Abstract
The availability of genetically engineered mice allows unraveling the role of specific proteins in mechanisms of ischemic brain injury. Due to the high variability of their vascular anatomy, mouse models of global cerebral ischemia are rather complex. In the present study, we describe a simple model of mouse forebrain ischemia where the bilateral common carotid artery occlusion (BCCO) is combined with isoflurane-induced hypotension. The forebrain ischemia was induced by BCCO that was preceded by increase of the isoflurane level from 1.5% to 5% in the respiratory gases. This caused a decrease of the mean arterial blood pressure (MABP) to about 30 mmHg and the cerebral blood flow dropped to 5% of the control after the BCCO. During the 10 min ischemic period both MABP and CBF remained stable and the reperfusion was induced by reducing the isoflurane level to 0% followed by removal of the carotid clamps. Mice were allowed 1, 2, 3 or 5 days survival followed by histologic analysis. The number of CA1 uninjured neurons was assessed utilizing a stereological approach.
Neurodegeneration was observed at two days after the onset of reperfusion. At 3 days of recovery, about 40% of neurons survived and the cell death did not further increase at 5 days. Degenerative neurons were also detected in the striatum and sporadically in the cortex. This study demonstrates the feasibility of using the described model in mice that can be utilized to examine the effect of new neuroprotective compounds or use transgenic animals to test new hypothesis.
Keywords: mouse, global cerebral ischemia, model, hypotension, isoflurane, cell death
Introduction
A reproducible model of global cerebral ischemia is essential for elucidating the molecular mechanisms of ischemic brain injury and to discover new treatments. The mouse model is particularly important because of the abundance of genetically engineered mouse strains that can be used to test specific hypotheses. Although many models, including gerbils and rats, have been widely used for global ischemia studies (Pulsinelli and Brierley, 1979, Smith et al., 1984, Ginsberg and Busto, 1989, Pegorini et al., 2005), a simple model of forebrain ischemia in the mouse has not been fully established.
A simple method of bilateral common carotid artery occlusion is the most frequently used mouse model of global brain ischemia. However, a drawback of this model is the high variability in ischemic outcome (Yang et al., 1997, Kitagawa et al., 1998). One possible reason for this variability is the application of a universal occlusion time in strains of mice from different genetic backgrounds. It is now apparent that sensitivities to ischemic insult between different mouse strains is a problematic issue (Sheldon et al., 1998, Wellons et al., 2000). In addition, there is significant variability due to individual differences in collateral flow through the circle of Willis (Beckmann, 2000, Fujii et al., 1997, Kitagawa et al., 1998, Wellons et al., 2000, Zhen and Dore, 2007) because mice express highly variable arterial cerebrovascular structures even within the same strain. To reduce the variability in histological outcome and optimize the bilateral carotid occlusion model, animals that possess the posterior communicating artery (PcomA) were excluded (Murakami et al., 1998, Zhen and Dore, 2007). This endpoint was approached by simultaneous measurement of residual cerebral blood flow in both hemispheres during ischemia (Kitagawa et al., 1998, Yonekura et al., 2004) or by examination of mouse vasculature for the presence of PcomA following animal perfusion-fixation. Furthermore, models of three-vessel occlusion were developed, however these exhibit major limitations and are surgically challenging (Panahian et al., 1996). Global ischemia induced by cardiocirculatory arrest is clinically the most relevant model and was first developed for rats (Bottiger et al., 1998) and later adapted by the same lab for mice (Bottiger et al., 1999). The two-vessel occlusion plus hypotension model developed for rats (Smith et al. 1984) was also adapted for mice (Sheng et al., 1999, Wellons et al., 2000). This model gives a typical reproducible brain damage, however it requires cannulation of both the internal jugular vein and the femoral artery to continuously monitor mean arterial blood pressure (MABP) during ischemia.
We have developed a similar model of forebrain cerebral ischemia where the bilateral common carotid artery occlusion is combined with hypotension induced by increased levels of anesthesia. The MABP is maintained at 30 to 35 mm Hg when a 5% isoflurane concentration is used in the respiratory gas mixture of N2O and O2 (70:30). This significantly simplifies the surgical procedure and offers a model more suitable for screening protective compounds. No artery cannulation is required since the common carotid arteries are clamped for 10 min after the isofluorane level is increased to 5%. Reperfusion is induced by reducing the isoflurane level to 0% and removing the clamps. Our data suggest that this model demonstrates a similar degree of hippocampal injury as observed in presently used models of mouse forebrain ischemia.
Materials and Methods
Animals
All animal protocols were approved by the Animal care and Use Committee of the University of Maryland Baltimore, in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Adult male C57Bl6 mice (10–11 weeks old; Charles River Laboratories, Wilmington, MA) were used. The animals were maintained in a 12-hours light/dark cycle.
Global ischemia
Before the surgery the animals were fasted overnight with free access to tap water. Mice were anesthetized with 5% isoflurane in a mixture of N2O:O2 (70:30; flow rates 35:15 ml per minute), intubated, and mechanically ventilated with 1.5% isoflurane in N2O:O2 as above. The common carotid arteries were isolated via a neck incision and encircled with loose ligatures for later clamping. Pericranial temperature was monitored with a needle thermistor placed subcutaneously adjacent to the skull. The body and head temperature were maintained at 37.0 ± 0.5 °C through the use of a homoeothermic-heating pad and heating lamp.
Forebrain ischemia (10 min) was induced by increasing the isoflurane concentration to 5% then 2 min later, the carotid arteries were clamped with micro-vessel clamps. The isoflurane level was set to 0% 4 min before the release of the carotid artery clamps and onset of the reperfusion. The neck incision was then closed with 3-0 aseptic non-absorbable silk sutures, in a simple interrupted pattern. The animals were extubated following resumption of spontaneous breathing, and then moved into warm (T = 36–37 °C) recovery chamber (V-1200, Harvard Apparatus) to prevent post-ischemic hypothermia (Wellons et al., 2000, Zhen and Dore, 2007). Animals were subjected to 10 min of transient forebrain ischemia and randomly divided into several recovery groups. Following the ischemic insult, mice underwent 1, 2, 3 or 5 days of recovery (n = 6 to 8 per group) and at the end of the recovery period the animals were perfusion-fixed and their brains processed for histological examination or the animals were tested for neurologic outcomes (see below). As a control, sham operated animals subjected to 5 % of isoflurane for 8 min were used (n = 6 for histological examination and n = 10 for behavioral tests).
Monitoring of mean arterial blood pressure (MABP)
In a separate group of animals (n = 6), an additional incision was made to expose and cannulate the femoral artery (PE 10 tubing) for heparin administration (300 IU.kg−1), blood pressure recording, and blood sampling. After surgical preparation, 0.1 cc of blood was drawn for blood gas analysis and ventilation adjusted accordingly to give an arterial PaCO2 of 34–38 mm Hg and a PaO2 of about 130 mm Hg. The animals were mechanically ventilated at a rate of 120 breaths per min and the tidal volume was adjusted to give normal physiological blood gas values of (34–38 mm Hg PaCO2 and 120–140 mm Hg of PaO2). The MABP was monitored before, during ischemia, and following the first 5 min of reperfusion.
Measurement of regional Cerebral blood flow (CBF)
Regional blood flow was determined by laser-Doppler flowmetry using a flexible 0.5 mm fiber-optic extension. The tip of the probe was affixed to the surface of the skull over a brain region devoid of major vasculature 3 mm posterior to bregma and 2 mm lateral to midline, contralateral to the thermal probe. The CBF recording started 5 min before onset of the 5% isoflurane concentration in the respiratory gases and continued during the 10 min of common carotid arteries occlusion and the first 10 min of reperfusion. Six animals were used to determine the ischemia/reperfusion-induced changes in regional cerebral blood flow.
Histology
To process the brain tissue for histological or immunohistochemical examination, the animals were perfusion-fixed under deep anesthesia of ketamine plus xylazine. The thorax was opened and a perfusion needle transcardially inserted into the ascending aorta, the left atria was cut to remove blood and excess perfusate from the body. The brains were first rinsed with saline for 1 minute and then perfusion-fixed with 4% paraformaldehyde for histology/immunohistochemistry. After perfusion-fixation, brains were immersed in cold fixative overnight and transferred to a 30% sucrose solution. Thirty μm-thick coronal sections were cut on a freezing microtome and stored in a cryoptotectant solution at −20 °C.
Fluoro-Jade B and cresyl violet stainings
To identify brain regions affected by the ischemic insult we utilized Fluoro-Jade staining that visualizes degenerative neurons. Sections were washed three times for 10 min in KPBS, mounted on glass slides and dried overnight. For Fluoro-Jade staining, slides were immersed in absolute ethanol for 3 min followed by 1 min in 70% ethanol and 1 min in distilled water. After incubation in 0.06% solution of potassium permanganate for 15 min, the slides were rinsed in distilled water for 1 min. They were then transferred to a solution containing 0.01% Fluoro-Jade (Histo-Chem Inc., Jefferson, AR) and 0.1% acetic acid (1:10), placed on a shaker for 30 min, and after three washes for 1 min in distilled water, dried, immersed in xylene for 10 min then coverslipped.
Since Fluoro-Jade B stains not only degenerating cells but also cells that might not die (Larsson et al. 2001) we assessed the cell death using cresyl-violet staining. Sections were rinsed in distilled water, immersed in 0.5% cresyl violet and rinsed again in distilled water. The slides were dehydrated, immersed in xylene, and coverslipped. Examination of Fluoro-Jade B and cresyl violet staining was performed using fluorescence and light microscopy, respectively.
Stereologic Quantifications
Every sixth 30 μm-thick section located between 1.7 and 2.5 mm posterior to bregma (6 sections), which comprises the dorsal hippocampus, was stained with cresyl violet and used for estimating the total neuronal numbers of normal and dying neurons in the different regions of the hippocampus. Quantitative analysis was performed on a computer-assisted image analysis system consisting of a Nikon Eclipse 800 photomicroscope equipped with a computer-controlled motorized stage, and a computer utilizing the StereoInvestigator program, a custom-designed morphology and stereology software (Vereczki et al., 2006). Cell numbers were quantified according to the optical fractionator method (Larsson et al. 2001, West et al. 1991). The stage-controlling computer randomly places the counting frame on the first counting area, then systematically moves it until the entire delineated field is sampled. Only the top layer of cells between 5 – 15 μm are counted. For cresyl violet staining, dying neurons are defined as those exhibiting either clear accumulation of dense, globular materials in the cytoplasm with evidence of nuclear fragmentation, or cells showing shrunken perikarya and darkly stained nuclei of reduced size (Vereczki et al., 2006).
Motor test
Five days after the ischemic insult, mice were subjected to neurological examination designed to detect motor deficits (Sheng et al., 1999). The mouse underwent a prehensile traction test. The time that the mouse was able to hang onto a horizontal rope by its forepaws was recorded, to a maximum of 5 seconds. The traction portion involves the animal’s ability to bring one of its rear limbs up to the rope and depends on equilibrium and muscle strength. Mouse forepaws were placed on the rope, and the animal was released. Time to falling was noted as well as whether or not the mouse brought a rear limb up to the rope. The scoring of this test was performed according Combs et all. 1987.
Screen test
Mice were placed on a 20 X 30 inch screen (0.2 X 0.2 cm grid size) that could be rotated from horizontal to vertical position. The mouse was placed on the horizontal screen and the screen was rotated into the vertical position. The duration of time that the mouse was able to hold onto the vertical screen was recorded to a maximum of 15 sec (allowing a total 3 points)(Combs et all. 1987).
From these two tests, a maximal score of 6 and minimal score of 0 could be obtained.
Analysis of exploratory behavior
This test measures the activity and habituation response of animals on placement in a novel environment (Dean et al. 1981, Inoue et al. 1996). Following five days of recovery the animals were placed in a translucent polypropylene box and the activity was measured by using the Any-maze video tracking system. Mice are placed in the activity chamber in a quiet room with dimmed light. The total distance traveled by the animal was recorded during a 15 min testing period in 3 min consecutive intervals.
Statistical analysis
Physiological values and hippocampal CA1 histologic damage were compared among groups by one-way ANOVA followed by the Bonferroni post hoc test. For motor performance scores Mann-Whitney U test for rank data was used. Values are presented as mean ± SD. A p value of < 0.05 was considered to be statistically significant.
Results
Evaluation of physiological parameters
Physiological parameters are shown in Table 1 and Table 2. Six mice were subjected to physiological study. MABP, PaO2, and pH showed normal physiological levels with a ventilator setting of 120 strokes per min and about 0.3 cc of stroke volume. PaCO2 was slightly hypocapnic. There was no difference in head and core temperature between the animals in sham and ischemic groups (Table 2). Similarly, there was no significant difference in the body weight of animals between the different experimental groups.
Table 1.
Physiological data
| MABP (mmHg) | 83 ± 3 |
| pH | 7.33 ± 0.1 |
| PaCO2 (mmHg) | 32.1 ± 2.53 |
| PaO2 (mmHg) | 136 ± 9 |
| Weight (g) | 22 ± 3.1 |
Values are mean ± SD, n = 6.
Table 2.
Head temperature and weight of animals used for the histological studies.
| Head temperature (°C) | Sham n = 6 | Ischemia + 3 days of recovery n = 6 | Ischemia + 5 days of recovery; histology; n = 6 | Ischemia + 5 days of recovery; neurologic tests; n = 8 |
|---|---|---|---|---|
| Pre-ischemia | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.2 |
| Intra-ischemia | 37.1 ± 0.1 | 37.0 ± 0.2 | 37.2 ± 0.2 | 37.0 ± 0.2 |
| Post-ischemia | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| Weight (g) | 22.9 ±0.17 | 23.2 ± 1.0 | 22.5 ± 1.1 | 23.3 ± 1.0 |
Intra-ischemia for sham group represents 5 % isoflurane with no BCCO. Values are mean ± SD.
Isofluorane-induced hypotension
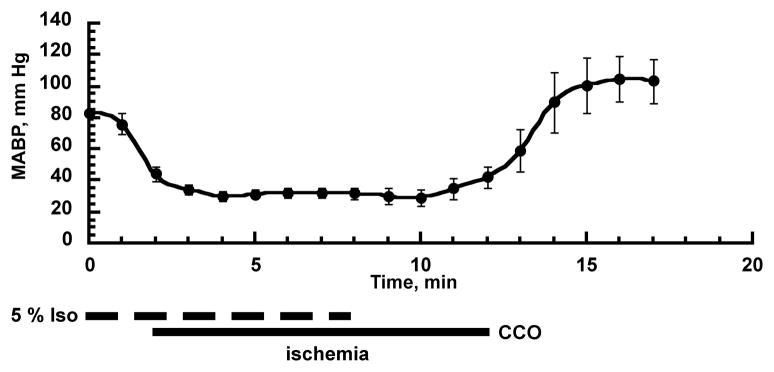
To reduce the collateral blood flow via vertebral arteries during the bilateral common carotid artery occlusion, we decreased the mean arterial blood pressure (MABP) by increasing the isoflurane concentration in respiratory gases from 1.5 to 5%. After 2 min, the MABP drops to about 40 mmHg at which time both common carotid arteries are occluded (Fig 1). The MABP stabilizes at 30 – 35 mmHg during the occlusion period without further manipulation of isoflurane levels. Four minutes before the end of the 10 min occlusion period, the isoflurane anesthesia was discontinued (0% isoflurane), then after 10 min of occlusion the microclips were removed. This protocol is simple, and as Fig 1 shows, the levels of MABP are very reproducible.
Fig 1.
Changes in mean arterial blood pressure (MABP) during common carotid arteries occlusion plus isoflurane-induced hypotension. The dashed line represents the 5% isoflurane concentration period, and the solid line the bilateral common carotid artery occlusion period that is identical to the forebrain ischemia (n = 6, mean ± SD).
Cerebral blood flow alterations
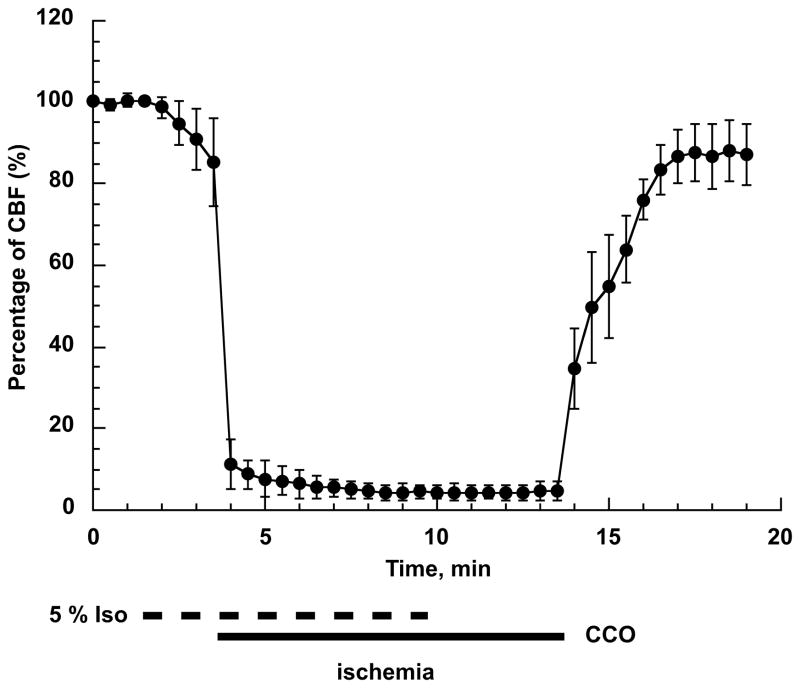
To determine the ischemic conditions in the brain, we examined changes in cerebral blood flow (CBF) using laser-Doppler flowmetry. This technique allows assessment of relative changes in tissue blood flow. The recorded values before the onset of ischemia were considered as 100% blood flow level (see Fig 2). After increasing the isofluorane concentration to 5% the CBF started to slowly decrease reaching about 85% of the control values after 2 minutes. Common carotids occlusion caused a rapid drop in CBF to about 10% of baseline. This was further decreased to less than 5 % within 1 – 2 min. The release of common carotids occlusion resumed the CBF raising it initially to about 40% then, within the next 2–3 min, the CBF reached 80%. During the first 5 min of reperfusion the cortical blood flow stabilized at this level.
Fig 2.
Changes in cortical blood flow during forebrain ischemia and reperfusion. First minute represents the baseline (100%) blood flow level. Then the isoflurane concentration was increased to 5% and at 3 min the common carotids were occluded (CCO) using microclips. The combination of the high isoflurane level and CCO reduced the blood flow to less than 5% of baseline. At 10 min of CBF recording, the anesthesia was discontinued (0% isoflurane). Following 10 min of CCO the microclips were removed which caused a rapid increase in CBF to about 40% followed by a slower rise. The CBF reached 80 – 90% of baseline at 3 minutes of reperfusion (n=4, error bars represent SD).
Motor and exploratory behavior test
There were no significant difference between sham and ischemic group performance levels in motor score. However, the open filed test showed a significant reduction of distance travelled by the animals subjected to forebrain ischemia during the first three 3 min intervals (fig 3).
Fig 3.
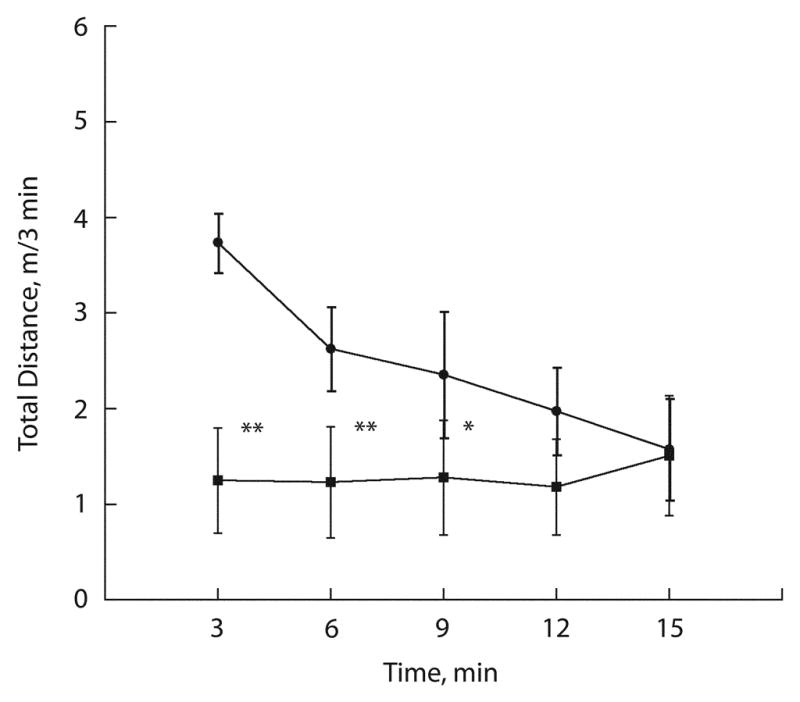
Exploratory behavior of control mice (filled circles) and mice subjected to forebrain ischemia (filled squares) following 5 days of recovery. During the 15 min test session and distance travelled each consecutive 3 min period was recorded. The data are expressed as mean ± SD (n=10 for sham control group and n = 8 for the post-ischemic group; ** p < 0.01; * p < 0.05 when compared to sham).
Fluoro-Jade B staining
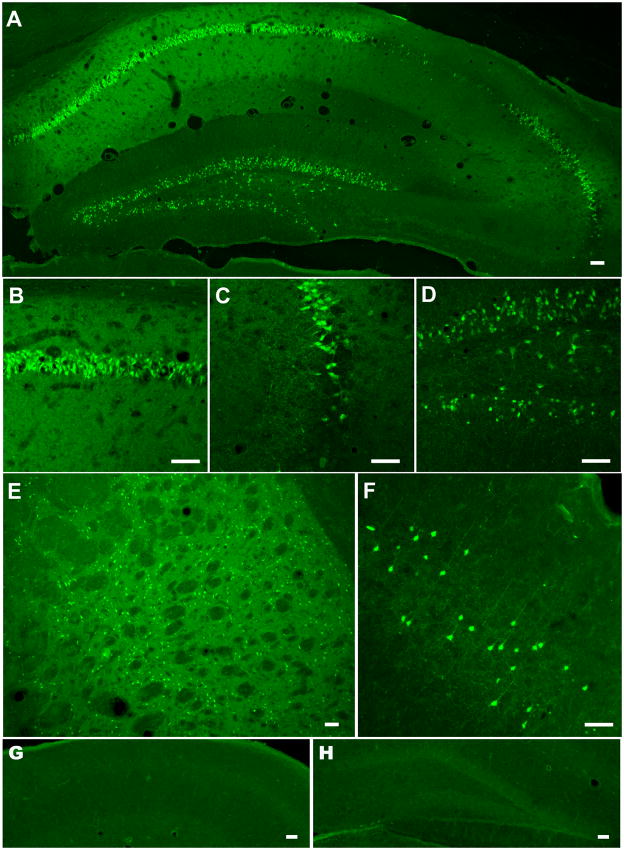
The Fluoro-Jade staining specifically visualizes dying neurons, which become fluorescent green (Schmued et al., 1997). There was no staining at 24 h of recovery. At 2 days of reperfusion, the Fluoro-Jade staining resulted in positively stained neurons particularly in the CA1 sector of the hippocampus and in the CA2-CA3 border area (data not shown). Similarly, we detected degenerative neurons in the striatum. As shown in Fig 4, three days after the ischemic insult there is an intense and dense staining of degenerative cells in the CA1 sector of the hippocampus, particularly in the medial region. Interestingly, 10 – 20% of cells in the dentate gyrus (DG) region were also positive for Fluoro-Jade B staining. Furthermore, some cells were Fluoro-Jade B positive at the border between the CA3 and CA2 sector (see Fig 3C). The Fluoro-Jade B staining also revealed neurodegeneration in the caudo putamen and in upper layers of the parietal cortex (Fig 3E, F). Brains from sham operated animals subjected to the surgery and 8 min of 5% isoflurane concentration without the bilateral common carotid artery occlusion did not show any cells with positive Fluoro-Jade B staining (Fig 3G, H).
Fig 4.
Fluoro-Jade B staining of the mouse brain hippocampus subjected to 10 min forebrain ischemia and 5 days of recovery (A). The enlarged views of the CA1 sector (B), the CA2–3 border (C), and DG (D). Note the high number of degenerating, Fluoro-Jade B positive cells in the CA1 pyramidal cell layer. There are relatively few cells stained in the CA3 region at the border with the CA2, and a subpopulation of neurons are stained in the DG. Fluoro-Jade B positive cells are also located in the caudo putamen (E) and layers 2–3 of the parietal cortex (F). Sham operated animals do not show Fluoro-Jade B positive cells in the CA1 (G) or DG (H) region of the hippocampus. Scale bar = 100 μm.
Cresyl violet staining and cell death assessment
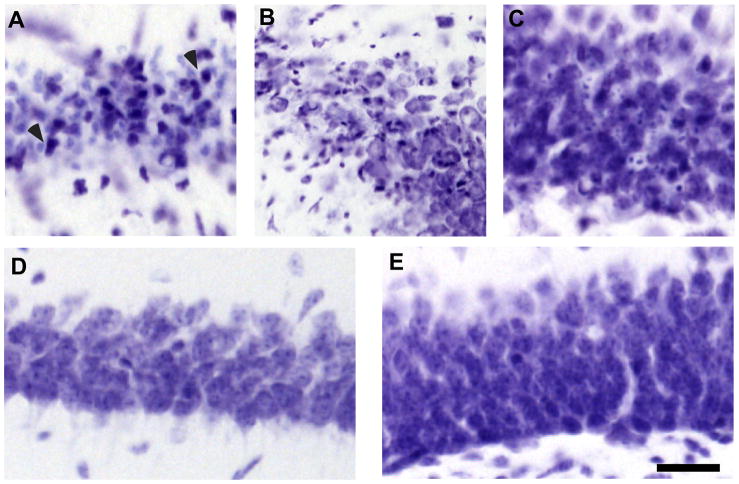
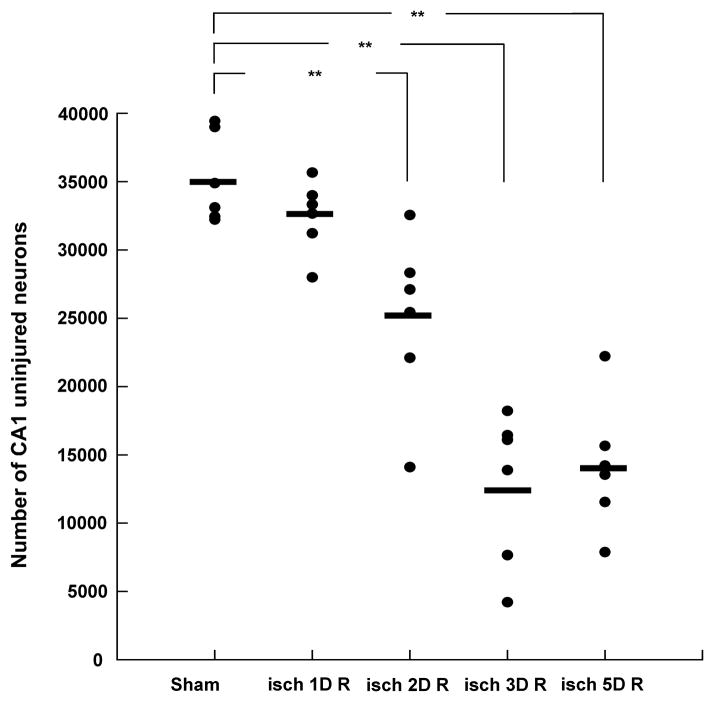
Cresyl violet staining also showed a dramatic loss of CA1 pyramidal neurons (Fig 5A). Similarly, neuronal death was observed in the CA2 region (Fig 5B) and a few dead cells can be identified in the cell body layer of the DG (Fig 5C). In sham operated animals (subjected only to 5 % isoflurane with no BCCO) all the CA1 neurons following cresyl violet staining appeared normal (Fig 5D). Similarly, neurons in the dentate gyrus did not exhibit pathologic morphology (Fig 5E). Consistent neuronal injury in the CA1 sector was observed at both the 3 and 5 days recovery periods. There were about 40% uninjured CA1 neurons and most of the pyramidal neurons exhibited pyknotic, shrunken nuclei (Fig 5A). There was no significant difference in the number or surviving neurons at 3 and 5 days of recovery (p= 0.32) (Fig 6).
Fig 5.
Cresyl violet staining of the post-ischemic mouse hippocampus following 5 days of recovery. Cell layer of the CA1 sector (A), the CA2–3 border (B), and DG (C). Arrowheads (A) indicate degenerative cells with a dark shrunken nuclei. The bottom panels show the CA1 (D) and DG (E) pyramidal cell layer of sham-operated animals following 5 days of recovery. Scale bar = 50 μm.
Fig 6.
Quantification of neuronal cell death in the CA1 sector of the hippocampus. The number of uninjured cells was estimated using a stereologic approach, as described in Materials and Methods. Filled circles represent the number of uninjured cells in individual animals. Horizontal bars represent the mean value. At firs day of recovery there was no significant difference in the number of undamaged CA1 neurons when compared to sham group (P=1.000). However, following 2, 3 and 5 days of recovery there was a significant loss of CA1 cells (p<0.009 sham vs. 2D R; p<0.001 sham vs. 3D R; p<0.001 sham vs. 5D R). There was no significant difference in the number of CA1-surviving cells between animals undergoing 3 and 5 days of recovery (p = 1.000). Similarly, there was no significant difference between the 1 and 2 days recovery group (p= 0.108). Sham - sham operated animals; isch 1D R – ischemia plus 1 day of recovery, isch 2D R – ischemia plus 2 days of recovery; isch 3D R – ischemia plus 3 days of reperfusion; isch 5D R – ischemia plus 5 days of reperfusion (n = 6 for each group; ** p < 0.01 when compared to the sham group).
Discussion
This study describes a simple model of mouse global cerebral ischemia where the bilateral common carotid artery occlusion (BCCO) is combined with isoflurane-induced hypotension. Several models of mouse global cerebral ischemia have been developed. The simplest one is the bilateral common carotid artery occlusion model. However, a drawback of this model is the inconsistency in histologic outcome due to significant variability in collateral flow through the circle of Willis. This is due to the irregular occurrence of the posterior communicating artery (PcomA) (Yang et al., 1997, Kitagawa et al., 1998, Murakami et al., 1998, Zhen and Dore, 2007). To increase the reproducibility of damage thus optimizing the BCCO model, one can exclude animals determined to possess the PcomA (Murakami et al., 1998, Zhen and Dore, 2007). Another approach is to decrease the cerebral blood flow (CBF) by combination of BCCO and reduction of blood flow via vertebral arteries. This approach is used in a three-vessel occlusion model where the occlusion of carotid arteries is combined with clamping of the basilar artery (Panahian et al., 1996, Yonekura et al., 2004).
Generally, in rat and also in mouse models of forebrain ischemia, to minimize the collateral blood flow, the BCCO is combined with reduction of the mean arterial blood pressure (MABP) to 30–35 mmHg. The hypotension is induced by withdrawal of blood via a venous catheter inserted into the jugular vein (Smith et al., 1984, Sheng et al., 1999, Wellons et al., 2000). Addition of controlled hypotension to CCO then leads to a more uniform reduction of CBF, and less inter-hemisphere differences in CA1 damage (Wellons et al., 2000).
We simplified this method so that no additional surgical procedure apart from the common carotid isolation is required. In our model, the reduction in MABP is induced by increasing the isoflurane level in respiratory gases from 1.5 to 5%. Thus, we first increased the isoflurane concentration to 5% then, after about 2 min the common carotid arteries were occluded. At this time the MABP dropped to 40 mmHg then further decreased to 30–35 mmHg remaining stable during the whole period of CCO. The time period between the setting of isoflurane to 5% and the drop of MABP to 40 mmHg depends on the diameter and length of the tubing used between the vaporizer and the animal, and also on the flow rate of respiratory gases. Therefore, for each surgical setup the duration of this time period needs to be determined in pilot experiments. Similarly, the time point when the isoflurane is set to 0% before the clamps are removed needs to be experimentally resolved. In our hands, the MABP recovery was reproducible when we stopped delivering isoflurane to the animals 4 minutes before the clamps were removed. This resulted in a relatively rapid and consistent increase in MABP. Discontinuing the isoflurane delivery at time points closer to the onset of reperfusion resulted in slow and variable recovery of MABP suggesting still significant anesthetic effects from residual isoflurane.
The combination of bilateral carotid arteries occlusion and isoflurane-induced hypotension was sufficient to reduce the CBF to 5% of control. Similar reduction of CBF was observed in standard two-vessel occlusion plus hemorrhage-induced hypotension models in rat or mouse (Smith et al., 1984, Sheng et al., 1999). This level of CBF reduction leads to the phenomenon of delayed cell death. Neurodegenerative neurons as identified with Fluoro-Jade B staining, were detected in the CA1 subregion of the hippocampus as early as following two days of recovery. The three and five day recovery data suggest that the cell death matures at 3 days of reperfusion with no further increase in cell death at 5 days after the ischemic insult (see also Sheng et al., 1999, Wellons et al., 2000). Similar damage development in the CA1 sector of the hippocampus was reported by utilizing the mouse three-vessel occlusion model (Panahian et al. 1996) or the two-vessel occlusion plus hypotension ischemia model (Wellons et al., 2000). Unlike in rat models of forebrain ischemia, cell death is also observed in subpopulations of cells within the dentate gyrus (see also Sheng et al., 1999). Furthermore, our model causes neuronal degeneration at the border of the CA2 and the CA3 hippocampal subregions. Similarly as in commonly used models of mouse global cerebral ischemia, we found extensive cell death within the striatum and neurodegenerative neurons also occurred sporadically within the parietal cortex (Murakami et al., 1998, Sheng et al., 1999, Wellons et al., 2000, Yonekura et al., 2004).
During the first hours of recovery, the animals show very limited motor activity. Therefore, to avoid hypothermia mice, were placed into warm recovery chamber (Zhen and Dore, 2007). Some models of global cerebral ischemia are associated with occasional seizure activities during the early reperfusion period. We did not observe any signs of seizures in our post-ischemic animals.
The commonly used BCCO plus hemorrhage-induced hypotension model is more complex than our model which requires only isolation of the carotid arteries. The former in addition to occlusion of carotid arteries, also requires cannulation of both the femoral (or tail) artery and the jugular vein. Due to cannulation of the femoral artery, there was reported evidence of hind limb ischemia on the side ipsilateral to the arterial catheter (Wellons et al., 2000). This could present an important limitation in behavioral studies of post-ischemic animals. Although our model of forebrain ischemia did not affect the mice muscle strength and their prehensile reflex, the exploratory activity in a novel environment was significantly decreased.
Apart from the complexity of the surgical process, three-vessel occlusion or bilateral common carotid artery occlusion plus hypotension models also have other drawbacks, including high mortality and post-ischemic seizures (Wellons et al., 2000).
In our model the animals are not subjected to hemorrhagic shock and the stress from the surgical procedure is significantly reduced. The disadvantage of this model is possible interaction of isoflurane with mechanisms leading to cell death during the intra-ischemic period. It has been shown that isoflurane reduces brain damage in cerebral ischemia models (Baughman et al., 1988, Miura et al., 1998, Mackensen et al., 1999, Blanck et al., 2000). However, this protection was observed when fentanyl/N2O was used as the anesthetic agent instead of isoflurane (Mackensen et al., 1999, Miura et al., 1998). The mechanism by which isoflurane improves histological outcome seems to be due to its ability to attenuate the peripheral sympathetic response (Mackensen et al., 1999). This protective effect of isoflurane was also assessed on long-term outcome following both global and focal ischemia. The reduction of infarct size following focal ischemic insult was observed following 2 weeks recovery (Sakai et al. 2007) when isoflurane was compared to fentanyl/N2O. After global ischemia the protective effect was observed only at 3 days of recovery. At 3 weeks or 3 months, there was no differential effect of intraischemic anesthetic on histology or behavioral outcome (Elsersy et al. 2004). In our model, during the ischemic period we increase the isoflurane concentration from 1.5 to 5%. However, in most laboratories isoflurane is used as a common anesthetic during surgical procedures. Since the hippocampal damage is very similar to that reported when using common models of mouse global ischemia, it seems unlikely that the intra-ischemic increase in the isoflurane level has a significant effect on the histological outcome when compared to surgical procedure when standard 1.5 % isoflurane levels are used.
The disadvantage of this model is that it does not allow monitoring of physiological parameters (blood gases) in each animal.
In summary, the bilateral common carotid artery occlusion in combination with isoflurane-induced hypotension is an efficient and simple global cerebral ischemia model that produces reproducible hippocampal damage in adult C57Bl6 mice. It is surgically easy and quick. This global ischemia model can be readily use for testing new drug effects and novel hypotheses when utilizing transgenic animals.
Highlights.
Article describes a simple model of forebrain ischemia in mouse
The described model of transient global ischemia give rise to cell death mainly in the CA1 sector and partially in the dentate gyrus sub-region of the hippocampus
The cell death is delayed and matures after 3 days of recovery
Acknowledgments
This work was supported by NIH R21 NS0585556 grant, U.S. Veterans Affairs Merit grant, and Maryland Stem Cell Research Fund grant to TK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baughman VL, Hoffman WE, Miletich DJ, Albrecht RF, Thomas C. Neurologic outcome in rats following incomplete cerebral ischemia during halothane, isoflurane, or N2O. Anesthesiology. 1988;69:192–8. doi: 10.1097/00000542-198808000-00007. [DOI] [PubMed] [Google Scholar]
- Beckmann N. High resolution magnetic resonance angiography non-invasively reveals mouse strain differences in the cerebrovascular anatomy in vivo. Magn Reson Med. 2000;44:252–8. doi: 10.1002/1522-2594(200008)44:2<252::aid-mrm12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Blanck TJ, Haile M, Xu F, Zhang J, Heerdt P, Veselis RA, Beckman J, Kang R, Adamo A, Hemmings H. Isoflurane pretreatment ameliorates postischemic neurologic dysfunction and preserves hippocampal Ca2+/calmodulin-dependent protein kinase in a canine cardiac arrest model. Anesthesiology. 2000;93:1285–93. doi: 10.1097/00000542-200011000-00023. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Schmitz B, Wiessner C, Vogel P, Hossmann KA. Neuronal stress response and neuronal cell damage after cardiocirculatory arrest in rats. J Cereb Blood Flow Metab. 1998;18:1077–87. doi: 10.1097/00004647-199810000-00004. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass P. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Brain Res Mol Brain Res. 1999;65:135–42. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Combs DJ, D'Alecy LG. Motor performance in rats exposed to severe forebrain ischemia: effect of fasting and 1,3-butanediol. Stroke. 1987;18:503–11. doi: 10.1161/01.str.18.2.503. [DOI] [PubMed] [Google Scholar]
- Dean RL, 3rd, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age-related differences in behavior across the life span of the C57BL/6J mouse. Exp Aging Res. 1981;7:427–51. doi: 10.1080/03610738108259823. [DOI] [PubMed] [Google Scholar]
- Elsersy H, Sheng H, Lynch JR, Moldovan M, Pearlstein RD, Warner DS. Effects of isoflurane versus fentanyl-nitrous oxide anesthesia on long-term outcome from severe forebrain ischemia in the rat. Anesthesiology. 2004;100:1160–6. doi: 10.1097/00000542-200405000-00018. [DOI] [PubMed] [Google Scholar]
- Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–10. doi: 10.1161/01.str.28.9.1805. discussion 11. [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–42. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, Watanabe T. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc Natl Acad Sci U S A. 1996;93:13316–20. doi: 10.1073/pnas.93.23.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Larsson E, Lindvall O, Kokaia Z. Stereological assessment of vulnerability of immunocytochemically identified striatal and hippocampal neurons after global cerebral ischemia in rats. Brain Res. 2001;913:117–32. doi: 10.1016/s0006-8993(01)02762-7. [DOI] [PubMed] [Google Scholar]
- Mackensen GB, Nellgard B, Miura Y, Chu CT, Dexter F, Pearlstein RD, Warner DS. Sympathetic ganglionic blockade masks beneficial effect of isoflurane on histologic outcome from near-complete forebrain ischemia in the rat. Anesthesiology. 1999;90:873–81. doi: 10.1097/00000542-199903000-00031. [DOI] [PubMed] [Google Scholar]
- Miura Y, Grocott HP, Bart RD, Pearlstein RD, Dexter F, Warner DS. Differential effects of anesthetic agents on outcome from near-complete but not incomplete global ischemia in the rat. Anesthesiology. 1998;89:391–400. doi: 10.1097/00000542-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780:304–10. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Panahian N, Yoshida T, Huang PL, Hedley-Whyte ET, Dalkara T, Fishman MC, Moskowitz MA. Attenuated hippocampal damage after global cerebral ischemia in mice mutant in neuronal nitric oxide synthase. Neuroscience. 1996;72:343–54. doi: 10.1016/0306-4522(95)00563-3. [DOI] [PubMed] [Google Scholar]
- Pegorini S, Braida D, Verzoni C, Guerini-Rocco C, Consalez GG, Croci L, Sala M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br J Pharmacol. 2005;144:727–35. doi: 10.1038/sj.bjp.0706115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–72. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–9. doi: 10.1097/00000542-200701000-00017. discussion 8–10. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–22. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Characterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Methods. 1999;88:103–9. doi: 10.1016/s0165-0270(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–35. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellons JC, 3rd, Sheng H, Laskowitz DT, Burkhard Mackensen G, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagihara T, Matsumoto M. C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res. 1997;752:209–18. doi: 10.1016/s0006-8993(96)01453-9. [DOI] [PubMed] [Google Scholar]
- Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24:151–8. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]
- Zhen G, Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods. 2007;166:73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]