Abstract
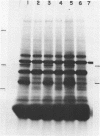
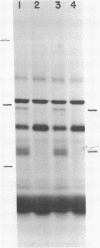
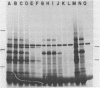
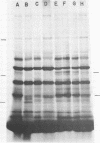
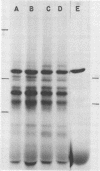
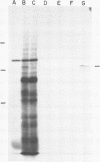
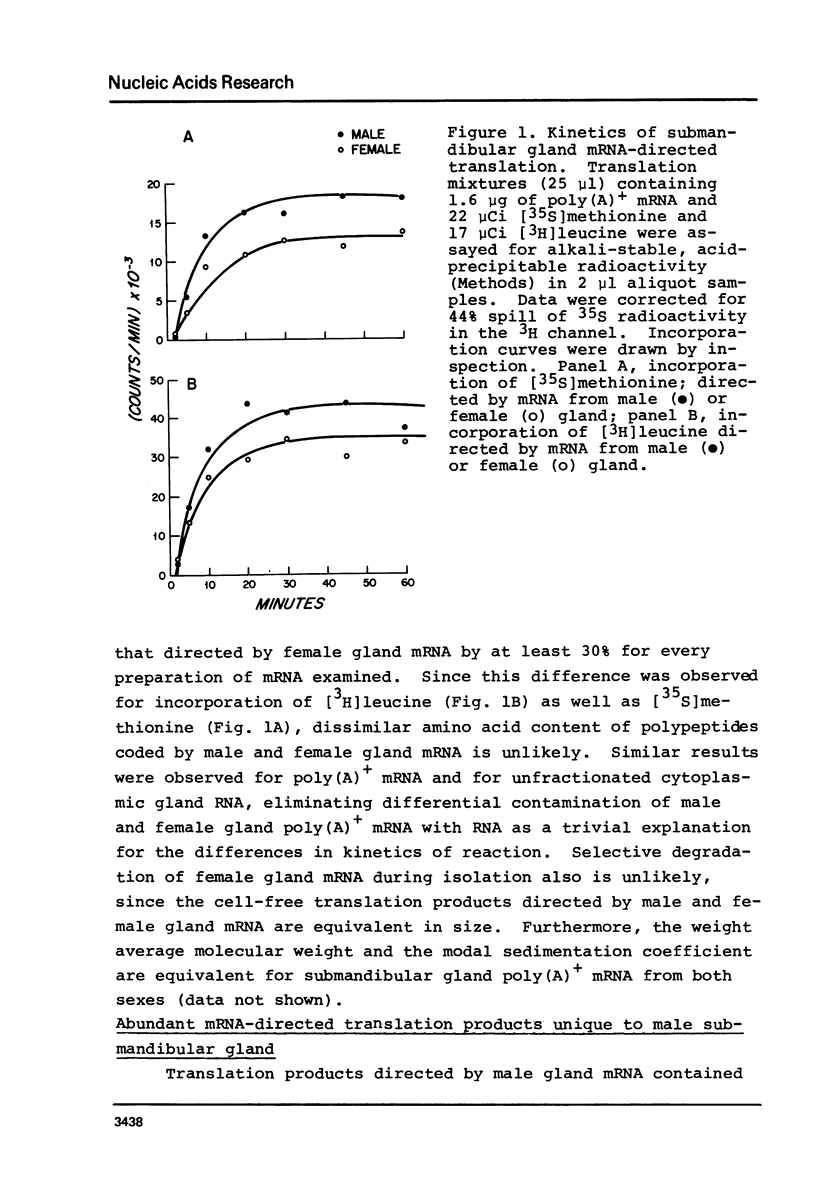
Submandibular glands of male mice contain at least four abundant mRNAs that occur at low concentrations in glands of females. The male-specific mRNAs code for polypeptides of 48,000, 43,000, 29,000, and 27,000 MW. Androgenic regulation of these mRNAs is illustrated by their apparent absence in glands of castrate males and by their accumulation in glands of females treated with testosterone. Selective hybrid-arrested translation experiments also indicate reduced levels of these male-specific sequences in female gland cytoplasm. The 48,000 MW male-specific polypeptide is reduced in translation products directed by gland mRNA from C57BL10/J mice (variants deficient in salivary renin), suggesting the corresponding mRNA codes for a renin precursor. The identity of this polypeptide is confirmed by immune selection with renin-specific antibody.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Bradshaw R. A. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. A., Shooter E. M. Biosynthesis of beta nerve growth factor in mouse submaxillary glands. J Biol Chem. 1978 Feb 10;253(3):804–810. [PubMed] [Google Scholar]
- Berger E. A., Shooter E. M. Evidence for pro-beta-nerve growth factor, a biosynthetic precursor to beta-nerve growth factor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3647–3651. doi: 10.1073/pnas.74.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Caramia F. Ultrastructure of mouse submaxillary gland. I. Sexual differences. J Ultrastruct Res. 1966 Dec;16(5):505–523. doi: 10.1016/s0022-5320(66)80003-5. [DOI] [PubMed] [Google Scholar]
- Caramia F. Ultrastructure of mouse submaxillary gland. II. Effect of castration in the male. J Ultrastruct Res. 1966 Dec;16(5):524–536. doi: 10.1016/s0022-5320(66)80004-7. [DOI] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Taylor J. M., Murakami K., Michelakis A. M., Inagami T. Isolation and characterization of renin-like enzymes from mouse submaxillary glands. Biochemistry. 1972 Nov 7;11(23):4286–4293. doi: 10.1021/bi00773a015. [DOI] [PubMed] [Google Scholar]
- Comstock J. P., Rosenfeld G. C., O'Malley B. W., Means A. R. Estrogen-induced changes in translation, and specific messenger RNA levels during oviduct differentiation. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2377–2380. doi: 10.1073/pnas.69.9.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Dzau V. J., Slater E. E., Haber E. Complete purification of dog renal renin. Biochemistry. 1979 Nov 13;18(23):5224–5228. doi: 10.1021/bi00590a029. [DOI] [PubMed] [Google Scholar]
- Faust C. H., Jr, Heim I., Moore J. Murine myeloma immunoglobulin heavy-chain mRNA. Isolation, partial purification, and characterization of gamma1, gamma2a, gamma2b, gamma3, micron and alpha heavy-chain mRNA'S. Biochemistry. 1979 Mar 20;18(6):1106–1119. doi: 10.1021/bi00573a027. [DOI] [PubMed] [Google Scholar]
- Frey P., Forand R., Maciag T., Shooter E. M. The biosynthetic precursor of epidermal growth factor and the mechanism of its processing. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6294–6298. doi: 10.1073/pnas.76.12.6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbüchle O., Bovey R., Young R. A. Tissue-specific expression of mouse-alpha-amylase genes: nucleotide sequence of isoenzyme mRNAs from pancreas and salivary gland. Cell. 1980 Aug;21(1):179–187. doi: 10.1016/0092-8674(80)90125-7. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A. Analysis of mRNA populations by cDNA.mRNA hybrid-mediated inhibition of cell-free protein synthesis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1217–1221. doi: 10.1073/pnas.75.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A., Toole J. J. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979 Jun;17(2):449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Parker M. G., Herries D. G. Effects of testosterone on sequence complexity of polyadenylated RNA from rat seminal vesicle. Eur J Biochem. 1978 Nov 15;91(2):327–334. doi: 10.1111/j.1432-1033.1978.tb12683.x. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Orth D. N. Concentrations of epidermal growth factor, nerve growth factor, and submandibular gland renin in male and female mouse tissue and fluids. Endocrinology. 1979 Dec;105(6):1382–1387. doi: 10.1210/endo-105-6-1382. [DOI] [PubMed] [Google Scholar]
- JUNQUEIRA L. C., FAJER A. Biochemical and histochemical observations on the sexual dimorphism of mice submaxillary glands. J Cell Physiol. 1949 Aug;34(1):129-58, incl 6 pl. doi: 10.1002/jcp.1030340109. [DOI] [PubMed] [Google Scholar]
- KOCHAKIAN C. D., HILL J., AONUMA S. Regulation of protein biosynthesis in mouse kidney by androgens. Endocrinology. 1963 Mar;72:354–363. doi: 10.1210/endo-72-3-354. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiho M., Nakamura T., Kumegawa M. Morphological studies on the synthesis of secretory granules in convoluted tubules of mouse submandibular gland. Anat Rec. 1975 Nov;183(3):405–419. doi: 10.1002/ar.1091830305. [DOI] [PubMed] [Google Scholar]
- Kochakian C. D., Tomana M., Strickland B. Role of cytosol and polysomes in the stimulation by androgen of protein biosynthesis in the mouse kidney,. Mol Cell Endocrinol. 1974 Apr;1(2):129–138. doi: 10.1016/0303-7207(74)90005-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Pennequin P., Schimke R. T. Induction of ovalbumin mRNA sequences by estrogen and progesterone in chick oviduct as measured by hybridization to complementary DNA. J Biol Chem. 1975 Oct 25;250(20):8105–8110. [PubMed] [Google Scholar]
- Murphy R. A., Saide J. D., Blanchard M. H., Young M. Molecular properties of the nerve growth factor secreted in mouse saliva. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2672–2676. doi: 10.1073/pnas.74.7.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M. A., Koch J., Hoffman H., Bender V., Hagopian H. Isolation and activity of a thymocyte-trasforming factor from the mouse submaxillary gland. Exp Cell Res. 1969 Sep;57(1):95–103. doi: 10.1016/0014-4827(69)90371-1. [DOI] [PubMed] [Google Scholar]
- Oparil S., Haber E. The renin-angiotensin system (first of two parts). N Engl J Med. 1974 Aug 22;291(8):389–401. doi: 10.1056/NEJM197408222910805. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Caplan A. I. High diversity in the polyadenylated RNA populations of embryonic myoblasts. J Biol Chem. 1978 Nov 10;253(21):7683–7691. [PubMed] [Google Scholar]
- Ouellette A. J. Purification by benzoylated cellulose chromatography of translatable messenger ribonucleic acid lacking polyadenylate. J Biol Chem. 1980 Apr 10;255(7):2740–2746. [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Parker M. G., Mainwaring W. I. Effects of androgens on the complexity of poly(A) RNA from rat prostate. Cell. 1977 Oct;12(2):401–407. doi: 10.1016/0092-8674(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T. The androgenic regulation of abundant mRNA in rat ventral prostate. Eur J Biochem. 1978 Apr 17;85(2):399–406. doi: 10.1111/j.1432-1033.1978.tb12252.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Poulsen K., Vuust J., Lykkegaard S., Nielsen A. H., Lund T. Renin is synthesized as a 50,000 dalton single-chain polypeptide in cell-free translation systems. FEBS Lett. 1979 Feb 1;98(1):135–138. doi: 10.1016/0014-5793(79)80169-6. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schibler U., Tosi M., Pittet A. C., Fabiani L., Wellauer P. K. Tissue-specific expression of mouse alpha-amylase genes. J Mol Biol. 1980 Sep 5;142(1):93–116. doi: 10.1016/0022-2836(80)90208-9. [DOI] [PubMed] [Google Scholar]
- Server A. C., Shooter E. M. Nerve growth factor. Adv Protein Chem. 1977;31:339–409. doi: 10.1016/s0065-3233(08)60221-1. [DOI] [PubMed] [Google Scholar]
- Stach R. W., Server A. C., Pignatti P. F., Piltch A., Shooter E. M. Characterization of the gamma subunits of the 7S nerve growth factor complex. Biochemistry. 1976 Apr 6;15(7):1455–1461. doi: 10.1021/bi00652a016. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Cohen S., Mitchell W. M. Epidermal growth factor: high and low molecular weight forms. Proc Natl Acad Sci U S A. 1970 Sep;67(1):164–171. doi: 10.1073/pnas.67.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole J. J., Hastie N. D., Held W. A. An abundant androgen-regulated mRNA in the mouse kidney. Cell. 1979 Jun;17(2):441–448. doi: 10.1016/0092-8674(79)90170-3. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Males J. L., Cohen S. Synthesis and storage of epithelial-epidermal growth factor in submaxillary gland. Cancer Res. 1971 Mar;31(3):252–256. [PubMed] [Google Scholar]
- Weimar V. L., Haraguchi K. H. A potent new mesodermal growth factor from mouse submaxillary gland. A quantitative, comparative study with previously described submaxillary gland growth factors. Physiol Chem Phys. 1975;7(1):7–21. [PubMed] [Google Scholar]
- Wetekam W., Mullinix K. P., Deeley R. G., Kronenberg H. M., Eldridge J. D., Meyers M., Goldberger R. F. Effect of estrogen on gene expression: purification of vitellogenin messenger RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3364–3368. doi: 10.1073/pnas.72.9.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Dunn J. F., Wilson J. D. Genetic control of renin activity in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1185–1189. doi: 10.1073/pnas.74.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Wilson J. D., Taylor B. A. Location on chromosome 1 of Rnr, a gene that regulates renin in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5623–5626. doi: 10.1073/pnas.75.11.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]