Abstract
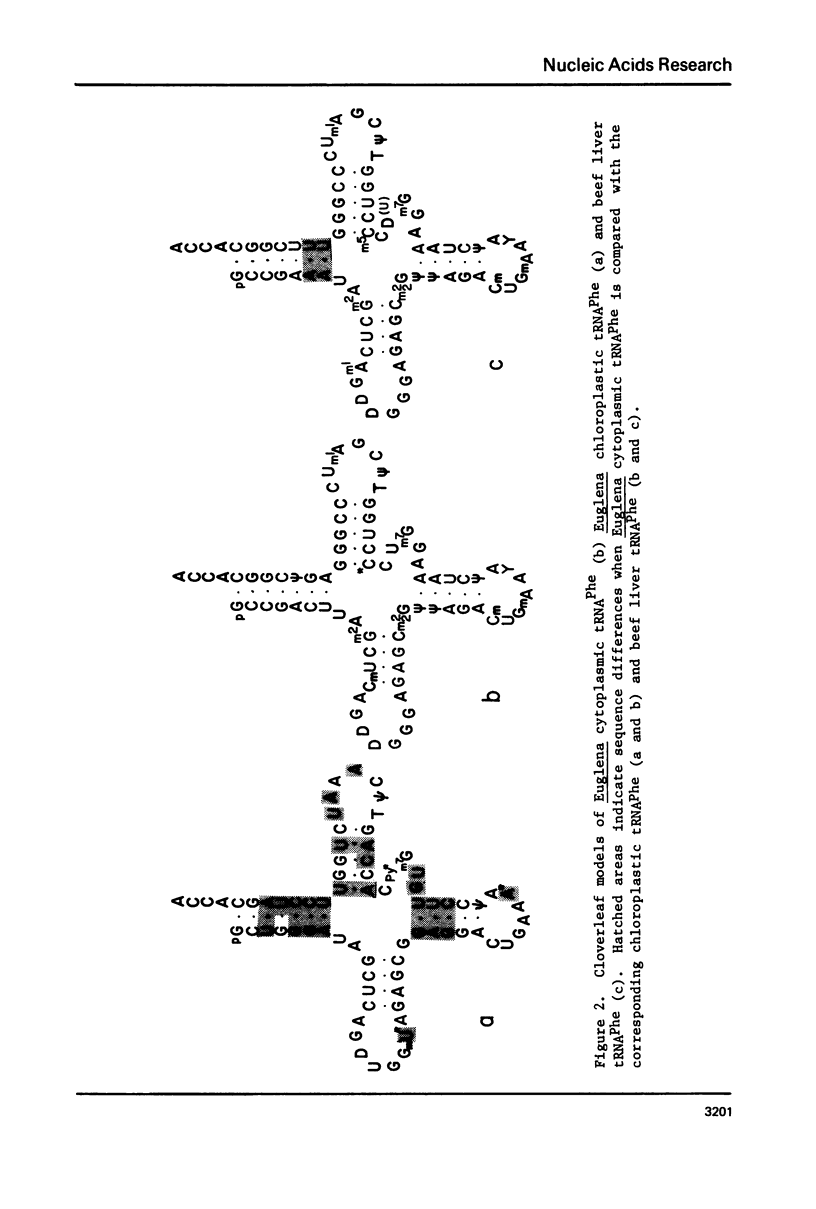
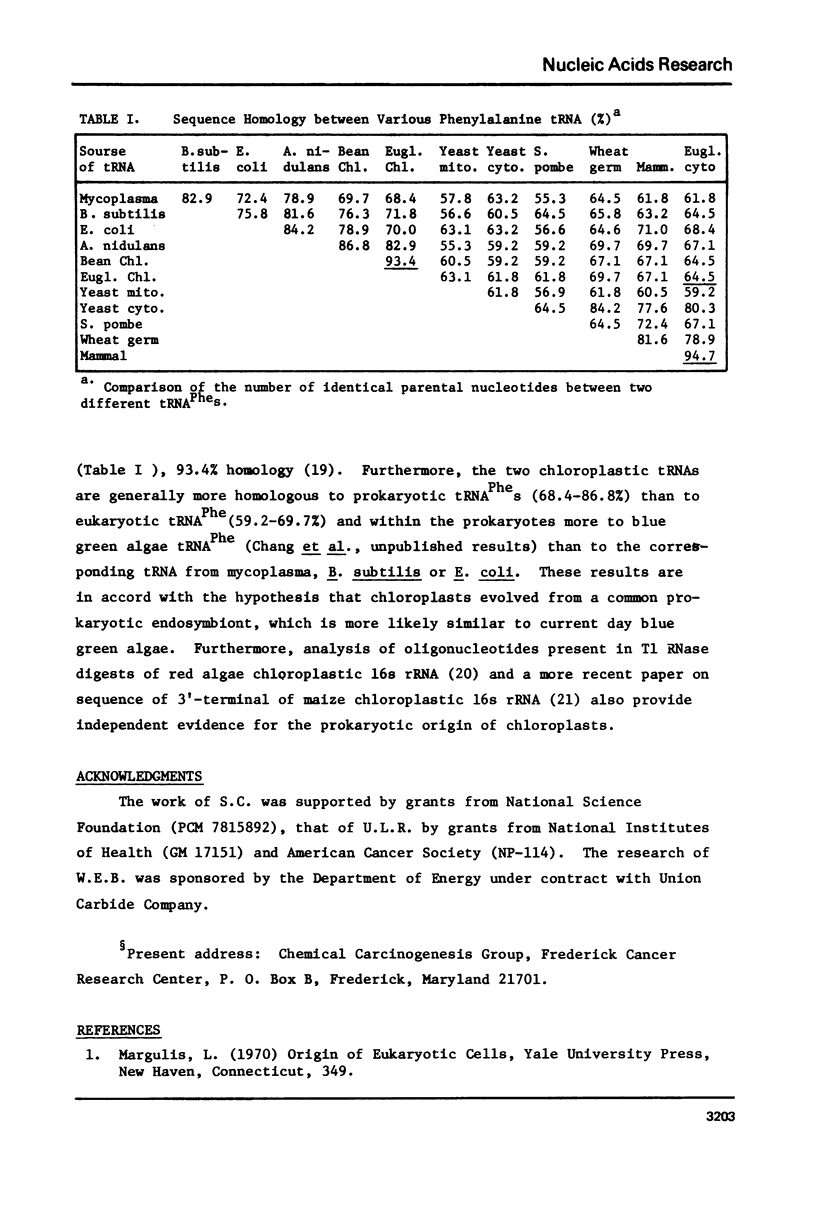
The nucleotide sequence of cytoplasmic phenylalanine tRNA from Euglena gracilis has been elucidated using procedures described previously for the corresponding chloroplastic tRNA [Cell, 9, 717 (1976)]. The sequence is: pG-C-C-G-A-C-U-U-A-m(2)G-C-U-Cm-A-G-D-D-G-G-G-A-G-A-G-C-m(2)2G-psi-psi-A-G-A-Cm -U-Gm-A-A-Y-A-psi-C-U-A-A-A-G-m(7)G-U-C-*C-C-U-G-G-T-psi-C-G-m(1)A-U-C-C-C-G-G- G-A-G-psi-C-G-G-C-A-C-C-A. Like other tRNA Phes thus far sequenced, this tRNA has a chain length of 76 nucleotides. The sequence of E. gracilis cytoplasmic tRNA Phe is quite different (27 nucleotides out of 76 different) from that of the corresponding chloroplastic tRNA but is surprisingly similar (72 out of 76 nucleotides identical) to that of tRNA Phe from mammalian cytoplasm. This extent of sequence homology even exceeds that found between E. gracilis and wheat germ cytoplasmic tRNA Phe. These findings raise interesting questions on the evolution of tRNAs and the taxonomy of Euglena.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apgar J., Everett G. A., Holley R. W. Analyses of large oligonucleotide fragments obtained from a yeast alanine transfer ribonucleic acid by partial digestion with ribonuclease T1. J Biol Chem. 1966 Mar 10;241(5):1206–1211. [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Partial sequences of 16S rRNA and the phylogeny of blue-green algae and chloroplasts. Nature. 1976 Jun 24;261(5562):669–673. doi: 10.1038/261669a0. [DOI] [PubMed] [Google Scholar]
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Brum C. K., Siberklang M., RajBhandary U. L., Hecker L. I., Barnett W. E. The first nucleotide sequence of an organelle transfer RNA: chloroplastic tRNAphe. Cell. 1976 Dec;9(4 Pt 2):717–723. doi: 10.1016/0092-8674(76)90135-5. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Keith G. Primary structure of bean chloroplastic tRNAPhe. Comparison with Euglena chloroplastic tRNAPhe. FEBS Lett. 1977 Dec 15;84(2):351–356. doi: 10.1016/0014-5793(77)80723-0. [DOI] [PubMed] [Google Scholar]
- HOLLEY R. W., APGAR J., EVERETT G. A., MADISON J. T., MARQUISEE M., MERRILL S. H., PENSWICK J. R., ZAMIR A. STRUCTURE OF A RIBONUCLEIC ACID. Science. 1965 Mar 19;147(3664):1462–1465. doi: 10.1126/science.147.3664.1462. [DOI] [PubMed] [Google Scholar]
- Keith G., Ebel J. P., Dirheimer G. The primary structure of two mammalian tRNAs Phe: identity of calf liver and rabbit liver tRNAs Phe. FEBS Lett. 1974 Nov 1;48(1):50–52. doi: 10.1016/0014-5793(74)81059-8. [DOI] [PubMed] [Google Scholar]
- Keith G., Picaud F., Weissenbach J., Ebel J. P., Petrissant G., Dirheimer G. The primary structure of rabbit liver tRNA Phe and its comparison with known tRNA Phe sequences. FEBS Lett. 1973 May 1;31(3):345–347. doi: 10.1016/0014-5793(73)80138-3. [DOI] [PubMed] [Google Scholar]
- McCutchan T., Silverman S., Kohli J., Söll D. Nucleotide sequence of phenylalanine transfer RNA from Schizosaccharomyces pombe: implications for transfer RNA recognition by yeast phenylalanyl-tRNA synthetase. Biochemistry. 1978 May 2;17(9):1622–1628. doi: 10.1021/bi00602a007. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Mahler H. R. The non symbiotic origin of mitochondria. Science. 1972 Aug 18;177(4049):575–582. doi: 10.1126/science.177.4049.575. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Anandaraj M. P., Chia L. S., Randerath E., Gupta R. C., Randerath K. Sequence studies on tRNAPhe from placenta: comparison with known sequences of tRNAPhe from other normal mammalian tissues. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1097–1105. doi: 10.1016/0006-291x(75)90470-2. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Simsek M., Petrissant G., Rajbhandary U. L. Replacement of the sequence G-T-phi-C-G(A)- by G-A-U-C-G- in initiator transfer RNA of rabbit-liver cytoplasm. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2600–2604. doi: 10.1073/pnas.70.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzell T., Spolsky C. Mitochondria and plastids as endosymbionts: a revival of special creation? Am Sci. 1974 May-Jun;62(3):334–343. [PubMed] [Google Scholar]



