Abstract
Childhood multicentric Castleman disease (MCD) is a rare and unexplained lymphoproliferative disorder. We report a human herpesvirus-8 (HHV-8)-infected child, born to consanguineous Comorian parents, who displayed isolated MCD in the absence of any known immunodeficiency. We also systematically review the clinical features of the 32 children previously reported with isolated and unexplained MCD. The characteristics of this patient and the geographic areas of origin of most previous cases suggest that pediatric MCD is associated with HHV-8 infection. Moreover, as previously suggested for Kaposi sarcoma, MCD in childhood may result from inborn errors of immunity to HHV-8 infection.
KEY WORDS: Children, human herpes virus 8, multicentric Castleman disease, systematic review
Castleman disease (CD), first described in 1956, is a rare lymphoproliferative disorder characterized by the massive growth of lymphoid tissue, with follicular hyperplasia, vascular proliferation, and plasmocytosis.1 The lesions may also affect nonlymphoid tissues. Three distinct histologic subtypes of CD have been described. The hyaline vascular type, usually unicentric, tends to be a mild form with few or no systemic symptoms. The plasma cell type, often multicentric, is associated with a marked proliferation of plasma cells and is highly aggressive. The mixed-type is pathologically a mixture of the other 2 types. Patients with multicentric CD (MCD) present multiple lesions, potentially affecting all organs, lymph nodes, liver, and spleen, and is accompanied by constitutional symptoms and hematologic abnormalities.2 MCD is the rarest form of CD at all ages, but particularly in children, with only few cases reported to date.3,4 However, MCD usually follows a more favorable course in children than in adults.2 MCD is driven by human herpesvirus-8 (HHV-8), a γ2-herpesvirus5 phylogenetically classified into 5 subtypes (A–E). This lymphotropic and oncogenic virus is required for the development of Kaposi sarcoma (KS),6 and its detection in adults with MCD suggests that it may also play a role in MCD pathogenesis.7 Moreover, MCD occurs preferentially in patients with underlying immunodeficiency, such as HIV infection or immunosuppressive therapy, consistent with impaired immunity to HHV-8 leading to MCD.4 Interestingly, HHV-8-driven MCD has never been identified in children. We report here the case of a child with MCD and HHV-8 infection who was born to consanguineous parents. We also present a systematic review of the literature of pediatric MCD.
Case Report
A 7-year-old girl presented with recurrent fever episodes since age 3 years, generalized lymphadenopathy, hepatosplenomegaly, and anemia with no identifiable infectious or malignant etiology. She was born to consanguineous parents originating from and living in a small village (<1000 inhabitants) in the Comores where she lived with her family (parents and 2 siblings) until the onset of the disease. Only her mother was tested for HHV-8 and had a positive serology. At age 7, she suffered a new episode of fever, generalized lymphadenopathy, hepatosplenomegaly, and signs of hemophagocytic syndrome (hyponatremia, anemia –4 g hemoglobin/dL, thrombocytopenia –115 000/mm3). She tested negative for common bacterial infections, toxoplasmosis, Epstein-Barr virus (blood polymerase chain reaction [PCR], see Supplemental Information, but with serological results consistent with past infection: positive immunoglobulin [Ig]G anti–viral capsid antigens and IgG anti-Epstein-Barr nuclear antigens, negative IgM anti–viral capsid antigens), varicella-zoster virus, HHV-6, cytomegalovirus, enterovirus, adenovirus, arboviruses, HIV, human T-lymphotropic virus-1 and -2, and hepatitis B and C (serum antibodies). Detailed immunologic analyses ruled out an underlying immunodeficiency: flow cytometry studies showed 13% CD19+ cells (ie, 270 cells/mm3), 78% CD3+ cells (ie, 1630 cells/mm3), 35% CD4+ cells (ie, 730 cells/mm3), 33% CD8+ cells (ie, 700 cells/mm3) among the 2100 circulating lymphocytes/mm3 present, with normal mitogen and antigen stimulations of T cells. Serum immunoglobulin levels were high (IgG: 24.2 g/L; IgA: 1.8 g/L; IgM: 1.4 g/L).
Lymph node biopsy showed mixed-type MCD (Fig 1). HHV-8 genes were also detected in a blood sample by specific PCR (5779 copies/150 000 cells). The virus was identified as a B1 subtype HHV-8, from a subgroup of the sub-Saharan African B subtype (Supplemental Fig 2).8–10 Plasma samples were tested for HHV-8-specific IgG, in immunofluorescence assays (HHV-8 indirect fluorescent assay, ABI, Columbia, MD). IgG antibodies directed against lytic (IgGlytic) but not against latent antigens were detected in our patient (IgGlytic positive at dilution 1/5120). We assessed further the degree of consanguinity by genotyping the patient with the genome-wide single-nucleotide polymorphism (SNP) array 6.0 (Affymetrix, Santa Clara, CA) including >900 000 SNPs. The proportion of homozygous regions (of at least 300 kb or based on at least 50 contiguous SNPs) within the whole genome, as estimated by PLINK software,11 was 2.95% indicating a high level of homozygosity (that expected for a marriage between second cousins).
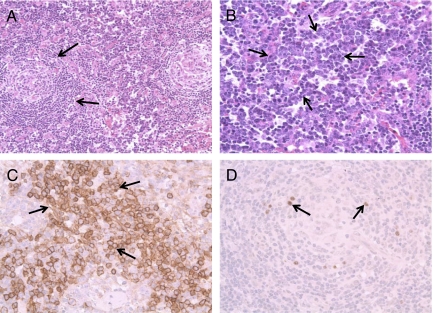
FIGURE 1.
Histologic features of axillary lymph node sections from our patient. Pathologic analyses showed lymph nodes with abnormal germinal centers of variable size and shape, and atretic follicles with prominent hyalinization penetrating blood vessels in the germinal center. The lamination of the mantle cell layers led to a typical “onionskin” appearance of the follicle. Immunohistochemical staining of lymph node sections showed numerous plasma cells (anti-CD138 antibody staining) in the interfollicular region. Latent nuclear antigens (LANA) protein was detected in situ, in plasmacytes. A, Low magnification (×20) showing abnormal atretic hyaline-vascular germinal centers, “onionskin” cuffing of lymphocytes, and a central hyaline vascular region. B, High magnification (×40) revealing sheets of plasma cells in the interfollicular region (arrows). This biopsy showed features of both the hyaline vascular and plasma cell type types (mixed type) of Castleman disease. C, Immunohistochemistry with anti-CD138 antibody at high magnification (×40) confirming the presence of numerous plasma cells in the interfollicular region (arrows). D, Immunohistochemistry for LANA protein results in the staining of cells around the germinal center (arrows).
The severity of the disease led to this patient being treated with a 4-week course of rituximab (375 mg/m2/injection/week) and etoposide (150 mg/m2/injection). The patient responded to treatment but relapsed 6 months later, requiring another 4-week-long course of the same drugs at the same doses. Complete remission was then achieved, and the child remains free of relapse 4 years later, at age 13.
Systematic Review of the Literature
We conducted a systematic, computerized literature search (see Methods in the Supplemental Information) to identify case reports or series of pediatric MCD cases. We identified 468 potentially eligible articles (Supplemental Fig 3), ending up with 22 relevant articles, relating 35 cases of pediatric MCD (see references in the Supplemental Information). Three patients in whom primary immunodeficiency was suspected (Klinefelter syndrome, humoral deficiency, and T-cell deficiency; references 21 and 22 in the Supplemental Information) were excluded from the analysis. We investigated the potential pathogenic influence of HHV-8 on the occurrence of MCD by focusing on the 32 remaining cases of MCD in which no inherent condition could be detected (references 1–20 and Supplemental Table 2). None of the 32 children had been treated with immunosuppressive drugs, and all 11 (34%) patients tested for HIV were found to be negative.
Twenty (63%) were boys, and the mean age of the reported patients was 10.2 years (SD: 5.0 years; median: 10.0 years; range: 2–18 years; Table 1). All children presented multiple lymphadenopathies. Hepatomegaly and/or splenomegaly, and clinical and/or biological signs of inflammation were found in most patients. This disease was found to affect all organs and systems: the blood was affected in half the patients and the kidneys (glomerulonephritis, nephrotic syndrome) in a quarter; the lungs, joints, brain (stroke, seizures), eyes, and the ear, nose, and throat system were affected less frequently. Four (13%) children presented cancer (2 KS, 2 T- or B-cell lymphomas), and 3 (9%) children died (2 in the months immediately after MCD diagnosis and 1 after 5.5 years of follow-up during treatment of lymphoma). None of the case reports provided information about parental consanguinity. Only 7 (22%) children were screened for HHV-8 infection (references 8,13,18,19 in the Supplemental Information), all between 1996 and 2008, with techniques providing various degrees of diagnostic accuracy for HHV-8 infection; also, the time lag between disease onset and testing was not always clearly defined. All these children tested negative: 6 tests were based on biopsy (4 PCR analyses and 2 immunohistochemical analyses, with screening for latent nuclear antigens (LANA) protein in 1 case), with 1 of these 6 cases also testing negative by blood PCR, and the final case was tested serologically (unspecified method). Neither of the 2 patients also displaying KS or lymphoma was investigated for HHV-8.
TABLE 1.
Characteristics of the Included Cases According to the HHV-8 Endemicity Status of the Region From Which They Originated
| Characteristics | All Casesa | Cases From Regions in Which HHV-8 Is Not Endemicb | Cases From Regions in Which HHV-8 Is Endemicb | P Value When Comparing Cases From Endemic and Nonendemic HHV-8 Regionsc |
|---|---|---|---|---|
| Male | 20 (63) | 9 (45) | 11 (55) | .2 |
| Age (years) | 10.0 (±5.0) | 11.7 (±5.1) | 8.0 (± 4.3) | .008 |
| Clinical features | ||||
| Mass(es) | 8 (25) | 2 (25) | 6 (75) | .1 |
| HSM/HM/SM | 25 (78) | 10 (40)d | 15 (60) | .03 |
| Inflammatory syndromee | 18 (56) | 4 (22)d | 14 (78) | < .001 |
| Renal involvementf | 9 (28) | 4 (44) | 5 (56) | .7 |
| Ascites, edema | 6 (19) | 5 (83) | 1 (17) | .07 |
| Hematologic disorders | 20 (63) | 12 (60) | 8 (40) | .1 |
| Anemia | 20 (63) | 12 (60) | 8 (40) | .1 |
| AIHA | 9 (28) | 5 (56) | 4 (44) | .7 |
| Thrombocytopenia | 11 (34) | 9 (82) | 2 (18) | .009 |
| Neutropenia | 2 (6) | 2 (100) | 0 (0) | .1 |
| Lung diseasesg | 4 (13) | 3 (75) | 1 (25) | .3 |
| Skin involvement (rash) | 7 (22) | 5 (71) | 2 (29) | .2 |
| Arthritis/arthralgia | 3 (9) | 1 (33) | 2 (67) | .5 |
| Cerebral involvement | 3 (9) | 0 (0) | 3 (100) | .07 |
| Hepatitis | 7 (22) | 5 (71) | 2 (29) | .2 |
| Uveitis | 1 (3) | 0 (0) | 1 (100) | .3 |
| Sinusitis | 1 (3) | 1 (100) | 0 (0) | .3 |
| Cancer occurrence | 4 (13) | 0 (0)d | 4 (100) | .03 |
| Lymphoma occurrenceh | 2 (6) | 0 (0) | 2 (100) | .1 |
| Kaposi sarcoma | 2 (6) | 0 (0) | 2 (100) | .1 |
| Death | 3 (9) | 1 (33) | 2 (67) | .5 |
| Histologic features | ||||
| Hyaline vascular type | 10 (32) | 7 (70) | 3 (30) | .3d |
| Plasma cell type | 15 (48) | 8 (53) | 7 (47) | |
| Mixed type | 6 (19) | 2 (33) | 4 (67) |
AIHA, autoimmune hemolytic anemia; HM, hepatomegaly; HSM, hepatosplenomegaly; SM, splenomegaly.
Values are expressed in n (%), with the percentage calculated using the total number of cases (n = 32); age is expressed as a mean value (± SD).
Values are expressed in n (%), with the percentage calculated from the number of cases with the corresponding characteristics; age is expressed as a mean value (± SD). HHV-8 was considered to be endemic to the following regions: Africa, Oman, and the Mediterranean region (Turkey, Jordan, Italy, Israel, and Spain).
χ2 or nonparametric tests were carried out.
Comparing the 3 histologic types together.
Clinical (fever, fatigue, loss of wt, growth retardation, etc) and/or biological inflammatory syndrome.
Three cases of glomerulonephritis, and 2 of nephrotic syndrome.
Two cases of pneumonia, 1 of pleural effusion, and 1 of respiratory distress syndrome before death.
One was a B-cell lymphoma, the other was a T-cell lymphoma.
Sixteen (50%) patients were reported to be from regions in which HHV-8 is endemic (Africa, Oman, and the Mediterranean region—Jordan, Turkey, Italy, Israel, Spain6), on the basis of their ethnic origin or the location of the hospital to which they were admitted (see Table 1). Patients from regions of endemic HHV-8 infection tended to be younger (P = .08), were more likely to present hepatomegaly and/or splenomegaly (P = .02), clinical and/or biological signs of inflammation (P = .001), and were also more likely to have suffered from cancer (2 KS and 2 lymphomas vs 0, P = .02). Other disorders potentially related to immunologic mechanisms (renal diseases, arthritis, autoimmune hemolytic anemia, etc) were not significantly associated with regions of endemic HHV-8 infection, but sample sizes were small (Table 1).
Discussion
We describe here the first child with proven HHV-8 infection and MCD. This diagnosis was based on (1) clinical observation, with typical clinical features (lymphadenopathies in more than two areas, hepatosplenomegaly); (2) typical histopathologic MCD lesions with the detection of HHV-8 antigen by immunohistochemistry in situ; (3) the detection of HHV-8 DNA in blood. The etiology of MCD remains unclear, but the known occurrence of HHV-8 in adult cases of MCD suggests that this virus may be involved in its pathogenesis. HHV-8 may produce a molecule homologous to human interleukin-6, leading to the systemic signs of the disease, lymphoproliferation, and plasma cell differentiation.12 Our case report suggests that the association between MCD and HHV-8 can be extended to children. Moreover, systematic literature review revealed that almost half of the cases of pediatric MCD originated from areas in which HHV-8 is endemic: even though the proxy used to classify regions as HHV-8-endemic may need refinement, this indicates the possibility of such an association in other children. It would therefore be interesting to check for HHV-8 infection in children with MCD.
The child described here was also born to consanguineous parents. This suggests that MCD in childhood may be favored by rare inborn errors of immunity against HHV-8 infection, including, as in this particular patient, autosomal recessive predisposition.13–15 On infection with HHV-8, this deficiency may lead to excessive proliferation of B lymphocytes and plasmocytes in lymphoid organs. This genetic hypothesis is consistent with the rarity of MCD in childhood, with only 32 cases reported worldwide since 1965, half of whom originated from regions in which HHV-8 is endemic. It is also supported by the observation of KS in 2 patients with MCD, given that some primary immunodeficiencies, including IFNγRI deficiency16 and Wiskott-Aldrich syndrome,17 have been shown to confer predisposition to KS and other infections. Two children have also been reported with familial hemophagocytic lymphohistiocytosis related to perforin mutations; in these cases, HHV-8 infection was responsible for the onset of the clinical signs revealing the underlying genetic disease.18 Moreover, 3 unrelated Turkish children with classic, isolated KS born to first-cousin parents with no relevant familial history were recently reported.19 One of these children was recently shown to suffer from STIM1 deficiency.20 Collectively, these findings suggest that inborn errors of immunity to HHV-8 may provide a context favoring the rare occurrence of HHV-8-associated classic KS and/or MCD in children. Additional investigations are required to increase our understanding of the full consequences for the immune response of these inborn errors of immunity.
Supplementary Material
Acknowledgments
Sandrine Leroy is supported by a grant from the Fondation pour la Recherche Médicale and The French Foreign Office. Sabine Plancoulaine is supported in part by Assistance Publique-Hôpitaux de Paris. The Branches of the Laboratory of Human Genetics of Infectious Diseases at Necker and Rockefeller are supported by grants from Institut National de la Santé et de la Recherche Médicale, University Paris Descartes, the Rockefeller University Center for Clinical and Translational Science (grant 5UL1RR024143-03), the Rockefeller University, the Agence Nationale de la Recherche, the BNP Paribas Foundation, the March of Dimes, and the Dana Foundation. Jean-Laurent Casanova was an International Scholar of the Howard Hughes Medical Institute.
We thank Laurent Abel for helpful comments.
Glossary
- CD
Castleman disease
- HHV-8
human herpes virus 8
- Ig
immunoglobulin
- KS
Kaposi sarcoma
- MCD
multicentric Castleman disease
- PCR
polymerase chain reaction
- SNP
single-nucleotide polymorphism
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Castleman B, Iverson L, Menendez VP. Localized mediastinal lymphnode hyperplasia resembling thymoma. Cancer. 1956;9(4):822–830 [DOI] [PubMed] [Google Scholar]
- 2.McClain KL, Natkunam Y, Swerdlow SH. Atypical cellular disorders. Hematology Am Soc Hematol Educ Program. 2004;283–296 [DOI] [PubMed] [Google Scholar]
- 3.Parez N, Bader-Meunier B, Roy CC, Dommergues JP. Paediatric Castleman disease: report of seven cases and review of the literature. Eur J Pediatr. 1999;158(8):631–637 [DOI] [PubMed] [Google Scholar]
- 4.Smir BN, Greiner TC, Weisenburger DD. Multicentric angiofollicular lymph node hyperplasia in children: a clinicopathologic study of eight patients. Mod Pathol. 1996;9(12):1135–1142 [PubMed] [Google Scholar]
- 5.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266(5192):1865–1869 [DOI] [PubMed] [Google Scholar]
- 6.Ganem D. Kaposi’s sarcoma-associated herpesvirus. In: BF Fields, DM Knipe, PM Howley, eds. Fields Virology 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2846–2888. [Google Scholar]
- 7.Jenson HB. Human herpesvirus 8 infection. Curr Opin Pediatr. 2003;15(1):85–91 [DOI] [PubMed] [Google Scholar]
- 8.Lacoste V, Kadyrova E, Chistiakova I, Gurtsevitch V, Judde JG, Gessain A. Molecular characterization of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 strains from Russia. J Gen Virol. 2000;81(Pt 5):1217–1222 [DOI] [PubMed] [Google Scholar]
- 9.White T, Hagen M, Gudza I, White IE, Ndemera B, Gwanzura Let al. Genetic diversity of the Kaposi’s sarcoma herpesvirus K1 protein in AIDS-KS in Zimbabwe. J Clin Virol. 2008;42(2):165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kajumbula H, Wallace RG, Zong JC, et al. Ugandan Kaposi’s sarcoma-associated herpesvirus phylogeny: evidence for cross-ethnic transmission of viral subtypes. Intervirology. 2006;49(3):133–143 [DOI] [PubMed] [Google Scholar]
- 11.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger R, Neipel F, Fleckenstein B, Savino R, Ciliberto G, Kalden JR, Gramatzki M. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood. 1998;91(6):1858–1863 [PubMed] [Google Scholar]
- 13.Alcaïs A, Abel L, Casanova JL. Human genetics of infectious diseases: between proof of principle and paradigm. J Clin Invest. 2009;119(9):2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casanova JL, Abel L. Primary immunodeficiencies: a field in its infancy. Science. 2007;317(5838):617–619 [DOI] [PubMed] [Google Scholar]
- 15.Alcaïs A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann N Y Acad Sci. 2010;1214:18–33 [DOI] [PubMed] [Google Scholar]
- 16.Camcioglu Y, Picard C, Lacoste V, et al. HHV-8-associated Kaposi sarcoma in a child with IFNgammaR1 deficiency. J Pediatr. 2004;144(4):519–523 [DOI] [PubMed] [Google Scholar]
- 17.Picard C, Mellouli F, Duprez R, et al. Kaposi’s sarcoma in a child with Wiskott-Aldrich syndrome. Eur J Pediatr. 2006;165(7):453–457 [DOI] [PubMed] [Google Scholar]
- 18.Grossman WJ, Radhi M, Schauer D, Gerday E, Grose C, Goldman FD. Development of hemophagocytic lymphohistiocytosis in triplets infected with HHV-8. Blood. 2005;106(4):1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin G, Palanduz A, Aydogan G, et al. Classic Kaposi sarcoma in 3 unrelated Turkish children born to consanguineous kindreds. Pediatrics. 2010;125(3):e704–e708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byun M, Abhyankar A, Lelarge V, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic Kaposi sarcoma. J Exp Med. 2010;207(11):2307–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.