Abstract
Presently, many consumer products contain nano-sized materials (NMs) to improve material properties, product quality and ease of use. NMs in food additives and in cosmetic articles (e.g., tooth paste) may be taken up by the oral route. As adverse effects of environmental nanoparticles, like ultrafine particles, have been reported, consumers worry about potential risks when using products containing NMs. The review focuses on metal and metal oxide NMs as common additives in tooth paste and in food industry and exposure by the oral route. Testing of NMs for oral exposure is very complex because differences in the diet, in mucus secretion and composition, in pH, in gastrointestinal transit time and in gastrointestinal flora influence NM uptake. Acellular (mucus, saliva) and epithelial layer of the orogastrointestinal barrier are described. Expected exposure doses, interaction of the NMs with mucus and permeation through the epithelium as well as in vivo data are mentioned. The role of in vitro models for the study of parameters relevant for ingested NMs is discussed.
Keywords: Nanomaterials, Epithelial barriers, Consumer products, Titanium dioxide, Silicon oxide, Silver
1. Introduction
Nanomaterials (NMs) are already included in many consumer products (clothing, food, cosmetics, etc.) to improve handling, stability and efficacy of these products. In nanomedicine nanoparticles may find application in drug delivery, bio-imaging and regenerative medicine. Whereas developments in nanomedicine aim to improve cellular uptake and permeation of NMs to improve efficacy, consumers and workers worry about the risks of non-intended uptake. This article is focused on the evaluation of risk by exposure to consumer products. Sources of NMs relevant for oral exposure comprise mainly cosmetics (sunscreen, lipsticks, skin creams, toothpaste) and food (packaging, storage life sensors, food additives, juice clarifiers). Whereas NMs in food are intended to be ingested, nanoparticles for instance in cosmetics and ingredients in food packaging may accidently get into the gastrointestinal tract. Major materials used in these products are: silver, and metal oxides of zinc, silica, and titanium (Hansen et al., 2008)). Nanosilver (Ag) is used in food packaging, amorphous silica (SiO2) in food additives, titanium dioxide (TiO2), gold (Au), platinum (Pt) and zinc oxide (ZnO) nanoparticles in cosmetics, especially sunscreens and toothpastes. According to the Woodrow Wilson Nanotechnology Consumer Products Inventory 2011 Ag nanoparticles are the most commonly used new NM in consumer products followed by TiO2, ZnO, platinum (Pt) and silicium oxide NMs (http://www.nanotechproject.org/inventories/consumer/). Although gold NMs are also used in cosmetics, food packaging, beverage and toothpaste their main applications are in the medical field.
Decrease of particle size in the nanoscale has been identified as a main parameter for the increased toxicity of different materials. Polystyrene, for instance, is a very biocompatible polymer used in cell culture. Nanoparticles, however, made from this material are cytotoxic (Mayer et al., 2009). Accumulation of metal and metal oxide NMs is seen also in lower animals such as fruit flies, mussels, planktonic crustaceans, rainbow trouts and in plants (Harris and Bali, 2008; Pan and Wang, 2004; Panacek et al., 2011; Scown et al., 2009; Zhu et al., 2009). In laboratory animals accumulation of these particles especially in liver, spleen and kidney is seen (Bu et al., 2010; Chen et al., 2006; Kim et al., 2009, 2010; Meng et al., 2007; Park et al., 2011; Wang et al., 2007a; Zhang et al., 2010a).
2. Estimation of exposure
For physiologically relevant testing it would be important to have an approximate idea on the levels of NMs to which humans are exposed. This estimation is quite difficult to make. Models based on per capita daily intake of various foods combined with expected distributions of chemicals or biological hazards in food work less well with NMs. The content of ingredients in form of nanoparticles is generally not indicated in food, interaction with food compounds is expected and physical changes of particles in the gastrointestinal tract are likely.
Concentrations of metal and metal oxide in water and soil have been reported to reach 15.2 ng/l and 1.28 μg/kg for TiO2, 0.76 ng/l and 22.7 ng/kg for silver and 0.01 μg/l and 0.093 μg/kg for ZnO, respectively (Gottschalk et al., 2009). Compared to other metal and metal oxide nanoparticles intake of TiO2 by food is relatively high: Powell et al. (2010) estimate ingestion of 5 mg TiO2/person/d with an unknown part of it in nanoform. Total dietary intake only of nano-TiO2 is estimated to be 2.5 mg/individual/d (0.036 mg/kg for a person of 70 kg; (Lomer et al., 2000)). The intake of nano- and microparticles, however, shows great variations: 0–112 mg/individual/d has been reported (Lomer et al., 2004). Metal and metal oxide nanoparticles can accumulate in plants (Harris and Bali, 2008) and in animals of the food chain (Lankveld et al., 2010) and may reach considerably higher levels in humans. Consequently, chronic effects rather than acute toxic effects on the human organism are expected.
NMs are subjected to wide variations in the orogastrointestinal tract. pH variations from slightly acid to neutral in the oral cavity and in the small intestine to a very acid pH in the stomach have a strong effect on surface charge of the particles and, as a consequence, on agglomeration and cellular uptake. Differences in the pH between fasted and fed state are prominent in the stomach (Horter and Dressman, 2001). Even in healthy individuals gastrointestinal transit is by far not constant and shows considerable variation through the large intestine (Coupe et al., 1991). These effects are known to influence oral bioavailability of conventional drugs but are even more important for the effects of NMs because NMs readily adsorb proteins (Cedervall et al., 2007; Lynch et al., 2009), which on the one hand, determines biological actions and, on the other, influence the dispersion of nanoparticles. Carboxyl polystyrene particles, for instance show a high tendency of aggregation, when suspended in FBS-containing medium (Mayer et al., 2009; Xia et al., 2006). For other NMs like carbon nanotubes, protein has a dispersing effect (Bihari et al., 2008; Heister et al., 2010; Sager et al., 2007).
3. Uptake of particles
Permeation through the gastrointestinal barrier has been shown for micro- and nanoparticles. The absorption is estimated to be about 15–250 times higher for nanoparticles (Desai et al., 1996). These barriers consist of cellular (epithelium) and acellular parts (dead cells, mucus).
3.1. Acellular layers of the orogastrointestinal tract
For the entire tract, composed of the oral cavity, the esophagus, the stomach and the intestine, mucus represents an efficient acellular barrier. Mucus consists of mucin proteins (highly glycosylated extracellular proteins with characteristic gel-forming properties), antiseptic proteins (lysozyme) and other proteins (lactoferrin), inorganic salts and water. The major functions are the protection and the lubrication of the underlying tissue.
The saliva, which is produced by the salivary glands, mainly consists of water (up to 99.5%), inorganic salts, proteins, and mucins. The high molecular weight mucin MG1 can bind to the surface of the epithelium and build the so-called mucus layer, displaying the acellular barrier of the oral cavity (Bykov, 1996, 1997). The thickness of this mucus layer is different before and after swallowing and measures between 70 and 100 μm (Collins and Dawes, 1987; Harris and Robinson, 1992; Lagerlof and Dawes, 1984). It displays a thick gelatinous like layer, structured as a 3-dimensional network with high water-holding capacity. It is highly viscoelastic and displays a shear thinning gel acting as lubricant. It protects the epithelial cell layers from pathogens, toxins and particles and enables the exchange of nutrients, water and gases (Knowles and Boucher, 2002).
Once substances are swallowed they pass the esophagus. Esophageal glands, which are located throughout the esophagus, secrete mucus directly onto the surface (Squier and Kremer, 2001). Additionally, exocrine glands in the submucosa produce a secretion with high bicarbonate concentration. This is necessary to neutralize refluxing stomach acid (Long and Orlando, 1999).
The mucus of the following parts, stomach and small and large intestine, is mainly produced by intraepithelial cells. In the first part of the small intestine (duodenum) also exocrine glands in the submucosa are located. The thickness of the mucus layer shows high variations depending on the localization in the gastrointestinal tract. The mucus layer demonstrates maximal thickness in the stomach; thickness increases from proximal to distal parts of the small and large intestine (Atuma et al., 2001; Matsuo et al., 1997). Depending on the method used for the determination, the thickness of the mucus layer shows marked variation. Fixation of the tissues usually is accompanied by shrinking and lower values are obtained. Endoscopic ultrasound measurements indicate thickness of the mucus in the stomach of 897–1354 μm and in the rectum of 730–1136 μm (Huh et al., 2003) but variation may be quite high because the thickness is dictated by the interplay between mucus secretion by goblet cells and mucus erosion by mechanical shear and bacterial digestion, particularly in the lower gut (Corfield et al., 1992; Hoskins and Boulding, 1981).
Additionally, pH can vary. The pH of the mucus in the oral cavity is estimated to range around pH 6.6. Gastric mucus shows a wide pH range from 1 to 2 (luminal) to ∼7 (epithelial surface); (Schreiber and Scheid, 1997)).
3.2. Interaction of NMs with the mucus layer
The characteristics facilitating the passage through human mucus are relatively well known: electrostatic repulsion from negatively charged sugar moieties favors the penetration of positively charged hydrophilic molecules; the passage of lipophilic compounds is slow (Avdeef and Testa, 2002).
It was thought that nanoparticles are incapable to penetrate the mucus layer since recent studies demonstrated that specific viruses, like the Norwalk virus with a size of 38 nm and human papilloma virus with a size of 55 nm diffused in human mucus as rapidly as they do in water (Olmsted et al., 2001; Saltzman et al., 1994). The surface of viruses, which are able to permeate the mucus, is densely coated with positive and negative charges, thus, this net neutral surface charge prevents mucoadhesion (Olmsted et al., 2001). Since the pore size in (cervical) mucus is approximately 100 nm, it is suggested that small particles might also be capable to diffuse through mucus. Olmsted et al. (2001) demonstrated that small viruses diffused unhindered through mucus, whereas polystyrene microspheres in a size of 59 nm and covalently modified with carboxyl-groups, bound more tightly to mucins and bundled them into thick cables. Additional work by Dawson et al. (2003) reported that carboxyl and amine-modified polystyrene particles (100, 200 and 500 nm) were embedded in cystic fibrosis sputum. The positively charged particles penetrated more rapidly in the sputum than the negatively charged particles. Furthermore, smaller particles underwent a significantly faster transport. Lai et al. (2007) investigated polystyrene particles in a size range of 100–500 nm. The surfaces of the particles were covalently modified with a high density of low M.W. polyethylene glycol (PEG) and the diffusion in human mucus was studied. The results demonstrated that the neutral surface charge increased the diffusion rate of all particles. The large particles (200 nm and 500 nm) demonstrated a 6-fold and 4-fold lower effective diffusion-coefficient than that in water, whereas 100 nm particles were found to be immobile in mucus. Other studies showed that 14 nm latex particles, which were slightly negatively charged, cross the distal colon mucus gel layer within 2 min and 415 nm larges ones in 30 min, whereas 1 μm larges ones did not cross (Szentkuri, 1997). Non-biodegradable latex particles can rapidly permeate human mucus when they are coated with PEG. Surprisingly, 200 nm particles crossed the mucin layer faster than <100 nm NMs (Wang et al., 2007b). These findings suggest that the surface charge plays a crucial role in the transport rates of nanoparticles through a mucus layer.
Mucus lifetime is short and the fastest turnover (i.e., clearance time) is observed at surfaces with thinnest mucus layers. Thus, nanoparticles have to permeate quickly through this barrier to reach the underlying epithelia (Cone, 2009).
Local effects after oral exposure to NMs include abnormal mucus production, induced by TiO2 nanoparticles in cultured ChaGo-K1 cells (Chen et al., 2011) and by silver nanoparticles in vivo (Jeong et al., 2010). Additionally, pH changes induced by NMs can change the pH-dependent aggregation of mucins (Bhaskar et al., 1991). In addition, positively charged NMs impede mucin swelling and thereby increase viscosity (Chen et al., 2010).
3.3. Epithelial layers of the orogastrointestinal tract
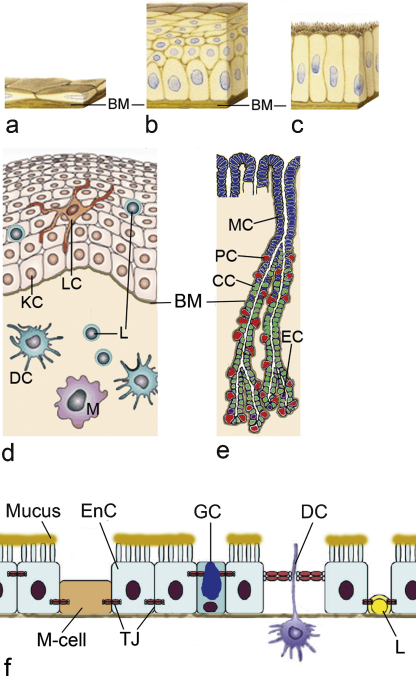
The epithelium generally represents the highest resistance against the passage of chemical compounds and NMs. Epithelial cells are polarized, they possess an apical surface facing an internal or external surface and a basal site, where they face the underlying tissue. Epithelia may consist of several layers and may vary in the height of the cells. Penetration through a monostratified squamous epithelium, like in endothelia (Fig. 1a), is easier than through the simple columnar epithelium in stomach and intestine (Fig. 1b) and the squamous epithelium of the oral cavity and the esophagus (Fig. 1c). The thickness of the non-keratinized squamous epithelium in the oral cavity ranges between 550 and 800 μm (Collins and Dawes, 1987; Harris and Robinson, 1992; Lagerlof and Dawes, 1984). The squamous epithelium of the esophagus shows a thickness of 300–500 μm (Takubo, 2009). The epithelium of the esophagus has the same structure as that of the buccal mucosa but is thinner and less variable (Diaz del Consuelo et al., 2005). The simple columnar epithelium in the gastrointestinal tract measures 20–25 μm (Atuma et al., 2001; Matsuo et al., 1997). In general, only one cell type forms the structural basis of the barrier: keratinocytes for the oral cavity and the esophagus, gastric epithelial cells for the stomach and enterocytes for the small and large intestine. The epithelial cells are linked together by intercellular junctions, which give the epithelial layer mechanical strength and restrict passage between cells. In the oral mucosa immune cells (Langerhans cells, lymphocytes) are embedded in the keratinocytes layer (Fig. 1d). The epithelium of the stomach contains mucus producing mucus neck cells, pepsinogen-producing gastric chief cells, gastric acid and intrinsic factor producing parietal cells and a variety of hormones (gastrin, serotonin, somatostatin, etc.) producing enteroendocrine cells (Fig. 1e). In the small intestine, cells belonging to the immune system (M-cells), enteroendocrine cells and goblet cells are embedded in a layer of enterocytes (Fig. 1f). M-cells are preferentially located in the epithelium overlying the Peyer's Patches, which is also called Follicle Associated Epithelium (FAE), and deliver foreign substances to the underlying tissues (mucosa lymphoid) to induce immune responses (Gerbert et al., 1996). M-cells, however, are also a potential portal for nanoparticles. The epithelium of the large intestine consists of enterocytes and goblet cells. When different cell types adjoin the barrier function of the epithelium is altered because the location and structure of these junctions differ between the cell types (Eom and Choi, 2009). All epithelia reside on a basal membrane, which separates them from the underlying connective tissue containing capillaries, lymph vessels, lymph follicles and peripheral nerves. To reach the systemic circulation by capillaries NMs have also to cross the basal membrane and the connective tissue.
Fig. 1.
Different thickness and heterogeneity of orogastrointestinal epithelia. Compared to the monostratified squamous epithelium of blood vessels (a) epithelia of the orogastrointestinal tract are much thicker: stratified non-keratinized squamous epithelium of the oral cavity and the esophagus (b) and simple columnar epithelium present in the stomach, small and large intestine. All epithelia reside on a basal lamina (BM). All epithelial layers are composed of different cell types. (d) In the oral cavity, Langerhans cells (LC) and intraepithelial lymphocytes (L) in addition to keratinocytes (KC) are present in the epithelial layer. The connective tissue below the epithelial layer contains dendritic cells (DC), macrophages (M) and lymphocytes (L). (e) The mucosa of the stomach consists of mucus-producing cells (MC), gastric acid producing cells (parietal cells, PC), pepsinogen-producing cells (chief cells, CC) and endocrine hormone producing enteroendocrine cells (EC). Cells of the immune systems are not shown. (f) In the small intestine, enterocytes (EnC), microfold (M-cells), goblet cells (GC), dendritic cells (DC) and intraepithelial lymphocytes (L) are linked together by tight junctions (TJ). These junctions show small differences in composition and location in the cell.
Figure adapted from Eom and Choi (2009).
3.4. Permeation through orogastrointestinal barriers in vitro
Epithelia can be permeated either by passage through the cells (transcellular) or by passage between the cells (paracellular). Physiological methods to evaluate interactions with biological barriers and to predict the effect of nanoparticles are highly demanded. Studies addressing permeation usually use transwell systems, where cells are cultured on filters. Moreover, diffusion-cells can be used to evaluate the penetration/permeation of NMs across excised tissues (Sudhakar et al., 2006). Studies on cell monolayers show that polystyrene particles can readily permeate the alveolar epithelium (Yacobi et al., 2008). By contrast, the rate of permeation of enterocyte (Caco-2 cell) monolayers by polystyrene particles without surface coating appears low (Geiser et al., 2005; Pietzonka et al., 2002). Gaumet et al. (2009) showed that small polystyrene particles were observed intracellularly in Caco-2 cells. Also TiO2 nanoparticles appear to cross Caco-2 monolayers without disruption of junctional complexes and without causing cytotoxicity (Koeneman et al., 2010).
Since the plasma membrane of the cells forming the epithelial barrier is lipophilic, lipophilic substances are taken up passively by the transcellular route whereas hydrophilic drug compounds use the paracellular route. The penetration area of the paracellular route is extremely small compared to the transcellular route and restricted to polar substances below 1000 D. Paracellular transport is only passive. Nanoparticles are not expected to be able to use the paracellular route, because they are considerably larger than 1000 D. To get an idea about the relation of molecular weight and size: serum albumin with 66 kD has an almost sphere-like shape of 3 nm × 8 nm × 8 nm (Takizawa et al., 1992).
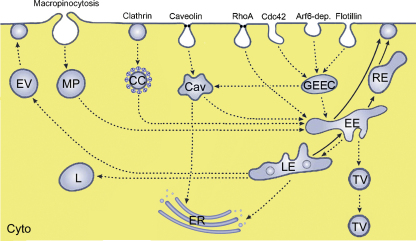
Transcellular passage by passive diffusion appears to be rare: although passage of cells by 22 nm TiO2 particles was suggested to occur by passive diffusion (Geiser et al., 2005), other researchers described that Au-nanoparticles in sizes of 5–8 nm could not enter cells by passive diffusion ((Stoeger et al., 2006)). Active uptake by endocytosis is the likely mode of cellular uptake for metal and metal oxide NMs. Several endocytotic routes have been characterized, which are classified according to the coating with clathrin and the involvement of dynamin in the uptake. Main mechanisms are termed clathrin-mediated endocytosis, macropinocytosis and caveolae-dependent. Different classifications are used for the clathrin-independent and caveolae-independent routes. The classification by Sahay et al. (2010) is mainly based on the GTPases involved (Arf6-dependent, Cdc42/Arf1-dependent and RhoA-dependent endocytosis) and on the coat protein (Flotillin-dependent). Another nomenclature employs the term clathrin-independent carriers/glycophosphatidylinositol (GPI)-anchored protein enriched compartment (GEEC)-type endocytosis as synonym for Cdc42/Arf1-dependent endocytosis and IL-2Rβ-dependent endocytosis for RhoA-dependent endocytosis (Doherty and McMahon, 2009). Independent of the route of entry, the cargos are mainly transported via endosomes to lysosomes (Fig. 2). Non-functionalized silver, TiO2 and SiO2 particles are mainly taken up by clathrin-mediated endocytosis (Chung et al., 2007; Greulich et al., 2011; He et al., 2009; Huang et al., 2005; Singh et al., 2007; Sun et al., 2008).
Fig. 2.
Active uptake mechanisms of NMs into cells. Major routes are macropinocytosis, clathrin-mediated endocytosis and caveolae-mediated and non-clathrin, non-caveolae mediated uptake. The later are subdivided into RhoA- (or IL-2Rβ-) dependent endocytosis, Cdc42/Arf1 or clathrin-independent cargo/glycophosphatidylinositol (GPI)-anchored protein enriched compartment-dependent (GEEC) endocytosis, Arf6- dependent endocytosis and flotillin-dependent endocytosis. The content of macropinosomes (MP), clathrin-coated vesicles (CC) and GEEC is transported via early endosomes (EE) and late endosomes (LE) to lysosomes (L). Material taken up by caveolae-mediated endocytosis is transported via caveolosomes (Cav) either to the endoplasmic reticulum (ER) or to early endosomes. NMs may be removed from the cells by exocytotic vesicles (EV). Early endosomes may also fuse with the plasma membrane directly or through recycling endosomes (RE). In polarized cells transcytosis occurs via transport vesicles (TV). Cyto: cytoplasm.
Nanoparticles can leave the cells either by transcytosis or by exocytosis. Exocytosis of nanoparticles is not well studied and conflicting results were obtained: exocytosis of quantum dots was not consistently seen in the studies (Clift et al., 2008; Jiang et al., 2010). Transcytosis can occur through receptor-mediated uptake or via adsorptive-mediated uptake. Receptors for BSA, transferrin and opioid peptides functionalized NMs are expressed on several cell types and BSA-coated nanoparticles have been shown to transcytose through endothelial cells (Wang et al., 2009). For the gastrointestinal tract, however, this type of uptake is not relevant. Absorptive-mediated transcytosis is mediated by the interaction of positively charged substances with anionic sites of the plasma membrane: cationic nanoparticles had a greater potential than neutral or negatively charged ones (Harush-Frenkel et al., 2008). Additionally uncoated, not positively charged TiO2 nanoparticles can cross the intestinal epithelium by the transcellular route (Koeneman et al., 2010).
As mentioned in Section 3.3, the epithelium of the gastrointestinal tract is composed of various cell types and permeation through some types of cells is easier than through others. This situation can be mimicked also in vitro. Kerneis et al. (1997) constructed an intestinal in vitro co-culture model consisting of Caco-2 on inverted inserts and immune cells isolated from murine Peyer's patches. The first M-cell model was developed by Gullberg et al. (2000) using Caco-2 (normally oriented inserts) and Raji cells. The group of des Rieux (des Rieux et al., 2005, 2007) improved the in vitro epithelial cell model and investigated the influence of the physicochemical properties on the transport (mechanism) of nanoparticles by M-cells. To this aim, Caco-2 and Raji B cells were co-cultured in transwells (to induce M-cell development). Both negatively charged and positively charged polystryrene particles were taken up by M-cells via the transcellular route. The transport was dependent on the concentration, the temperature and the size. Furthermore, the presence of cationic groups enhanced the transport due to electrostatic interactions between the particle surface structure and the cell surface. Compared with investigations carried out with a monoculture, the particle transport in the transwell system was 50-fold higher (des Rieux et al., 2005, 2007; Ruponen et al., 2004). Gullberg et al. (2006) studied the FAE and demonstrated that integrin-targeted nanoparticles are preferentially transported across the FAE into the Peyer's patches. These data suggest that integrin interaction is a dominating mechanism for improved particle uptake across the FAE. Although M cells are also located outside the FAE (villous-M cells), the transport of antigens and/or nanoparticles is mainly carried out by the FAE-M cells, since the mucus layer limits the particle uptake across the villous epithelium (Jang et al., 2004).
Some research has been carried out so far on the buccal mucosa. The permeability through excised porcine buccal mucosa was investigated with Franz diffusion cells to study the transport of nanoparticles across this tissue. The results demonstrated that polystyrene particles penetrated into the tissue due to endocytotic mechanisms (Roblegg et al., 2011). The most relevant barrier for negatively charged particles was the mucus layer together with the top third region of the epithelium. Positively charged particles, however, showed no interaction with the mucus layer and penetrated into deeper regions of the epithelium.
3.5. Permeation through barriers in vivo
Uptake of metallic silver from the environment is 10–20% in GI mainly in the stomach and the duodenum (Armitage et al., 1996). Recovery of 10% of the applied dose was also obtained for 60 nm polystyrene particles dosed at 14 mg/kg for 5 d to rats (Hillery et al., 1994). Fluorescent polystyrene particles in sizes between 2 and 20 μm are found in the Peyer Plaques of the ileum; 2 μm particles in addition also in mesenterial lymph nodes (Carr et al., 1996). The accumulation in the lymphatic tissues started 5 min after the application and was higher in proximal than in distal lymph nodes (Hazzard et al., 1996). Jani et al. (1990) evaluated the effect of the different sizes (50–100–200–300–1000–3000 nm) of polystyrene particles on gastrointestinal uptake. They found a size-dependent decrease of the uptake from 34% for 50 nm particles to 26% for 100 nm particles. The uptake rate of the larger particles was minimal. 6.6% of the dose was detected in liver, spleen, blood and bone marrow compared to 0.8% for 1000 nm particles.
In addition to particle size, dose and duration of the exposure are important for the interpretation of the data (Overview provided in Table 1). Independent from the material used, NMs up to 100 nm distribute into the organism after one single application (Jani et al., 1990). When multiple applications are performed also larger particles distribute outside of the gastrointestinal system (Jani et al., 1994). High dosing, species differences, choice of the tracer and methodology used for organ distribution complicate comparisons between different studies, as well as conclusions on nanoparticle effects. For instance, local effects at the gastrointestinal mucosa, liver and kidney damage and impairment of the immune system have been reported. Based on environmental data for nano-TiO2, concentrations much higher than 0.4 mg/kg for acute toxicity appear unrealistic (Lomer et al., 2000). As many metals and metal oxides may accumulate, the evaluation of higher doses is justified. Nevertheless, data from repeated applications of ≥1 g/kg are not physiologically relevant. In broiler chicken hatchlings, which were treated with doses below 250 ng/kg silver nanoparticles (Ahmadi and Kurdestany, 2010; Ahmadi, 2009; Ahmadi et al., 2009), adverse effects were already detected at these low concentrations. The higher toxicity of the silver nanoparticles may either be due to interspecies differences or to the low age of the chicken. For correct tracing of the organ distribution the choice of the label and the mode of detection appear important. In the study of Jani et al. (1990) the label was potentially not stable and the localization of the label may not correspond to that of the particles. If NMs are only detected by chemical analysis it is not clear if they are accumulated in a dissolved form or as intact particles.
Table 1.
Oral exposure of rodents and broiler chicken (*) to metal and metal oxide particles contained in consumer products. The doses were delivered either by gavage and intragastrial injection (mg/kg) or in the drinking water (ppm, mg/l).
| Particle (size) | Dose | Effect | Reference |
|---|---|---|---|
| Ag (14 nm)* | 5–15–25 ppm for 42 d | Indication for oxidative stress in bood and for decreased immune function | Ahmadi and Kurdestany (2010) |
| Ag (14 nm)* | 300–600–900 ppm for 56 d | No effect on blood chemistry and blood count, weight reduced at 900 ppm, no change in organ histology | Ahmadi (2009), Ahmadi et al. (2009) |
| Ag (15 nm) | 2.5 mg/kg for 3 d | Local inflammation of the stomach | Cha et al. (2008) |
| Ag (56 nm) | 30–125–500 mg/kg for 90 d | Liver damage at 125 mg/kg | Kim et al. (2010) |
| Ag (60 nm) | 30–300–1000 mg/kg for 28 d | Highest tissue levels in stomach, kidney, liver and lungs, LOAEL of 300 mg/kg | Kim et al. (2008) |
| Ag (22–42–71–323 nm) | 1 mg/kg for 14 d | Indication for liver damage, activation of B-lymphocytes | Park et al. (2010) |
| Au (4–10–28–58 nm) | 200 mg/l for 7 d | Accumulation of smaller particles in kidney, liver, spleen, lung, brain. Larger particles accumulated in the GI tract | Hillyer and Albrecht (2001) |
| Au (13.5 nm) | 137.5–2200 μg/kg for 14–28 d | Spleen enlargement, damage of intestinal mucosa | Zhang et al. (2010b) |
| Au (14 nm) | 75–150–300 ppm for 28 d | No changes in blood chemistry, body weight and organ histology | Dhar et al. (2010) |
| Pt (1–6 nm) | 9.75 mg/kg for 5 d | Little effect on protein expression | Katao et al. (2011) |
| TiO2 (5 nm) | 62.5–125–250 mg/kg for 30 d | Reduction of body weight, indication for liver damage, pathological blood count at ≥125 mg/kg | Duan et al. (2010) |
| TiO2 (25 nm) | 60–600 mg/l for 5 d | DNA-damage in various tissues | Trouiller et al. (2009) |
| TiO2 (<50 nm) | 0.16–0.4–1 g/kg | Indication for liver damage, disturbance of energy and amino acid metabolism at 1 g/kg | Bu et al. (2010) |
| TiO2 (50–120 nm) | 5 g/kg for 7 d | Liver and kidney damage only in combination with lead acetate | Zhang et al. (2010a) |
| TiO2 (25–80–155 nm) | Single dose of 5 g/kg | Minimal uptake, indication for liver and kidney damage | Wang et al. (2007a) |
| TiO2 (140 nm) | Single dose of 175–550–1750–5000 mg/kg | No mortality, no gross lesions at necropsy | Warheit et al. (2007) |
| TiO2 (500 nm) | 12.5 mg/kg for 10 d | Uptake of particles 7% in GI tract, 5% in liver, lung, spleen, heart, kidney | Jani et al. (1994) |
| ZnO (120 nm) | 5 g/kg for 14 d | Distribution in bone, liver, kidney, pancreas | Wang et al. (2008) |
4. Action on compromised barriers
Few data have been published regarding the permeation through diseased barriers. Changes in mucus composition induced by Ag nanoparticles (Jeong et al., 2010), polystyrene particles and diesel exhaust increased mucus permeability and permeation of small molecules by a factor of 5 (McGill and Smyth, 2010). The adherence of polystyrene nanoparticles to inflamed colonic mucosa was much higher than to normal mucosa (Lamprecht et al., 2001). Also in the elaborated co-culture in vitro model developed by Leonhard et al. (2010) smaller particles (50 nm) polystyrene particles adhered better to the inflamed monolayer and were taken up into the cells, whereas larger particles only adhered to the cell surface. Inflammation appears to increase uptake and permeation of NMs in vitro and in vivo. Inflammation caused by Yersinia pseudotuberculosis increases the uptake of 100 nm carboxyl polystyrene particles in cell monolayers and in intestinal biopsies (Ragnarsson et al., 2008). In contrast to that, in the in vitro study by Leonhard et al. (2010) no influence on the translocation of the polystyrene particles was observed. Since in the in vitro studies lipopolysaccharide and not intact bacteria were used, effects by the living bacteria on cells, mucus production and/or viscosity may account for the observed differences.
5. Conclusion
The assessment of penetration and biological effects of ingested NMs presents many problems because it is very complex. Inter-individual differences in the composition, pH and thickness of the mucus layer, in the gastrointestinal flora and in gastrointestinal passage time complicate in vivo experiments. In the study of Loeschner et al. (2011) on organ distribution of 60 nm Ag nanoparticles great inter-individual variations were noticed although all animals were fed the same diet. Also differences in the diet are important.
For in vivo testing, rodents also may not be ideal models. Although men and rodents are omnivorous, function (e.g., region for absorption of food) and morphology of the gastrointestinal tract (e.g., absence of gall bladder in rats) show considerable differences between rodents and humans (Kararli, 1995).
Apart from permeating themselves, NMs may have permeation enhancing properties for other substances. This phenomenon termed as ‘Trojan horse’ effect, was first identified for metal nanoparticles. Whereas plasma membranes restrict the cellular access for metal ions like silver cations, silver nanoparticles were readily internalized and intracellular silver concentrations were much higher than for silver ions (Navarro et al., 2008). Studies for uptake and toxicity should, therefore, include AgNO3 for silver nanoparticles (Trojan horse effect) or bulk material.
Other important effects are linked to the tendency of NMs to absorb macromolecules. By adsorption of organic compounds also unintended molecules (undigested and unmetabolized compounds) may be absorbed by the gastrointestinal tract. On the other hand adsorption to NMs may also prevent the uptake of necessary molecules (Alkhamis et al., 2009). Absorption may also be altered by a changed metabolization by enterocytes. Polystyrene and silver particles have been shown to inhibit the activity of cytochrome P450 enzymes (Fröhlich et al., 2010; Lamb et al., 2010).
To obtain more information about penetration of the orogastrointestinal barriers and subsequent biological effects physiologically relevant in vitro models should be used, which enable the controlled variation of the most important parameters involved. Particle properties should be recorded in mucus of different pH and the extent of binding to proteins and other macromolecules should be studied. Physiologically relevant in vitro (co-culture) models including mucus should be established to investigate also the effect of changed mucus structure, inflammation and pH changes. It is obvious that also in vivo experiments are needed but without a good knowledge on the influence of the orogastrointestinal variations on particle parameters and penetration in vivo data may be difficult to interpret.
Conflict of interest statement
The authors declare that there are no conflicting interests.
Acknowledgements
The authors acknowledge funding by the Austrian Science Fund (FWF) grant P22576-B18 and by the Austrian Research Promotion Agency (FFG) project 826136.
References
- Ahmadi F., Kurdestany A. The impact of silver nano particles on growth performance. Global Veterinaria. 2010;5:366–370. [Google Scholar]
- Ahmadi J. Application of different levels of silver nanoparticles in food on the performance and some blood parameters of broiler chickens. World Appl. Sci. J. 2009;7:24–27. [Google Scholar]
- Ahmadi J., Irani M., Choobchian M. Pathological study of intestine and liver in broiler chickens after treatment with different levels of silver nanoparticles. World Appl. Sci. J. 2009;7:28–32. [Google Scholar]
- Alkhamis K., Salem M., Khanfar M. Determination of the mechanism of uptake of organic vapors by chitosan. Pharm. Dev. Technol. 2009;14:90–95. doi: 10.1080/10837450802409453. [DOI] [PubMed] [Google Scholar]
- Armitage S.A., White M.A., Wilson H.K. The determination of silver in whole blood and its application to biological monitoring of occupationally exposed groups. Ann. Occup. Hyg. 1996;40:331–338. doi: 10.1016/0003-4878(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Atuma C., Strugala V., Allen A., Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- Avdeef A., Testa B. Physicochemical profiling in drug research: a brief survey of the state-of-the-art of experimental techniques. Cell. Mol. Life Sci. 2002;59:1681–1689. doi: 10.1007/PL00012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar K.R., Gong D.H., Bansil R., Pajevic S., Hamilton J.A., Turner B.S., LaMont J.T. Profound increase in viscosity and aggregation of pig gastric mucin at low pH. Am. J. Physiol. 1991;261:G827–G832. doi: 10.1152/ajpgi.1991.261.5.G827. [DOI] [PubMed] [Google Scholar]
- Bihari P., Vippola M., Schultes S., Praetner M., Khandoga A.G., Reichel C.A., Coester C., Tuomi T., Rehberg M., Krombach F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008;5:14. doi: 10.1186/1743-8977-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q., Yan G., Deng P., Peng F., Lin H., Xu Y., Cao Z., Zhou T., Xue A., Wang Y., Cen X., Zhao Y.L. NMR-based metabonomic study of the sub-acute toxicity of titanium dioxide nanoparticles in rats after oral administration. Nanotechnology. 2010;21:125105. doi: 10.1088/0957-4484/21/12/125105. [DOI] [PubMed] [Google Scholar]
- Bykov V.L. The tissue and cell defense mechanisms of the oral mucosa. Morfologiia. 1996;110:14–24. [PubMed] [Google Scholar]
- Bykov V.L. The functional morphology of the epithelial barrier of the oral mucosa. Stomatologiia (Mosk) 1997;76:12–17. [PubMed] [Google Scholar]
- Carr K.E., Hazzard R.A., Reid S., Hodges G.M. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm. Res. 1996;13:1205–1209. doi: 10.1023/a:1016064320334. [DOI] [PubMed] [Google Scholar]
- Cedervall T., Lynch I., Foy M., Berggard T., Donnelly S.C., Cagney G., Linse S., Dawson K.A. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew. Chem. Int. Ed. Engl. 2007;46:5754–5756. doi: 10.1002/anie.200700465. [DOI] [PubMed] [Google Scholar]
- Cha K., Hong H.W., Choi Y.G., Lee M.J., Park J.H., Chae H.K., Ryu G., Myung H. Comparison of acute responses of mice livers to short-term exposure to nano-sized or micro-sized silver particles. Biotechnol. Lett. 2008;30:1893–1899. doi: 10.1007/s10529-008-9786-2. [DOI] [PubMed] [Google Scholar]
- Chen E.Y., Garnica M., Wang Y.C., Chen C.S., Chin W.C. Mucin secretion induced by titanium dioxide nanoparticles. PLoS One. 2011;6:e16198. doi: 10.1371/journal.pone.0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E.Y., Wang Y.C., Chen C.S., Chin W.C. Functionalized positive nanoparticles reduce mucin swelling and dispersion. PLoS One. 2010;5:e15434. doi: 10.1371/journal.pone.0015434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Meng H., Xing G., Chen C., Zhao Y., Jia G., Wang T., Yuan H., Ye C., Zhao F., Chai Z., Zhu C., Fang X., Ma B., Wan L. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Chung T.H., Wu S.H., Yao M., Lu C.W., Lin Y.S., Hung Y., Mou C.Y., Chen Y.C., Huang D.M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials. 2007;28:2959–2966. doi: 10.1016/j.biomaterials.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Clift M.J., Rothen-Rutishauser B., Brown D.M., Duffin R., Donaldson K., Proudfoot L., Guy K., Stone V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol. Appl. Pharmacol. 2008;232:418–427. doi: 10.1016/j.taap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Collins L.M., Dawes C. The surface area of the adult human mouth and thickness of the salivary film covering the teeth and oral mucosa. J. Dent. Res. 1987;66:1300–1302. doi: 10.1177/00220345870660080201. [DOI] [PubMed] [Google Scholar]
- Cone R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Corfield A.P., Wagner S.A., Clamp J.R., Kriaris M.S., Hoskins L.C. Mucin degradation in the human colon: production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 1992;60:3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe A.J., Davis S.S., Wilding I.R. Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm. Res. 1991;8:360–364. doi: 10.1023/a:1015849700421. [DOI] [PubMed] [Google Scholar]
- Dawson M., Wirtz D., Hanes J. Enhanced viscoelasticity of human cystic fibrotic sputum correlates with increasing microheterogeneity in particle transport. J. Biol. Chem. 2003;278:50393–50401. doi: 10.1074/jbc.M309026200. [DOI] [PubMed] [Google Scholar]
- des Rieux A., Fievez V., Theate I., Mast J., Preat V., Schneider Y.J. An improved in vitro model of human intestinal follicle-associated epithelium to study nanoparticle transport by M cells. Eur. J. Pharm. Sci. 2007;30:380–391. doi: 10.1016/j.ejps.2006.12.006. [DOI] [PubMed] [Google Scholar]
- des Rieux A., Ragnarsson E.G., Gullberg E., Preat V., Schneider Y.J., Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005;25:455–465. doi: 10.1016/j.ejps.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Desai M.P., Labhasetwar V., Amidon G.L., Levy R.J. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm. Res. 1996;13:1838–1845. doi: 10.1023/a:1016085108889. [DOI] [PubMed] [Google Scholar]
- Dhar S., Mali V., Bodhankar S., Shiras A., Prasad B.L., Pokharkar V. Biocompatible gellan gum-reduced gold nanoparticles: cellular uptake and subacute oral toxicity studies. J. Appl. Toxicol. 2010;1:9. doi: 10.1002/jat.1595. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Diaz del Consuelo I., Falson F., Guy R.H., Jacques Y. Transport of fentanyl through pig buccal and esophageal epithelia in vitro. Influence of concentration and vehicle pH. Pharm. Res. 2005;22:1525–1529. doi: 10.1007/s11095-005-6020-y. [DOI] [PubMed] [Google Scholar]
- Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Ann. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Duan Y., Li N., Liu C., Liu H., Cui Y., Wang H., Hong F. Interaction between nanoparticulate anatase TiO2 and lactate dehydrogenase. Biol. Trace Elem. Res. 2010;136:302–313. doi: 10.1007/s12011-009-8548-x. [DOI] [PubMed] [Google Scholar]
- Eom H.J., Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol. In Vitro. 2009;23:1326–1332. doi: 10.1016/j.tiv.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Fröhlich E., Kueznik T., Samberger C., Roblegg E., Wrighton C., Pieber T.R. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol. Appl. Pharmacol. 2010;242:326–332. doi: 10.1016/j.taap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Gaumet M., Gurny R., Delie F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur. J. Pharm. Sci. 2009;36:465–473. doi: 10.1016/j.ejps.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Geiser M., Rothen-Rutishauser B., Kapp N., Schurch S., Kreyling W., Schulz H., Semmler M., Im Hof V., Heyder J., Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbert A., Rothkotter H.J., Pabst R. M-cells in peyer's patches of the intestine. Int. Rev. Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- Gottschalk F., Sonderer T., Scholz R.W., Nowack B. Modeled environmental concentrations of engineered nanomaterials (TiO(2), ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- Greulich C., Diendorf J., Simon T., Eggeler G., Epple M., Koller M. Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater. 2011;7:347–354. doi: 10.1016/j.actbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Gullberg E., Keita A.V., Salim S.Y., Andersson M., Caldwell K.D., Söderholm J.D., Artusson P. Identification of cell adhesion molecules in the human follicle-associated epithelium that improve nanoparticle uptake into the peyer's patches. J. Pharmacol. Exp. Ther. 2006;319:632–639. doi: 10.1124/jpet.106.107847. [DOI] [PubMed] [Google Scholar]
- Gullberg E., Leonard M., Karlsson J., Hopkins A., Baryden D., Baird W., Artursson P. Expression of specific markers and particles transport in a new human intestinal M cell model. Biochem. Biophys. Res. Commun. 2000;279:803–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- Hansen S.F., Michelson E.S., Kamper A., Borling P., Stuer-Lauridsen F., Baun A. Categorization framework to aid exposure assessment of nanomaterials in consumer products. Ecotoxicology. 2008;17:438–447. doi: 10.1007/s10646-008-0210-4. [DOI] [PubMed] [Google Scholar]
- Harris A., Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008;10:691–695. [Google Scholar]
- Harris D., Robinson J.R. Drug delivery via the mucous membranes of the oral cavity. J. Pharm. Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- Harush-Frenkel O., Rozentur E., Benita S., Altschuler Y. Surface charge of nanoparticles determines their endocytic and transcytotic pathway in polarized MDCK cells. Biomacromolecules. 2008;9:435–443. doi: 10.1021/bm700535p. [DOI] [PubMed] [Google Scholar]
- Hazzard R.A., Hodges G.M., Scott J.D., McGuinness C.B., Carr K.E. Early intestinal microparticle uptake in the rat. J. Anat. 1996;189(Pt 2):265–271. [PMC free article] [PubMed] [Google Scholar]
- He Q., Zhang Z., Gao Y., Shi J., Li Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano- and microparticles. Small. 2009;5:2722–2729. doi: 10.1002/smll.200900923. [DOI] [PubMed] [Google Scholar]
- Heister E., Lamprecht C., Neves V., Tilmaciu C., Datas L., Flahaut E., Soula B., Hinterdorfer P., Coley H.M., Silva S.R., McFadden J. Higher dispersion efficacy of functionalized carbon nanotubes in chemical and biological environments. ACS Nano. 2010;4:2615–2626. doi: 10.1021/nn100069k. [DOI] [PubMed] [Google Scholar]
- Hillery A.M., Jani P.U., Florence A.T. Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles. J. Drug Target. 1994;2:151–156. doi: 10.3109/10611869409015904. [DOI] [PubMed] [Google Scholar]
- Hillyer J.F., Albrecht R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001;90:1927–1936. doi: 10.1002/jps.1143. [DOI] [PubMed] [Google Scholar]
- Horter D., Dressman J.B. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2001;46:75–87. doi: 10.1016/s0169-409x(00)00130-7. [DOI] [PubMed] [Google Scholar]
- Hoskins L.C., Boulding E.T. Mucin degradation in human colon ecosystems. Evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. J. Clin. Invest. 1981;67:163–172. doi: 10.1172/JCI110009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D.M., Hung Y., Ko B.S., Hsu S.C., Chen W.H., Chien C.L., Tsai C.P., Kuo C.T., Kang J.C., Yang C.S., Mou C.Y., Chen Y.C. Highly efficient cellular labeling of mesoporous nanoparticles in human mesenchymal stem cells: implication for stem cell tracking. FASEB J. 2005;19:2014–2016. doi: 10.1096/fj.05-4288fje. [DOI] [PubMed] [Google Scholar]
- Huh C.H., Bhutani M.S., Farfan E.B., Bolch W.E. Individual variations in mucosa and total wall thickness in the stomach and rectum assessed via endoscopic ultrasound. Physiol. Meas. 2003;24:N15–N22. doi: 10.1088/0967-3334/24/4/401. [DOI] [PubMed] [Google Scholar]
- Jang M.H., Kweon M.N., Iwatani K., Yamamoto M., Terahara C., Sasakawa C., Suzuki T., Nochi T., Yokota Y., Rennert P.D., Hiroi T., Tamagawa H., Irijima H., Kunisawa J., Yuki Y., Kiyono H. Intestinale villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jani P., Halbert G.W., Langridge J., Florence A.T. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- Jani P., McCarthy D., Florence A. Titanium dioxide (rutile) particles uptake from the rat GI tract and translocation to the systemic organs after oral administration. Int. J. Pharm. 1994;105:157–168. [Google Scholar]
- Jeong G.N., Jo U.B., Ryu H.Y., Kim Y.S., Song K.S., Yu I.J. Histochemical study of intestinal mucins after administration of silver nanoparticles in Sprague-Dawley rats. Arch. Toxicol. 2010;84:63–69. doi: 10.1007/s00204-009-0469-0. [DOI] [PubMed] [Google Scholar]
- Jiang X., Rocker C., Hafner M., Brandholt S., Dorlich R.M., Nienhaus G.U. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano. 2010;4:6787–6797. doi: 10.1021/nn101277w. [DOI] [PubMed] [Google Scholar]
- Kararli T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Katao K., Honma R., Kato S., Watanabe S., Imai J. Influence of platinum nanoparticles orally administered to rats evaluated by systemic gene expression profiling. Exp. Anim. 2011;60:33–45. doi: 10.1538/expanim.60.33. [DOI] [PubMed] [Google Scholar]
- Kerneis S., Bogdonona A., Kraehenbuhl J.P., Pringault E. Conversion by Peyer's patches lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Kim J., Park J.D., Ryu H.Y., Yu I.J. Histological study of gender differences in accumulation of silver nanoparticles in kidneys of Fischer 344 rats. J. Toxicol. Environ. Health. 2009;72:1279–1284. doi: 10.1080/15287390903212287. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Kim J.S., Cho H.S., Rha D.S., Kim J.M., Park J.D., Choi B.S., Lim R., Chang H.K., Chung Y.H., Kwon I.H., Jeong J., Han B.S., Yu I.J. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Song M.Y., Park J.D., Song K.S., Ryu H.R., Chung Y.H., Chang H.K., Lee J.H., Oh K.H., Kelman B.J., Hwang I.K., Yu I.J. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010;7:20. doi: 10.1186/1743-8977-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M.R., Boucher R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeneman B.A., Zhang Y., Westerhoff P., Chen Y., Crittenden J.C., Capco D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010;26:225–238. doi: 10.1007/s10565-009-9132-z. [DOI] [PubMed] [Google Scholar]
- Lagerlof F., Dawes C. The volume of saliva in the mouth before and after swallowing. J. Dent. Res. 1984;63:618–621. doi: 10.1177/00220345840630050201. [DOI] [PubMed] [Google Scholar]
- Lai S.K.a., O’Hanlon D.E.b., Harrold S.b., Man S.T.a., Wang Y.-Y.d., Cone R.b., Hanes J.a.c.d.e. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Nat. Acad. Sci. U.S.A. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J.G., Hathaway L.B., Munger M.A., Raucy J.L., Franklin M.R. Nanosilver particle effects on drug metabolism in vitro. Drug Metab. Dispos. 2010;38:2246–2251. doi: 10.1124/dmd.110.035238. [DOI] [PubMed] [Google Scholar]
- Lamprecht A., Schafer U., Lehr C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- Lankveld D.P., Oomen A.G., Krystek P., Neigh A., Troost-de Jong A., Noorlander C.W., Van Eijkeren J.C., Geertsma R.E., De Jong W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials. 2010;31:8350–8361. doi: 10.1016/j.biomaterials.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Leonhard F., Collnot E.M., Lehr C.M. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in-vitro. Mol. Pharm. 2010;7:2103–2119. doi: 10.1021/mp1000795. [DOI] [PubMed] [Google Scholar]
- Loeschner K., Hadrup N., Qvortrup K., Larsen A., Gao X., Vogel U., Mortensen A., Lam H.R., Larsen E.H. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 2011;8:18–20. doi: 10.1186/1743-8977-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomer M.C., Hutchinson C., Volkert S., Greenfield S.M., Catterall A., Thompson R.P., Powell J.J. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn's disease. Br. J. Nutr. 2004;92:947–955. doi: 10.1079/bjn20041276. [DOI] [PubMed] [Google Scholar]
- Lomer M.C., Thompson R.P., Commisso J., Keen C.L., Powell J.J. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. Analyst. 2000;125:2339–2343. doi: 10.1039/b006285p. [DOI] [PubMed] [Google Scholar]
- Long J.D., Orlando R.C. Esophageal submucosal glands: structure and function. Am. J. Gastroenterol. 1999;94:2818–2824. doi: 10.1111/j.1572-0241.1999.1422_b.x. [DOI] [PubMed] [Google Scholar]
- Lynch I., Salvati A., Dawson K.A. Protein-nanoparticle interactions: what does the cell see? Nat. Nanotechnol. 2009;4:546–547. doi: 10.1038/nnano.2009.248. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Ota H., Akamatsu T., Sugiyama A., Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Vadon M., Rinner B., Novak A., Wintersteiger R., Fröhlich E. The role of nanoparticle size in hemocompatibility. Toxicology. 2009;258:139–147. doi: 10.1016/j.tox.2009.01.015. [DOI] [PubMed] [Google Scholar]
- McGill S.L., Smyth H.D. Disruption of the mucus barrier by topically applied exogenous particles. Mol. Pharm. 2010;7:2280–2288. doi: 10.1021/mp100242r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H., Chen Z., Xing G., Yuan H., Chen C., Zhao F., Zhang C., Zhao Y. Ultrahigh reactivity provokes nanotoxicity: explanation of oral toxicity of nano-copper particles. Toxicol. Lett. 2007;175:102–110. doi: 10.1016/j.toxlet.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Navarro E., Piccapietra F., Wagner B., Marconi F., Kaegi R., Odzak N., Sigg L., Behra R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008;42:8959–8964. doi: 10.1021/es801785m. [DOI] [PubMed] [Google Scholar]
- Olmsted S.S., Padgett J.L., Yudin A.I., Whaley K.J., Moench T.R., Cone R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Wang W. Influences of dissolved and colloidal organic carbon on the uptake of Ag, Cd, and Cr by marine mussel Perna viridis. Environ. Pollut. 2004;129:467–477. doi: 10.1016/j.envpol.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Panacek A., Prucek R., Safarova D., Dittrich M., Richtrova J., Benickova K., Zboril R., Kvitek L. Acute and chronic toxicity effects of silver nanoparticles (NPs) on Drosophila melanogaster. Environ. Sci. Technol. 2011;45:4974–4979. doi: 10.1021/es104216b. [DOI] [PubMed] [Google Scholar]
- Park E., Kim H., Kim Y., Choi K. Repeated-dose toxicity attributed to aluminum nanoparticles following 28-day oral administration particularly on gene expression in mouse brain. Toxicol. Environ. Chem. 2011;93:110–119. [Google Scholar]
- Park E.J., Bae E., Yi J., Kim Y.S., Choi K., Lee S.H., Yoon J., Lee B.C., Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Pietzonka P., Rothen-Rutishauser B., Langguth P., Wunderli-Allenspach H., Walter E., Merkle H.P. Transfer of lipophilic markers from PLGA and polystyrene nanoparticles to caco-2 monolayers mimics particle uptake. Pharm. Res. 2002;19:595–601. doi: 10.1023/a:1015393710253. [DOI] [PubMed] [Google Scholar]
- Powell J.J., Faria N., Thomas-McKay E., Pele L.C. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J. Autoimmun. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Ragnarsson E.G., Schoultz I., Gullberg E., Carlsson A.H., Tafazoli F., Lerm M., Magnusson K.E., Soderholm J.D., Artursson P. Yersinia pseudotuberculosis induces transcytosis of nanoparticles across human intestinal villus epithelium via invasin-dependent macropinocytosis. Lab. Invest. 2008;88:1215–1226. doi: 10.1038/labinvest.2008.86. [DOI] [PubMed] [Google Scholar]
- Roblegg E., Fröhlich E., Meindl C., Teubl B., Zaversky M., Zimmer A. Evaluation of a physiological in vitro system to study the transport of nanoparticles through the buccal mucosa. Nanotoxicology. 2011:1–15. doi: 10.3109/17435390.2011.580863. [DOI] [PubMed] [Google Scholar]
- Ruponen M., Honkakoski P., Tammi M., Urtt A. Cell-surface glycosaminoglycans inhibit cation-mediated gene transfer. J. Gene Med. 2004;6:405–414. doi: 10.1002/jgm.522. [DOI] [PubMed] [Google Scholar]
- Sager T.M., Porter D., Robinson V., Lindsley W.G., Schwegler-Berry D.E., Castranova V. An improved method to disperse nanoparticles for in vitro and in vivo investigation of toxicity. Nanotoxicology. 2007;1:118–129. [Google Scholar]
- Sahay G., Alakhova D.Y., Kabanov A.V. Endocytosis of nanomedicines. J. Control. Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W.M., Radomsky M.L., Whaley K.J., Cone R.A. Antibody diffusion in human cervical mucus. Biophys. J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S., Scheid P. Gastric mucus of the guinea pig: proton carrier and diffusion barrier. Am. J. Physiol. 1997;272:G63–G70. doi: 10.1152/ajpgi.1997.272.1.G63. [DOI] [PubMed] [Google Scholar]
- Scown T.M., van Aerle R., Johnston B.D., Cumberland S., Lead J.R., Owen R., Tyler C.R. High doses of intravenously administered titanium dioxide nanoparticles accumulate in the kidneys of rainbow trout but with no observable impairment of renal function. Toxicol. Sci. 2009;109:372–380. doi: 10.1093/toxsci/kfp064. [DOI] [PubMed] [Google Scholar]
- Singh S., Shi T., Duffin R., Albrecht C., van Berlo D., Hohr D., Fubini B., Martra G., Fenoglio I., Borm P.J., Schins R.P. Endocytosis, oxidative stress and IL-8 expression in human lung epithelial cells upon treatment with fine and ultrafine TiO2: role of the specific surface area and of surface methylation of the particles. Toxicol. Appl. Pharmacol. 2007;222:141–151. doi: 10.1016/j.taap.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Squier C.A., Kremer M.J. Oxford Journals: JNCI Monographs; 2001. Biology of Oral Mucosa and Esophagus. pp. 7–15. [DOI] [PubMed] [Google Scholar]
- Stoeger T., Reinhard C., Takenaka S., Schroeppel A., Karg E., Ritter B., Heyder J., Schulz H. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ. Health Perspect. 2006;114:328–333. doi: 10.1289/ehp.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar Y., Kuotsu K., Bandyopadhyay A.K. Buccal bioadhesive drug delivery – a promising option for orally less efficient drugs. J. Control. Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Sun W., Fang N., Trewyn B.G., Tsunoda M., Slowing I.I., Lin V.S., Yeung E.S. Endocytosis of a single mesoporous silica nanoparticle into a human lung cancer cell observed by differential interference contrast microscopy. Anal. Bioanal. Chem. 2008;391:2119–2125. doi: 10.1007/s00216-008-2162-1. [DOI] [PubMed] [Google Scholar]
- Szentkuri L. Light microscopic observation on luminally administered dyes, dextrans, nanospheres and microspheres in the pre-epithelial mucus gel layer of the rat distal colon. J. Control. Release. 1997;46:233–242. [Google Scholar]
- Takizawa H., Ohtoshi T., Ohta K., Hirohata S., Yamaguchi M., Suzuki N., Ueda T., Ishii A., Shindoh G., Oka T. Interleukin 6/B cell stimulatory factor-II is expressed and released by normal and transformed human bronchial epithelial cells. Biochem. Biophys. Res. Commun. 1992;187:596–602. doi: 10.1016/0006-291x(92)91236-j. [DOI] [PubMed] [Google Scholar]
- Takubo K. Springer; Tokyo, Berlin, Heidelberg: 2009. Pathology of the Esophageus: An Atlas and Textbook. [Google Scholar]
- Trouiller B., Reliene R., Westbrook A., Solaimani P., Schiestl R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009;69:8784–8789. doi: 10.1158/0008-5472.CAN-09-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Feng W.Y., Wang M., Wang T., Gu Y., Zhu M., Ouyang H., Shi J., Zhang F., Zhao Y. Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J. Nanopart. Res. 2008;10:263–276. [Google Scholar]
- Wang J., Zhou G., Chen C., Yu H., Wang T., Ma Y., Jia G., Gao Y., Li B., Sun J., Li Y., Jiao F., Zhao Y., Chai Z. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007;168:176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Wang J.J., Sanderson B.J., Wang H. Cyto- and genotoxicity of ultrafine TiO(2) particles in cultured human lymphoblastoid cells. Mutat. Res. 2007;628:99–106. doi: 10.1016/j.mrgentox.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Wang Z., Tiruppathi C., Minshall R.D., Malik A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3:4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit D.B., Hoke R.A., Finlay C., Donner E.M., Reed K.L., Sayes C.M. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol. Lett. 2007;171:99–110. doi: 10.1016/j.toxlet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Xia T., Kovochich M., Brant J., Hotze M., Sempf J., Oberley T., Sioutas C., Yeh J.I., Wiesner M.R., Nel A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- Yacobi N.R., Demaio L., Xie J., Hamm-Alvarez S.F., Borok Z., Kim K.J., Crandall E.D. Polystyrene nanoparticle trafficking across alveolar epithelium. Nanomed.: Nanotechnol. Biol. Med. 2008;4:139–145. doi: 10.1016/j.nano.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang R., Niu Y., Li Y., Zhao C., Song B., Zhou Y. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ. Toxicol. Pharmacol. 2010;30:52–60. doi: 10.1016/j.etap.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Zhang X.D., Wu H.Y., Wu D., Wang Y.Y., Chang J.H., Zhai Z.B., Meng A.M., Liu P.X., Zhang L.A., Fan F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhu L., Chen Y., Tian S. Acute toxicities of six manufactured nanomaterial suspensions to Daphnia magna. J. Nanopart. Res. 2009;11:65–75. [Google Scholar]