Abstract
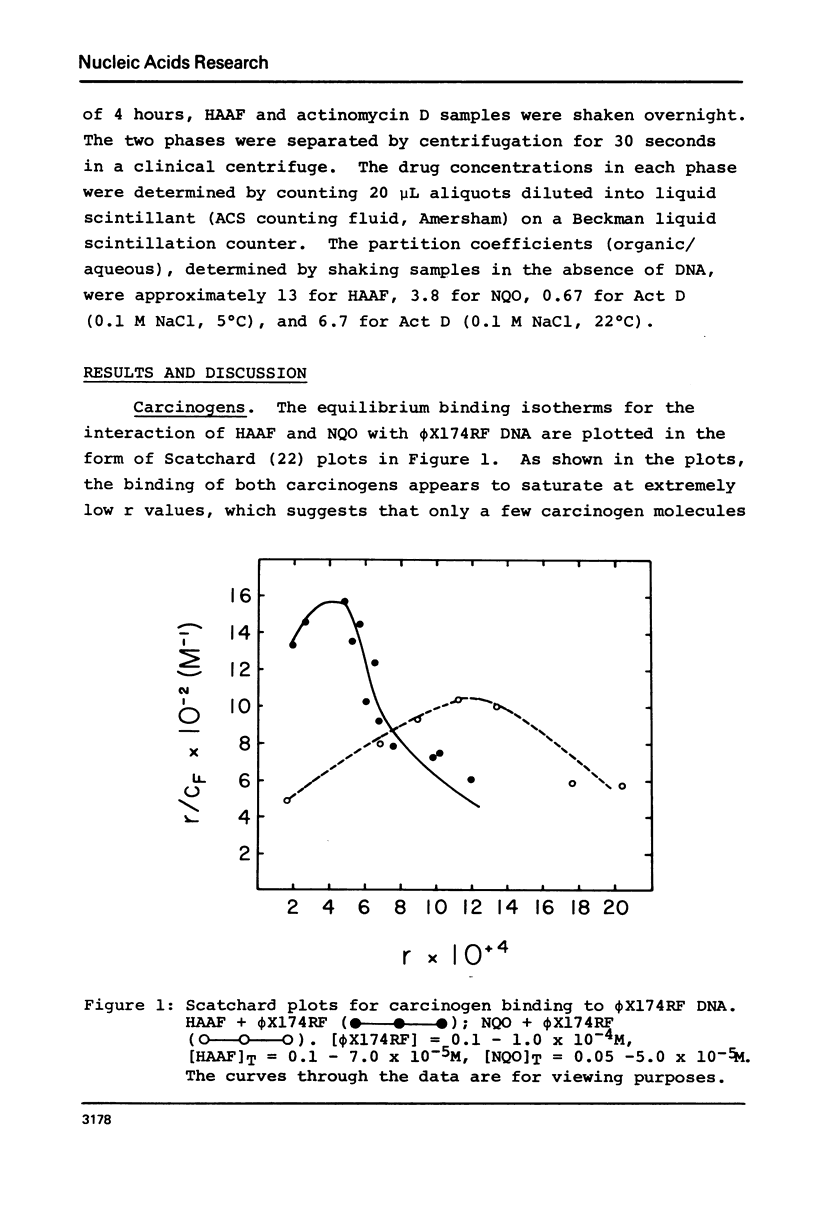
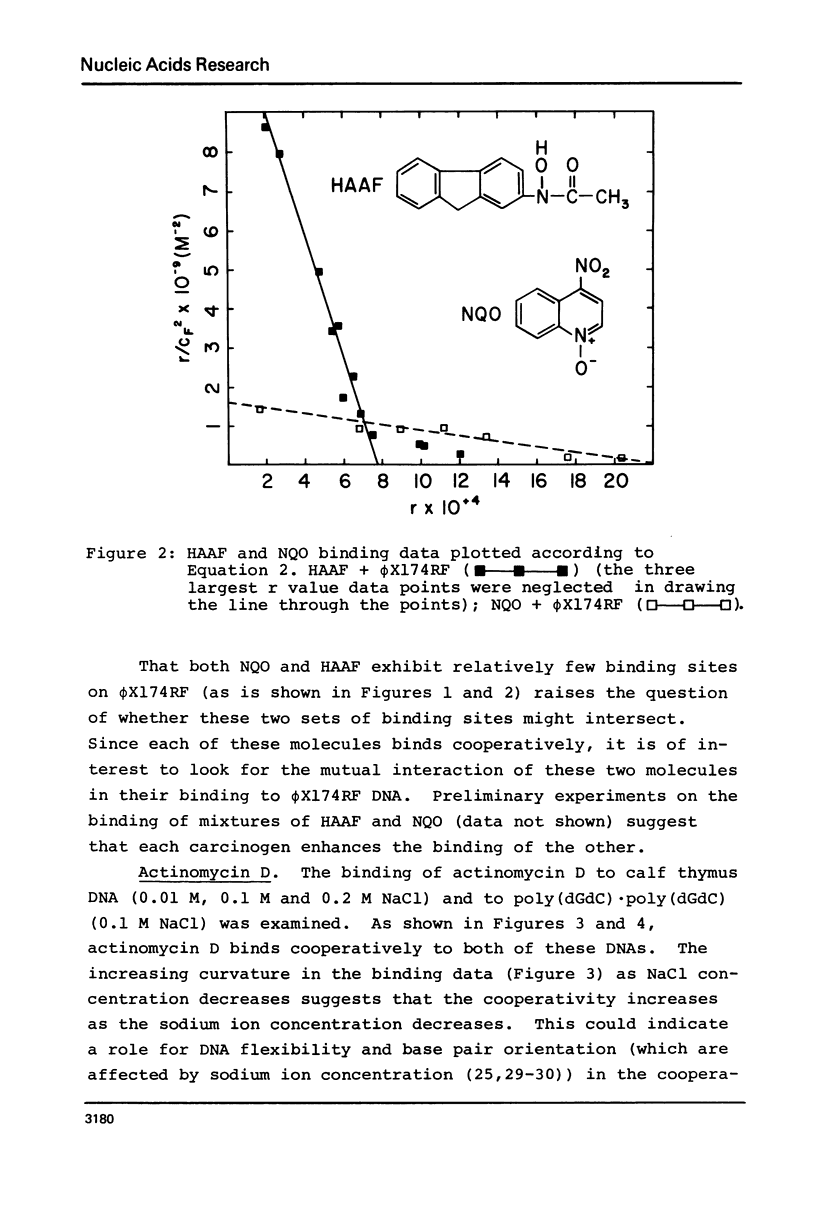
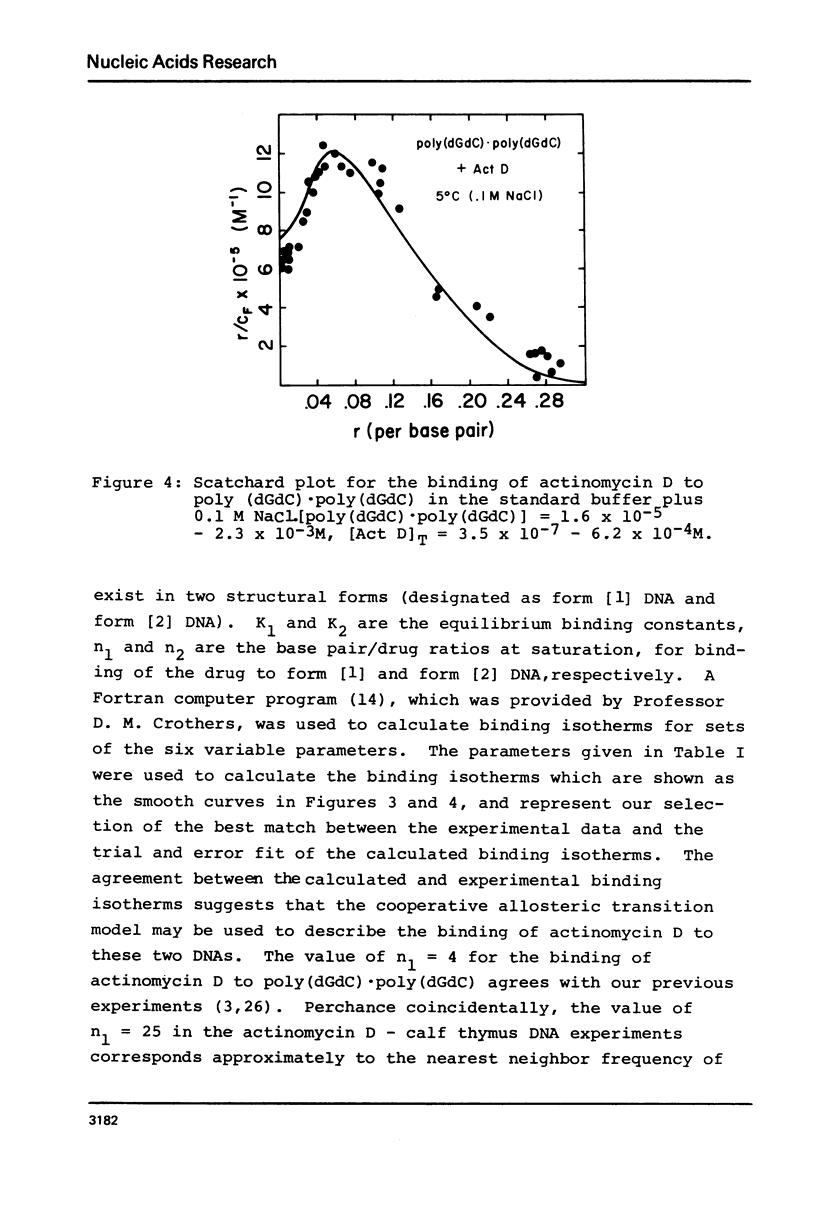
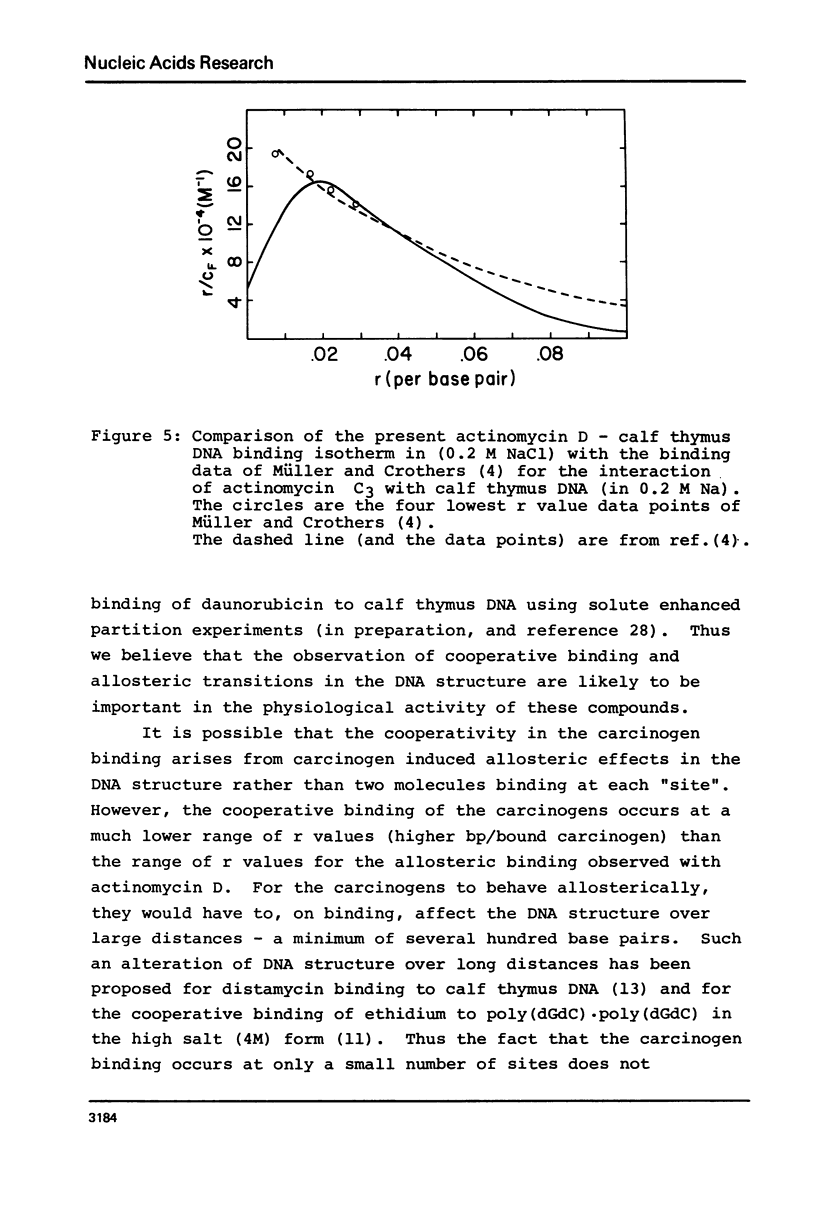
The equilibrium binding of the carcinogens N-hydroxy-N-acetyl-2-amino-fluorene (HAAF) and 4-nitroquinoline-1-oxide (NQO) to phi X174RF DNA have been studied by phase partition techniques. Both molecules bind in a cooperative manner with only a few carcinogen molecules binding to each phi X174RF DNA molecule. The binding data for both HAAF and NQO fit a model in which two carcinogens cluster into a small number of sites--four sites for HAAF and twelve sites for NQO. Phase partition techniques were also used to study the binding of actinomycin D to both calf thymus DNA and poly (dG-dC) . poly (dG-dC) at much lower r values than had been previously reported. These data exhibit humped Scatchard plots which are indicative of cooperative binding; the overall shape of the Scatchard plots are consistent with a model for drug induced allosteric transitions in the DNA structure. The cooperativity in the actinomycin D binding to calf thymus DNA increases with decreasing sodium chloride concentration, suggesting a role for DNA flexibility in allosteric binding.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dattagupta N., Hogan M., Crothers D. M. Interaction of netropsin and distamycin with deoxyribonucleic acid: electric dichroism study. Biochemistry. 1980 Dec 23;19(26):5998–6005. doi: 10.1021/bi00567a009. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Crothers D. M. Phase partition studies of actinomycin-nucleotide complexes. Biochemistry. 1976 Oct 5;15(20):4433–4438. doi: 10.1021/bi00665a015. [DOI] [PubMed] [Google Scholar]
- Fink L. M., Nishimura S., Weinstein I. B. Modifications of ribonucleic acid by chemical carcinogens. I. In vitro modification of transfer ribonucleic acid by N-acetoxy-2-acetylaminofluorene. Biochemistry. 1970 Feb 3;9(3):496–502. doi: 10.1021/bi00805a007. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transmission of allosteric effects in DNA. Nature. 1979 Apr 5;278(5704):521–524. doi: 10.1038/278521a0. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Koreeda M., Moore P. D., Yagi H., Yeh H. J., Jerina D. M. Alkylation of polyguanylic acid at the 2-amino group and phosphate by the potent mutagen (+/-)-7beta,8alpha-dihydroxy-9beta,10beta-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. J Am Chem Soc. 1976 Oct 13;98(21):6720–6722. doi: 10.1021/ja00437a061. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Reinhardt C. G. Evidence for sequence preferences in the intercalative binding of ethidium bromide to dinucleoside monophosphates. J Mol Biol. 1975 Sep 15;97(2):133–162. doi: 10.1016/s0022-2836(75)80031-3. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Winkle S. A., Graves D. E. Solute enhanced partition analysis-a novel method for measuring the binding of drugs to DNA. Biochem Biophys Res Commun. 1981 Jan 15;98(1):317–323. doi: 10.1016/0006-291x(81)91905-7. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Young M. A. Daunorubicin and adriamycin facilitate actinomycin D binding to poly(dA-dT)-poly(dA-dT). Nature. 1977 Oct 13;269(5629):627–628. doi: 10.1038/269627a0. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Nakanishi K., Kasai H., Cho H., Harvey R. G., Jeffrey A. M., Jennette K. W., Weinstein I. Absolute configuration of a ribonucleic acid adduct formed in vivo by metabolism of benzo[a]pyrene. J Am Chem Soc. 1977 Jan 5;99(1):258–260. doi: 10.1021/ja00443a053. [DOI] [PubMed] [Google Scholar]
- Nordén B. Structural evidence on DNA carcinogen interactions. N-acetoxy-N-2acetylaminofluorene binding to DNA. Biophys Chem. 1978 Sep;8(4):385–391. doi: 10.1016/0301-4622(78)80020-9. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M., Baehr W., Holbrook J. J. Ethidium bromide as a cooperative effector of a DNA structure. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3805–3809. doi: 10.1073/pnas.69.12.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Shanbhag V. P., Södergård R., Carstensen H., Albertsson P. A. A new method for the determination of the binding capacity of testosterone-estradiol-binding-globulin in human plasma. J Steroid Biochem. 1973 Sep;4(5):537–550. doi: 10.1016/0022-4731(73)90070-8. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P., Lee J. S. A solvent-partition method for measuring the binding of drugs to DNA. Application to the quinoxaline antibiotics echinomycin and triostin A. Biochim Biophys Acta. 1975 Oct 1;407(2):200–212. doi: 10.1016/0005-2787(75)90285-3. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Larson J. E., Wells R. D. Netropsin. A specific probe for A-T regions of duplex deoxyribonucleic acid. J Biol Chem. 1974 Nov 10;249(21):6719–6731. [PubMed] [Google Scholar]
- Wells R. D., Goodman T. C., Hillen W., Horn G. T., Klein R. D., Larson J. E., Müller U. R., Neuendorf S. K., Panayotatos N., Stirdivant S. M. DNA structure and gene regulation. Prog Nucleic Acid Res Mol Biol. 1980;24:167–267. doi: 10.1016/s0079-6603(08)60674-1. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E. Studies on the binding of actinomycin D to DNA and DNA model polymers. J Mol Biol. 1970 Apr 28;49(2):319–342. doi: 10.1016/0022-2836(70)90248-2. [DOI] [PubMed] [Google Scholar]


