Abstract
Our previous studies showed that an adenovirus (Ad) serotype 5 vector expressing Flt3 ligand (Ad-FL) as nasal adjuvant activates CD11c+ dendritic cells (DCs) for the enhancement of antigen (Ag)-specific IgA antibody (Ab) responses. In this study, we examined the molecular mechanism for activation of CD11c+ DCs and their roles in induction of Ag-specific Th1- and Th2- cell responses. Ad-FL activated CD11c+ DCs expressed increased levels of the Notch ligand (L)-expression and specific mRNA. When CD11c+ DCs from various mucosal and systemic lymphoid tissues of mice given nasal OVA plus Ad-FL were cultured with CD4+ T cells isolated from non-immunized OVA TCR-transgenic (OT II) mice, significantly increased levels of T cell proliferative responses were noted. Furthermore, Ad-FL activated DCs induced IFN-γ, IL-2 and IL-4 producing CD4+ T cells. Of importance, these APC functions by Ad-FL activated DCs were down-regulated by blocking Notch-Notch-L pathway. These results show that Ad-FL induces CD11c+ DCs to the express Notch-ligands and these activated DCs regulate the induction of Ag-specific Th1- and Th2- type cytokine responses.
Keywords: Dendritic cells, Notch ligands, Cytokines
1. Introduction
It has been shown that DCs are the most potent Ag presenting cells (APCs) for the activation of naïve T cells for the induction of adaptive immune responses [1, 2]. Thus, DCs have been found throughout the body including mucosal surfaces. Murine Peyer’s patches contain CD11c+, CD11b+, CD8− immature DCs with high endocytic activity and low levels of MHC and B7 molecule expression. These immature DCs form a dense layer in the sub-epithelial dome (SED) just underneath the epithelium of mucosal inductive sites in order to capture Ags [3, 4]. In addition, some DCs in the intestinal lamina propria extend their dendrites between epithelial cell junctions into the lumen for uptake of antigens (intraepithelial DCs) [5-7]. When Ag uptake occurs, these DCs change their phenotype by expressing high levels of MHC class II and co-stimulatory molecules and move to T cell areas for Ag presentation [1, 3, 4]. Thus, DCs and their derived cytokines play key roles in the induction of Ag-specific Th1-and Th2- type responses. In this regard, interactions between Notch expressed by CD4+ T cells and their ligand (L) expressed by DCs play a central role in the induction of adaptive immune responses [8-13]. It was reported that microbial Ag-stimulated DCs regulate the differentiation of naïve CD4+ T cells into Th1 and Th2 lineages based upon their Notch-L expression [8-10, 12, 13]. Delta-like (Dlk; 1, 3 and 4) and Jagged (1 and 2) are the two families of five Notch-Ls that have been reported in mice [11]. Among these, Dlk1 and Dlk4 promote Th1-type CD4+ T cells, while Jagged1 and Jagged2 elicit Th2- type CD4+ T cell differentiation independently of IL-4/STAT6 signaling [9, 10]. Further, recent studies showed that Dlk4 promotes IL-17 synthesis and specific transcription factor, RORγc activation [14].
Our previous studies showed that nasal immunization of mice with OVA plus Ad-FL as mucosal adjuvant elicited high levels of OVA-specific IgA Ab responses in external secretions and plasma as well as significant levels of cytotoxic T lymphocyte (CTL) responses. Interestingly, nasal Ad-FL as mucosal adjuvant elicited both Th1- and Th2- type cytokine responses. In this regard, we thought it important to determine how mucosal DCs stimulate CD4+ T cells for the induction of Th1- and Th2- type responses. In this study, we show that Ad-FL activated DCs directly regulate CD4+ T cell proliferation and their subsequent cytokine patterns through Notch-Notch-L interactions.
2. Materials and methods
2.1. Mice
Young adult (6- to 8- week-old) C57BL/6 mice were purchased from the Frederick Cancer Research Facility (National Cancer Institute, NIH, Frederick, MD). OVA TCR-transgenic (OT II: clone Thy1.1), were obtained from The Jackson Laboratory (Bar Harbor, ME). Upon arrival, all mice were transferred to microisolators, maintained in horizontal laminar flow cabinets, and provided sterile food and water in a specific-pathogen-free animal facility at the University of Alabama at Birmingham Immunobiology Vaccine Center. All mice used in these experiments were free of bacterial and viral pathogens.
2.2. Nasal immunization
Mice were nasally immunized three times at weekly intervals with 3 μl per nostril of PBS containing 1 × 108 PFU of either Ad-FL or Ad-expressing firefly luciferase (Ad-Luc) plus 100 μg of ovalbumin (OVA; Sigma-Aldrich, St. Louis, MO). Replication-incompetent Ad-FL or Ad-Luc, respectively, were constructed and prepared as described previously [15].
2.3. In vitro APC functional analysis
CD11c+ DCs from nasopharyngeal-associated lymphoreticular tissue (NALT), cervical lymph nodes (CLNs), nasal passages (NPs), submandibular glands (SMGs) and spleens were purified one week after the last immunization using an automated magnetic-activated cell sorter (AutoMACS) system (Miltenyi Biotec, Auburn, CA), as described previously [16]. This purified CD11c+ DC fraction (> 97 % CD11c+; > 99 % cell viability) was resuspended (1 × 105 cells/ml) in RPMI 1640 (Cellgro Mediatech, Washington, DC) supplemented with HEPES buffer (10 mM), L-glutamine (2 mM), a solution of non-essential amino acids (10 ml/l), sodium pyruvate (10 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), gentamicin (80 μg/ml) and 10 % FCS (complete RPMI 1640), and then cultured with FACS purified splenic CD4+ T cells (5 × 105 cells/ml) taken from naïve OT II mice with or without 1 mg/ml of OVA for 5 days. In some experiments, CD4+ T cells from OT II mice were cultured without DCs in the presence or absence of OVA. In some cultures, 10 μM of γ-secretase inhibitor IX N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) was added in order to inhibit the Notch pathway [17]. To assess OVA-specific T cell proliferative responses, an aliquot of 0.5 μCi of tritiated [3H] thymidine (TdR) (Amersham Biosciences, Arlington Heights, IL) was added during the final 18 h of incubation, and the amount of [3H]TdR incorporation was determined by scintillation counting. The supernatants of T cell cultures not incubated with [3H]TdR were then subjected to a cytokine-specific ELISA, while T cells were subjected to cytokine-specific intracellular FACS and quantitative RT-PCR analyses as described below.
2.4. Flow cytometric analysis
To characterize the Notch-L expression by DCs, mononuclear cells (2 × 105 cells) were stained with Alexa Fluor®488-conjugated anti-mouse Dlk1, PE-labeled anti-mouse Jagged 1 or Jagged 2 (all from eBiosciences), APC-tagged anti-Dlk4 (Biolegend®) and/or biotinylated anti-CD11c mAbs (BD Biosciences) followed by PerCP-Cy5.5-streptavidin. For intracellular interferon-gamma (IFN-γ) and interleukin-4 (IL-4) analyses, cells were incubated with ionomycin (1 mg/ml, Sigma) and phorbol 12-myristate 13-acetate (PMA, 25 ng/ml; Sigma) for 4 h in the presence of Monensin and then stained with FITC-labeled anti-CD4, before being stained intracellularly with PE-labeled anti-IFN-γ, or -IL-4 mAbs (BD Biosciences).
2.5. Expression of Notch-L-specific mRNA
CD11c+ DCs from NALT, CLNs, NPs, SMGs and spleens were purified one week after the last immunization using the AutoMACS system. Total RNA from DCs was subjected to quantitative real-time RT-PCR analysis using Notch-L-specific primer sets. Primer sequences used were as follows: Dlk1 (forward) 5′-CCC AGG TGA GCT TCG AGT G-3′ (reverse) 5′-GGA GAG GGG TAC TCT TGT TGA G-3′; Dlk3 (forward) 5′-ACT CTT GGT CAT CCA CGT T-3′ (reverse) 5′-CAA AGA GTC TCC AGT CGG T-3′; Dlk4 (forward) 5′-AGG TGC CAC TTC GGT TAC ACA T-3′ (reverse) 5′-CAA TCA CAC ACT CGT TCC TCT CTT C-3′; Jagged1 (forward) 5′-AGA AGT CAG AGT TCA GAG GCG TCC TCT-3′ (reverse) 5′-AGT AGA AGG CTG TCA CCA AGC AAC-3′; Jagged2 (forward) 5′-AGC CAC GGA GCA GTC ATT TG-3′ (reverse) 5′-TCG GAT TCC AGA GCA GAT AGC G-3′.
2.6. Cytokine-specific ELISA
Levels of IFN-γ, IL-2 and IL-4 in culture supernatants of CD4+ T cells purified from NALT, CLNs, NPs, SMGs and spleens were measured by cytokine-specific ELISA as described previously [15, 18-21]. The detection limits for each cytokine were: 106.3 pg/ml for IFN-γ, 15.6 pg/ml for IL-2 and 4.66 pg/ml for IL-4.
2.7. Quantitative analysis of cytokine-specific mRNA
The CD4+ T cells were harvested after 2 days of incubation for total RNA extraction. Aliquots of extracted RNA (25 μg/ml) were subjected to reverse transcriptase reaction and were treated with 1 μl of 10 μg/ml RNase H (Invitrogen™, Carlsbad, CA). The levels of synthesized cDNA were measured using a NanoView RNA/DNA calculator (GE Healthcare, Piscataway, NJ). Sample cDNA and external standards were amplified with cytokine-specific primers and SYBER green I by using a LightCycler® (Roche Applied Science, Indianapolis, IN). The concentration of sample cDNA was determined using linear, diluted external standards obtained by an identical PCR protocol with the LightCycler® [16, 19, 21].
2.8. Statistical analysis
The results are presented as the mean ± one standard error of the mean (SEM). Ad-FL plus OVA-immunized C57BL/6 mouse groups were compared with the mice immunized with Ad-Luc plus OVA using an unpaired Mann-Whitney U test with Statview software (Abacus Concepts, Cary, NC) designed for Macintosh computers. Values of p < 0.05 were considered significant.
3. Results
3.1. Nasal delivery of Ad-FL induces DC-mediated CD4+ T cell activation
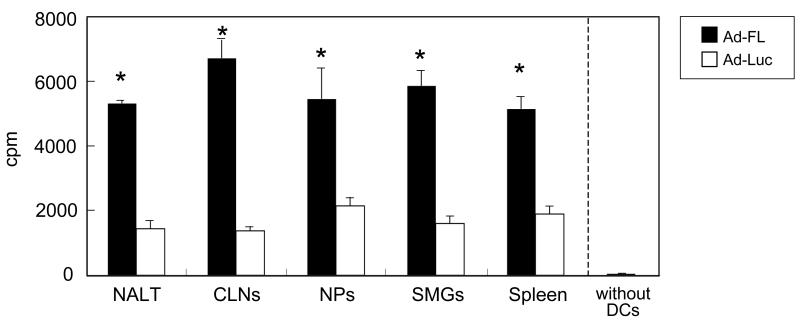
Since our previous study showed that nasal immunization with OVA and Ad-FL effectively elevated the numbers of mature-type DCs in both mucosal and systemic lymphoid tissues [15], we initially examined the ability of Ad-FL-induced CD11c+ DCs from various lymphoid tissues to activate OVA-specific CD4+ T cells. CD11c+ DCs from NALT, CLNs, NPs, SMGs and spleen of mice given nasal OVA plus Ad-FL induced significant levels of CD4+ T cell proliferative responses when compared with DCs from mice given nasal OVA plus Ad-Luc as controls (Fig. 1). When CD4+ T cells from OT II mice were cultured alone or with DCs in the absence of OVA, essentially no proliferative response was observed. Further, cultures containing CD4+ T cells from OT II mice incubated with OVA revealed essentially no proliferative response in the absence of exogenous CD11c+ DCs, which was the same as the cultures with CD4+ T cells alone (cpm: 338 ± 111).
Fig. 1.
Analysis of DC function for induction of CD4 T cell proliferation. Mice were nasally immunized weekly for three consecutive weeks with OVA plus Ad-FL as mucosal adjuvant or Ad-Luc as a vector control. One week after the last immunization, CD11c+ DCs were purified from NALT, CLNs, NPs, SMGs and spleens, and cultured with naïve splenic CD4+ T cells isolated from non-immunized OVA TCR-transgenic (OT II) mice with or without 1 mg/ml of OVA for 5 days. An aliquot of 0.5 μCi of tritiated [3H]TdR was added during the final 18 h of incubation. The results are presented as the cpm of wells with OVA following subtraction of the cpm of wells without OVA. * p < 0.05 when compared with mice immunized with Ad-Luc plus OVA.
3.2. Ad-FL induces Notch ligand expression by DCs
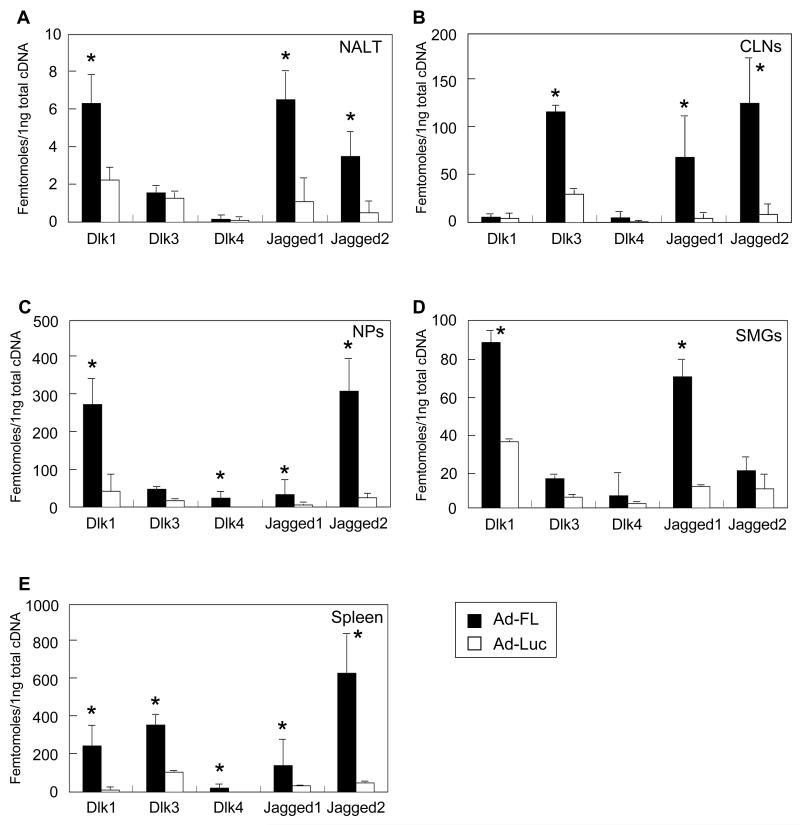
Since it has been reported that the Notch-Ls, Dlk and Jagged, preferentially induce Th1- and Th2- type CD4+ T cells, respectively, we next examined Notch-L expression by Ad-FL-induced DCs from various lymphoid tissues. DCs from NALT, CLNs, NPs, SMGs and spleens of mice given nasal Ad-FL plus OVA showed increased levels of Notch-L expression when compared with those from mice given nasal Ad-Luc plus OVA (Figures 2A-2D and Table 1). Significantly increased levels of Jagged1, Jagged2 and Delta-like 1 (Dlk1)- specific messages were noted in DCs from NALT (Fig. 2A). DCs from CLNs elicited significantly increased levels of Dlk3- specific mRNA as well as Jagged1 and Jagged2- specific mRNA (Fig. 2B. SMG DCs showed marked increases in Jagged1- and Dlk1- specific mRNA expression, while DCs from NPs contained elevated levels of Jagged1- and Jagged2- specific mRNA in addition to Dlk1- and Dlk4- specific mRNA (Fig. 2D and E). Further, increased levels of Dlk1, Jagged1 and Jagged2 expression by DCs from various lymphoid tissues of mice given nasal OVA plus Ad-FL were seen when compared with those from mice nasally immunized with OVA plus Ad-Luc (Table 1). These results showed that Ad-FL-induced DCs in the different lymphoid tissues express increased levels of Notch-Ls which potentially play central roles in the induction of Th1- and Th2- type cytokine responses by CD4+ T cells.
Fig. 2.
Expression of Notch ligand-specific mRNA. Mice were nasally immunized weekly for three consecutive weeks with Ad-FL plus OVA. One week after the last immunization, CD11c+ DCs were purified from NALT (A), CLNs (B), NPs (C), SMGs (D) and spleens (E). Total RNA was subjected to quantitative RT-PCR analysis. The values shown are the mean ± SEM taken from 25 mice in each experimental group. * p < 0.05 when compared with mice immunized with Ad-Luc plus OVA.
Table 1.
Notch ligand expression by CD11c+ DCs of various lymphoid tissuesa
| Tissues | Mice nasally immunized with OVA plus |
% of Total Lymphocytes |
% of CD11c+ DCs | |||
|---|---|---|---|---|---|---|
| CD11c | Dlk 1 | Dlk 4 | Jagged 1 | Jagged 2 | ||
| NALT | Ad-FL | b4.40 ± 0.36** | 17.73 ± 0.82* | 1.21 ± 0.35 | 13.03 ± 0.47* | 9.24 ± 0.86* |
| Ad-Luc | 2.27 ± 0.29 | 13.35 ± 2.08 | 2.49 ± 1.67 | 9.09 ± 2.08 | 7.33 ± 1.83 | |
|
| ||||||
| CLNs | Ad-FL | 3.00 ± 0.08** | 14.94 ± 0.83* | 1.34 ±0.55 | 13.03 ± 1.67** | 7.41 ± 0.96** |
| Ad-Luc | 1.69 ± 0.49 | 10.66 ± 1.08 | 2.37 ± 0.84 | 7.70 ± 1.60 | 5.33 ± 0.84 | |
|
| ||||||
| NPs | Ad-FL | 9.37 ± 0.55** | 25.57 ± 5.04* | 5.94 ± 0.69* | 34.54 ± 4.88* | 36.24 ± 2.14* |
| Ad-Luc | 1.57 ± 0.26 | 10.42 ± 0.97 | 2.13 ± 0.37 | 10.84 ± 1.28 | 7.02 ± 1.91 | |
|
| ||||||
| SMGs | Ad-FL | 13.21 ± 0.96** | 35.33 ± 4.07* | 1.03 ± 0.73 | 24.75 ± 2.24* | 12.52 ± 0.76* |
| Ad-Luc | 3.92 ± 0.24 | 30.56 ± 3.08 | 1.36 ± 0.64 | 10.47 ± 1.99 | 2.47 ± 0.15 | |
|
| ||||||
| Spleen | Ad-FL | 6.15 ± 0.22* | 29.12 ± 2.31* | 1.46 ± 0.59 | 17.95 ± 1.69* | 14.53 ± 0.68* |
| Ad-Luc | 3.31 ± 0.18 | 14.40 ± 0.35 | 1.01 ± 0.46 | 6.95 ± 1.32 | 11.28 ± 1.22 | |
Mice were nasally immunized weekly for three consecutive weeks with OVA plus Ad-FL. One week after the last immunization, mononuclear cells from NALT, CLNs, NPs, SMGs and spleen were stained with a combination of the respective mAbs and subjected to flow cytometry analysis by FACSCalibur®.
The values shown are the mean ± SEM of three independent experiments. Each experimental group consisted of five mice.
*p < 0.05, **p < 0.01 when compared with Ad-Luc group.
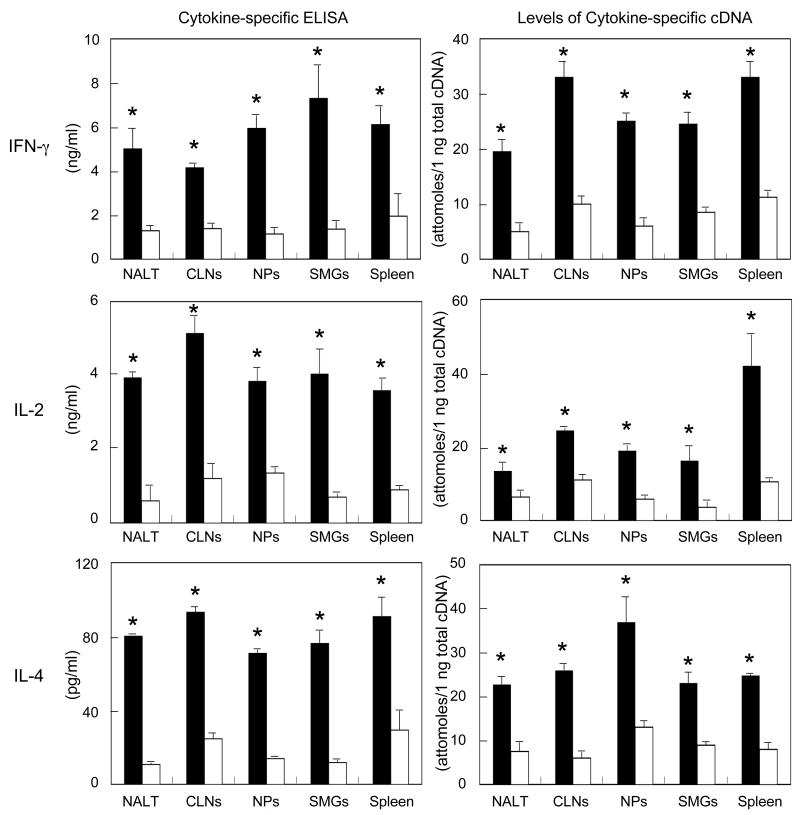
3.3. Ad-FL-activated DCs induce both Th1- and Th2-type CD4+ T cell responses
In order to test whether Ad-FL activated DCs expressing high levels of Notch-L directly interact with Ag-specific CD4+ T cells for the induction of Th1- and Th2-type cytokines, purified CD11c+ DCs were isolated from mice given nasal OVA plus Ad-FL and co-cultured with OVA-specific CD4+ T cells from OT-II mice. We noted that DCs from mice given Ad-FL as nasal adjuvant induced higher levels of Th1 (IFN-γ and IL-2)- and Th2 (IL-4)- type CD4+ T cells when compared with those seen in mice given Ad-Luc as a control (Fig. 3). These results indicate that nasal Ad-FL effectively up-regulates APC function by CD11c+ DCs in NALT and in mucosal effector tissues and spleens. Further, nasal Ad-FL induced CD11c+ DCs play a key role in the activation of CD4+ T cells by supporting Th1- and Th2- type cytokine responses.
Fig. 3.
Analysis of Th1- and Th2-cytokine production by OVA-specific CD4+ T cells. Mice were nasally immunized as described in Fig. 1 legend. One week after the last immunization, CD11c+ DCs were purified from NALT, CLNs, NPs, SMGs and spleens, and cultured with naïve splenic CD4+ T cells isolated from non-immunized OVA TCR-transgenic (OT II) mice with or without 1 mg/ml of OVA for 2 or 5 days. Culture supernatants were harvested after 5 days of incubation and analyzed for the respective cytokine by ELISA. Total RNA was extracted after 2 days of incubation and subjected to quantitative RT-PCR analysis. The values shown are the mean ± SEM taken from 25 mice in each experimental group. * p < 0.05 when compared with mice given nasal Ad-Luc plus OVA.
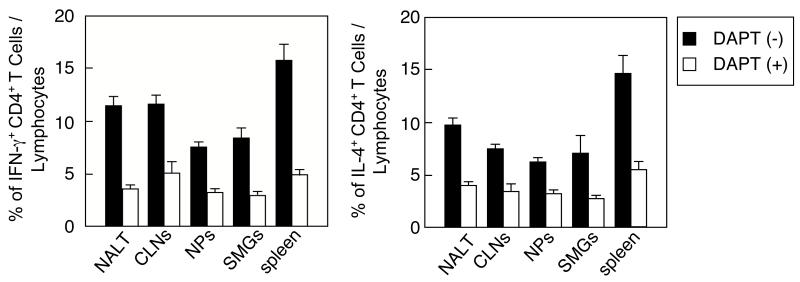
3.4 Blockade of the Notch signal pathway down-regulates Th1- and Th2-producing CD4+ T cells
We next examined the essential role of Notch-L expression by DCs in the induction of Th1-and Th2-type cytokine responses by blocking the Notch-Notch-L pathway. The cultures containing CD11c+ DCs from mice given nasal OVA plus Ad-FL and OVA-specific CD4+ T cells from OT-II mice were stimulated with OVA in the presence or absence of DAPT. Intracellular cytokine analyses showed that significantly reduced frequencies of both IFN-γ- and IL-4-producing CD4+ T cell subsets were seen in the cultures treated with DAPT when compared with those of non-treated wells (Fig. 4, Supplemental Figure). These results directly show that the Notch-Notch-L pathway is required for the induction of Th1- and Th2-type cytokine responses.
Fig. 4.
A blockade of the Notch-Notch-L pathway down-regulates the numbers of IFN-γ- and IL-4-producing T cells. Mice were nasally immunized as described in Fig. 1 legend. Purified CD11c+ DCs and splenic CD4+ T cells from OT II mice were co-cultured with 1 mg/ml of OVA in the presence or absence of DAPT for 5 days. Cells were stained with FITC conjugated anti-CD4 mAb followed by additional intracellular staining with PE-tagged anti-IFN-γ or -IL-4 mAbs. Samples were subjected to flow cytometric analysis by FACSCalibur®. The values shown are the mean ± SEM of 10 mice in each experimental group. *p < 0.05 when compared with the cultures in the absence of DAPT.
4. Discussion
This study directly shows that Notch-L expressing CD11c+ DCs from NALT, CLNs, NPs and SMGs of mice given nasal OVA plus Ad-FL elicited higher T cell proliferative responses and increased levels of Th1- and Th2- type cytokine responses by activated CD4+ T cells. It is well accepted that DCs are the most potent APCs for presenting Ag and providing cytokines and costimulatory molecules to naïve CD4+ T cells to drive expansion and differentiation into cytokine-secreting effector CD4+ Th cells [22]. Further, it was shown that retinoic acid-and TGF-β1- producing DCs also play key roles in the induction of Treg cells whereas Th17-type CD4+ T cells were induced by DCs producing only TGF-β1 [23-26]. Recent reports have shown that nasal DC-targeting adjuvants including Ad-FL elicited a balanced Ag-specific Th1- and Th2- type CD4+ T cell response [15, 16].
To this end, we further investigated Notch-L expression by DCs from mice given nasal Ad-FL since preferential expression of the Notch-Ls by DCs has been suggested to play a role in instructing Th1- or Th2- type responses, respectively [9, 10, 27, 28]. Further, one recent study showed that microbial Ag-stimulated DCs regulated the differentiation of naïve CD4+ T cells into Th1 and Th2 lineages based upon their Notch-L expression [28]. Dlk1 has been shown to be expressed by DCs, the principal APC involved in CD4+ Th1 cell activation in vivo [29, 30]. On the other hand, Jagged1 expressing DCs were involved in IL-4-producing Th2- type CD4+ T cell induction and intiation of allergic lung responsiveness [31]. In addition, Jagged2 expression was up-regulated in response to the helminth Schistosoma mansoni soluble egg Ag, which conditions DCs to induce Th2-type responses [32]. Based upon these observations, we expected to see increased levels of both Dlk and Jagged expression by DCs of mice given nasal Ad-FL when compared with those seen in mice given control Ad-Luc. Indeed, our results showed that significantly increased levels of Dlk1 or Dlk3, and Jagged1 and/or Jagged2 expression were noted in various mucosal tissues and spleen. Interestingly, Dlk4 expression in those tissues was limited even though some expression levels were higher than those seen in controls. To support our findings, it was recently reported that a unique subset of Th1 cells which produce IL-10 were induced by Dlk4 expressing DCs [33, 34]. Thus, Ad-FL as nasal adjuvant most likely induces classical Ag-specific Th1-type CD4+ T cells through DCs expressing Dlk1 or Dlk3.
It was of interest to note that DCs in mucosal NALT as a mucosal inductive site and CLNs as the draining lymph nodes showed equally required for Jagged1 and Jagged2 expression for Th2-type cytokines which are ultimately essential for IgA and IgG Ab induction. In contrast, NPs showed significantly higher levels of Jagged2 expression when compared with SMGs although both NPs and SMGs contained approximately equal levels of Jagged1. Both NPs and SMGs are known as IgA effector tissues; however, NPs contains significantly higher numbers of OVA-specific IgG producing cells than those seen in the SMGs when mice were nasally immunized with OVA plus Ad-FL [15]. In this regard, it is possible that Jagged1-expressing DCs are preferentially involved in the induction of Ag-specific IgG Ab responses. We are currently testing this hypothesis by blocking the Jagged1 or Jagged2 pathway using recombinant Jagged1- and Jagged2-Fc-Ig chimera protein.
In summary, the present study showed that Ad-FL as a nasal adjuvant induces Notch-L-expressing CD11b+ DCs which activate T cells and induce Th1- and Th2- type cytokine production. This is the first evidence that balanced Th1- and Th2-type cytokine responses are directly regulated by mucosal DCs which express both Dlk and Jagged. In addition, we show that DCs in mucosal inductive sites and DCs in mucosal effector tissues serve as APCs to upregulate CD4+ T cell function.
Supplementary Material
Highlights.
-
>
Nasal Ad-FL effectively up-regulates APC function by CD11c+ DCs in mucosal tissues.
-
>
Nasal Ad-FL induces Notch ligand (L)-expressing CD11c+ DCs.
-
>
Notch L-expressing DCs support the induction of Th1- and Th2-type cytokine responses.
Acknowledgements
We thank Dr. Jerry R. McGhee for his scientific discussions regarding these experiments. This work is supported by National Institutes of Health (NIH) grants DE012242 and AG025873 as well as the Japan Society of the Promotion of Science (JSPS) program entitled “Young Researcher Overseas Visits Program for Vitalizing Brain Circulation”, the Japan Foundation for Pediatric Research and Houjinkai fellowship award of the Department of Pediatrics at Osaka City University Graduate School of Medicine and The Mochida Memorial Foundation for Medical and Pharmaceutical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adiels M, Taskinen MR, Packard C, Caslake M, Soro-Paavonen A, Westerbacka J, Vehkavaara S, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, Haring HU, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]
- 4.Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, et al. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut. 2009;58:1281–1288. doi: 10.1136/gut.2008.151977. [DOI] [PubMed] [Google Scholar]
- 5.Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, Rijcken TH, Korevaar JC, van Aalderen WM, Jansen PL, et al. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96:669–674. doi: 10.1136/adc.2010.199760. [DOI] [PubMed] [Google Scholar]
- 6.Kugelmas M, Hill DB, Vivian B, Marsano L, McClain CJ. Cytokines and NASH: a pilot study of the effects of lifestyle modification and vitamin E. Hepatology. 2003;38:413–419. doi: 10.1053/jhep.2003.50316. [DOI] [PubMed] [Google Scholar]
- 7.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–2163. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oza N, Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Horie H, Ushirogawa M, et al. A pilot trial of body weight reduction for nonalcoholic fatty liver disease with a home-based lifestyle modification intervention delivered in collaboration with interdisciplinary medical staff. J Gastroenterol. 2009;44:1203–1208. doi: 10.1007/s00535-009-0115-x. [DOI] [PubMed] [Google Scholar]
- 10.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schafer S, Kantartzis K, Machann J, Venter C, Niess A, Schick F, Machicao F, et al. Lifestyle intervention in individuals with normal versus impaired glucose tolerance. Eur J Clin Invest. 2007;37:535–543. doi: 10.1111/j.1365-2362.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- 12.Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162–2168. doi: 10.1038/oby.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90:3191–3196. doi: 10.1210/jc.2004-1959. [DOI] [PubMed] [Google Scholar]
- 14.Thamer C, Machann J, Stefan N, Haap M, Schafer S, Brenner S, Kantartzis K, et al. High visceral fat mass and high liver fat are associated with resistance to lifestyle intervention. Obesity (Silver Spring) 2007;15:531–538. doi: 10.1038/oby.2007.568. [DOI] [PubMed] [Google Scholar]
- 15.Thamer C, Machann J, Stefan N, Schafer SA, Machicao F, Staiger H, Laakso M, et al. Variations in PPARD determine the change in body composition during lifestyle intervention: a whole-body magnetic resonance study. J Clin Endocrinol Metab. 2008;93:1497–1500. doi: 10.1210/jc.2007-1209. [DOI] [PubMed] [Google Scholar]
- 16.Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813–5819. doi: 10.3748/wjg.v12.i36.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, et al. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. doi: 10.1016/s0168-8278(97)80287-5. [DOI] [PubMed] [Google Scholar]
- 18.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr. 2011;93:1048–1052. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiikkainen M, Bergholm R, Vehkavaara S, Rissanen A, Hakkinen AM, Tamminen M, Teramo K, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 21.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.2008 Physical Activity Guidelines for Americans. Department of Health and Human Services; Hyattsville, MD: 2008. Retrieved April 12, 2011 from http://www.health.gov/paguidelines/guidelines/default.aspx. [Google Scholar]
- 23.ACSM, AHA Support Federal Physical Activity Guidelines Indianapolis. American College of Sports Medicine. 2011 Retrieved December 6, 2011 from http://www.acsm.org/about-acsm/media-room/acsm-in-the-news/2011/08/01/acsm-aha-support-federal-physical-activity-guidelines.
- 24.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijden G-J, Wang ZJ, Chu ZD, Sauer PJJ, Haymond MW, Rodriguez LM, Sunehag AL. A 12-Week Aerobic Exercise Program Reduces Hepatic Fat Accumulation and Insulin Resistance in Obese, Hispanic Adolescents. Obesity. 2009;18:384–390. doi: 10.1038/oby.2009.274. [DOI] [PubMed] [Google Scholar]
- 26.Perri MG, Anton SD, Durning PE, Ketterson TU, Sydeman SJ, Berlant NE, Kanasky WF, Jr., et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21:452–458. [PubMed] [Google Scholar]
- 27.Ekkekakis P, Lind E, Vazou S. Affective Responses to Increasing Levels of Exercise Intensity in Normal-weight, Overweight, and Obese Middle-aged Women. Obesity. 2009;18:79–85. doi: 10.1038/oby.2009.204. [DOI] [PubMed] [Google Scholar]
- 28.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes. 2005;30:652–660. doi: 10.1038/sj.ijo.0803052. [DOI] [PubMed] [Google Scholar]
- 29.Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 30.Genton L, Hans D, Kyle UG, Pichard C. Dual-Energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 31.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 32.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 33.Mittendorfer B, Patterson BW, Klein S. Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am J Physiol Endocrinol Metab. 2003;284:E549–556. doi: 10.1152/ajpendo.00379.2002. [DOI] [PubMed] [Google Scholar]
- 34.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 35.Magkos F, Patterson BW, Mittendorfer B. Reproducibility of stable isotope-labeled tracer measures of VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics. Journal of Lipid Research. 2007;48:1204–1211. doi: 10.1194/jlr.D600048-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 37.Patterson BW, Mittendorfer B, Elias N, Satyanarayana R, Klein S. Use of stable isotopically labeled tracers to measure very low density lipoprotein-triglyceride turnover. J Lipid Res. 2002;43:223–233. [PubMed] [Google Scholar]
- 38.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women Produce Fewer but Triglyceride-Richer Very Low-Density Lipoproteins than Men. Journal of Clinical Endocrinology & Metabolism. 2007;92:1311–1318. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 39.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–1648. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 40.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–153. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Devries MC, Samjoo IA, Hamadeh MJ, Tarnopolsky MA. Effect of endurance exercise on hepatic lipid content, enzymes, and adiposity in men and women. Obesity (Silver Spring) 2008;16:2281–2288. doi: 10.1038/oby.2008.358. [DOI] [PubMed] [Google Scholar]
- 42.Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–413. doi: 10.1007/s00125-006-0498-7. [DOI] [PubMed] [Google Scholar]
- 43.Kelley GA, Kelley KS, Tran ZV. Aerobic exercise and lipids and lipoproteins in women: a meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2004;13:1148–1164. doi: 10.1089/jwh.2004.13.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley GA, Kelley KS, Vu Tran Z. Aerobic exercise, lipids and lipoproteins in overweight and obese adults: a meta-analysis of randomized controlled trials. Int J Obes (Lond) 2005;29:881–893. doi: 10.1038/sj.ijo.0802959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 46.Tambalis K, Panagiotakos DB, Kavouras SA, Sidossis LS. Responses of blood lipids to aerobic, resistance, and combined aerobic with resistance exercise training: a systematic review of current evidence. Angiology. 2009;60:614–632. doi: 10.1177/0003319708324927. [DOI] [PubMed] [Google Scholar]
- 47.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:G619–626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 48.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, et al. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300:G874–883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic Fatty Liver Disease: Predictors of Nonalcoholic Steatohepatitis and Liver Fibrosis in the Severely Obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 50.Wong VW-S, Wong GL-H, Choi PC-L, Chan AW-H, Li MK-P, Chan H-Y, Chim AM-L, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 51.Dixon J, Bhathal P, O’Brien P. Weight Loss and Non-alcoholic Fatty Liver Disease: Falls In Gamma-Glutamyl Transferase Concentrations are Associated with Histologic Improvement. Obesity Surgery. 2006;16:1278–1286. doi: 10.1381/096089206778663805. [DOI] [PubMed] [Google Scholar]
- 52.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 53.Prati D, Taioli E, Zanella A, Torre ED, Butelli S, Del Vecchio E, Vianello L, et al. Updated Definitions of Healthy Ranges for Serum Alanine Aminotransferase Levels. Annals of Internal Medicine. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 54.Omagari K, Takamura R, Matsutake S, Ichimura M, Kato S, Morikawa S, Nagaoka S, et al. Serum alanine aminotransferase concentration as a predictive factor for the development or regression of fatty liver. J Clin Biochem Nutr. 2011;49:200–206. doi: 10.3164/jcbn.11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine Aminotransferase, γ-Glutamyltransferase, and Incident Diabetes. Diabetes Care. 2009;32:741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.