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Hui G. Formation and subsequent removal of O6-methylguanine from deoxyribonucleic acid in rat liver and kidney after small doses of dimethylnitrosamine. Biochem J. 1978 Sep 1;173(3):739–748. doi: 10.1042/bj1730739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Hui G. Removal of methylated purines from rat liver DNA after administration of dimethylnitrosamine. Cancer Res. 1978 Jul;38(7):2011–2017. [PubMed] [Google Scholar]
- Renard A., Verly W. G. A chromatin factor in rat liver which destroys O6-ethylguanine in DNA. FEBS Lett. 1980 May 19;114(1):98–102. doi: 10.1016/0014-5793(80)80868-4. [DOI] [PubMed] [Google Scholar]
- Robins P., Cairns J. Quantitation of the adaptive response to alkylating agents. Nature. 1979 Jul 5;280(5717):74–76. doi: 10.1038/280074a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Scherer E., Steward A. P., Emmelot P. Kinetics of formation of O6-ethylguanine in, and its removal from liver DNA of rats receiving diethylnitrosamine. Chem Biol Interact. 1977 Oct;19(1):1–11. doi: 10.1016/0009-2797(77)90038-2. [DOI] [PubMed] [Google Scholar]
- Singer B. All oxygens in nucleic acids react with carcinogenic ethylating agents. Nature. 1976 Nov 25;264(5584):333–339. doi: 10.1038/264333a0. [DOI] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Sklar R., Strauss B. Role of the uvrE gene product and of inducible O6-methylguanine removal in the induction of mutations by N-methyl-N'-nitro-N-nitrosoguanidine in Escherichia coli. J Mol Biol. 1980 Nov 15;143(4):343–362. doi: 10.1016/0022-2836(80)90217-x. [DOI] [PubMed] [Google Scholar]
- Swann P. F., Mace R. Changes in O6-methylguanine disappearance from rat liver DNA during chronic dimethylnitrosamine administration. A possible similarity between the system removing O6-methylguanine from DNA in rat liver and in Escherichia coli adapted to N-methyl-N'-nitro-N-nitrosoguanidine. Chem Biol Interact. 1980 Aug;31(2):239–245. doi: 10.1016/0009-2797(80)90012-5. [DOI] [PubMed] [Google Scholar]


