Abstract
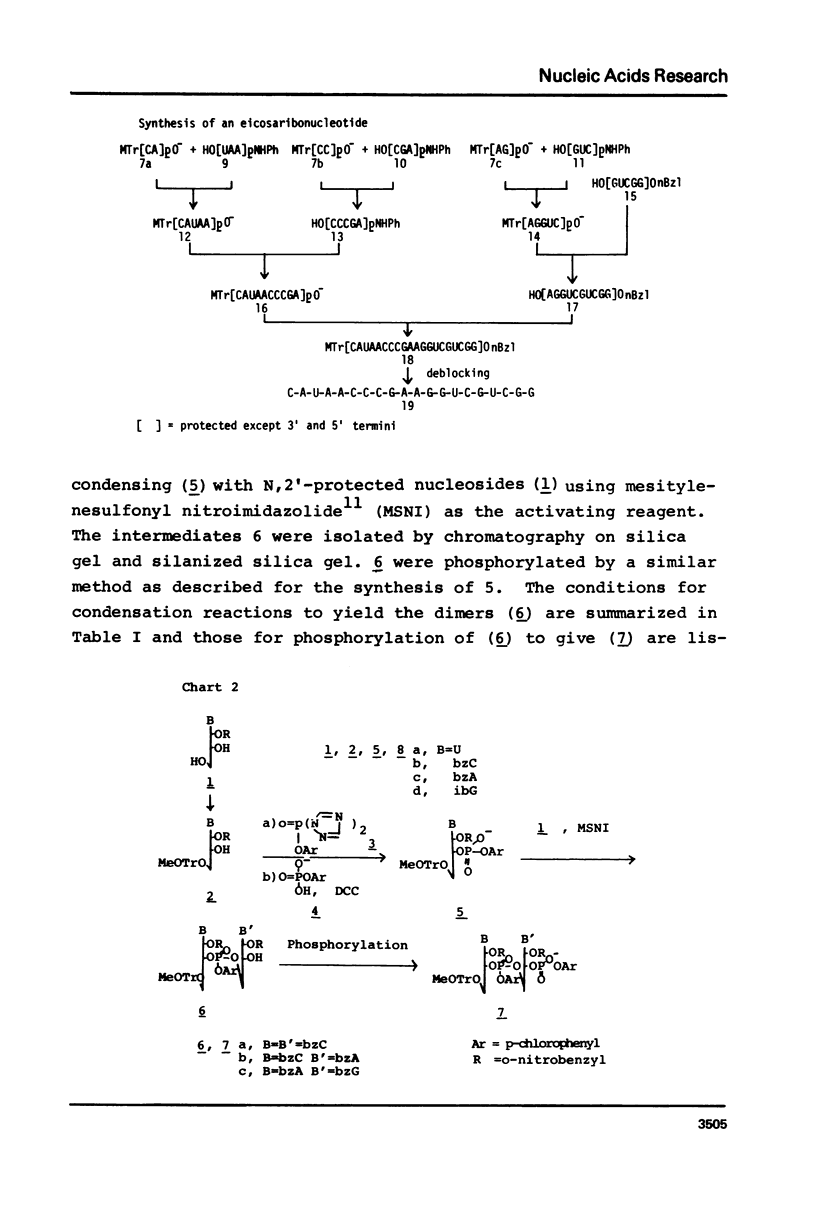
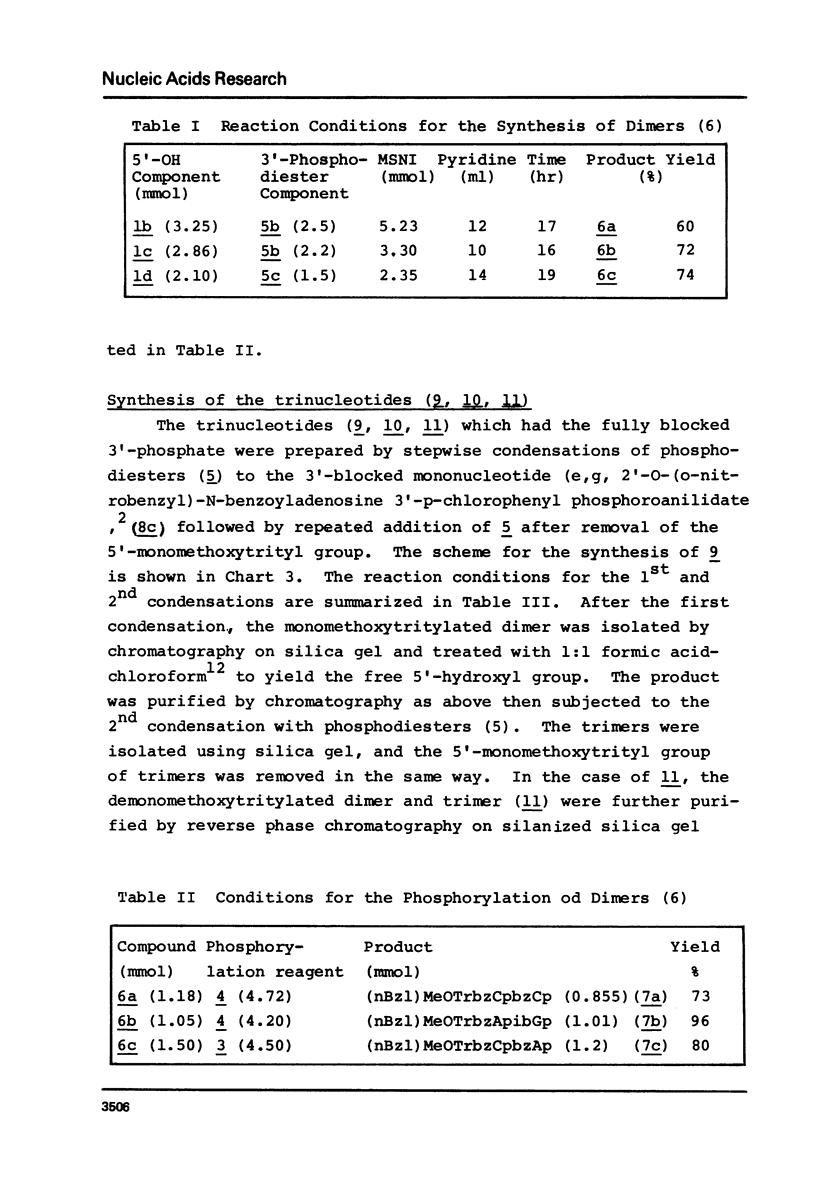
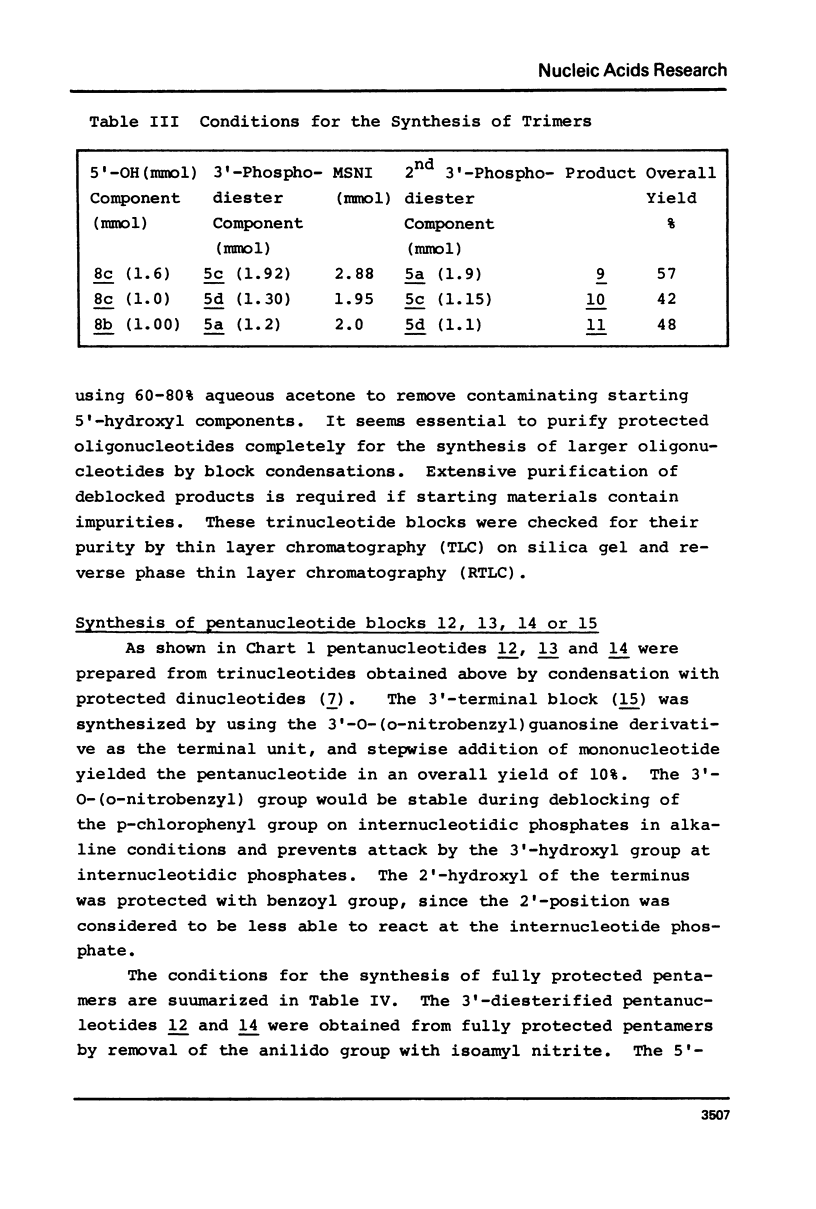
An E. coli tRNAfMet fragment [C-A-U-A-A-C-C-C-G-A-A-G-G-U-C-G-U-C-G-G (bases 35-f54)] containing the anticodon triplet has been synthesized by the phosphotriester method involving protected oligonucleotide blocks. Di- or tri-nucleotide blocks were prepared by condensation of 2'-O-(o-nitrobenzyl) nucleotide derivatives and used for the synthesis of pentanucleotide blocks. The 5'-hydroxy, heterocyclic amino and internucleotide linkage were protected with monomethoxytrityl, acyl and p-chlorophenyl groups, respectively. The 3'-phosphates of the pentanucleotides, except for the GUCGG block where 2'-O-benzoyl 3'-O-(o-nitrobenzyl) N-isobutyrylguanosine was used, were protected with p-chlorophenyl and anilido groups. The anilido groups were removed by treatment with isoamyl nitrite and the 3'-phosphodiesters of resulting pentamers were activated with mesitylenesulfonyl nitrotriazolide to give protected decanucleotides in yields of 61-89%. The two decanucleotides were condensed similarly to yield the protected eicosanucleotide in a yield of 59%. The product was deblocked and purified by ion-exchange chromatography on DEAE-Sephadex A-25 and characterized by enzymatic hydrolysis after labelling the 5'-end by phosphorylation using polynucleotide kinase and [gamma-32P]ATP.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleaney R. C., Jones A. S., Walker R. T. Synthetic analogues of polynucleotides. (Part) XIV. The synthesis of poly (3'-0-carboxymethyl-2'-deoxycytidine) and its interaction with polyinosinic acid. Nucleic Acids Res. 1975 May;2(5):699–706. doi: 10.1093/nar/2.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broka C., Hozumi T., Arentzen R., Itakura K. Simplications in the synthesis of short oligonucleotide blocks. Nucleic Acids Res. 1980 Nov 25;8(22):5461–5471. doi: 10.1093/nar/8.22.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gough G. R., Singleton C. K., Weith H. L., Gilham P. T. Protected deoxyribonucleoside-3' aryl phosphodiesters as key intermediates in polynucleotide synthesis. Construction of an icosanucleotide analogous to the sequence at the ends of Rous sarcoma virus 35S RNA. Nucleic Acids Res. 1979 Apr;6(4):1557–1570. doi: 10.1093/nar/6.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Markham A. F., Tanaka S., Tanaka T., Miyake T., Nakagawa E., Wakabayashi T., Taniyama Y., Fujiyama K., Nishikawa S. Total synthesis of tRNAfMet. Nucleic Acids Symp Ser. 1980;(7):335–343. [PubMed] [Google Scholar]
- Ohtsuka E., Nishikawa S., Fukumoto R., Uemura H., Tanaka T., Nakagawa E., Miyake T., Ikehara M. Synthesis of 5' fragments of formylmethionine transfer ribonucleic acid and their reconstitution with a natural three-quarter molecule. Eur J Biochem. 1980 Apr;105(3):481–487. doi: 10.1111/j.1432-1033.1980.tb04523.x. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. IX. Ribooligonucleotide synthesis using a photosensitive o-nitrobenzyl protection at the 2'-hydroxyl group. Nucleic Acids Res. 1974 Oct;1(10):1351–1357. doi: 10.1093/nar/1.10.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka T., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XXXI. Synthesis of the 3',5'-bisphosphorylated hexanucleotide corresponding to bases (72--77) of tRNAfMet from E. coli and its base transition analog. Chem Pharm Bull (Tokyo) 1980 Jan;28(1):120–125. doi: 10.1248/cpb.28.120. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Tanaka T., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XXXII. Synthesis of ribonucleotides corresponding to residues 1-5 and 6-10 of tRNAfMet from E. coli and their base conversion analogs. Nucleic Acids Res. 1979 Nov 10;7(5):1283–1296. doi: 10.1093/nar/7.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka E., Wakabayashi T., Ikehara M. Studies on transfer ribonucleic acids and related compounds. XXXVIII. A rapid method for the synthesis of ribooligonucleotides by using 3',5'-unsubstituted nucleosides. Synthesis of a hexanucleotide containing anticodon triplet of E. coli tRNA fMet. Chem Pharm Bull (Tokyo) 1981 Mar;29(3):759–765. doi: 10.1248/cpb.29.759. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Donelson J. E., Coulson A. R., Kössel H., Fischer D. Use of DNA polymerase I primed by a synthetic oligonucleotide to determine a nucleotide sequence in phage fl DNA. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1209–1213. doi: 10.1073/pnas.70.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]