The inositol trisphosphate receptor (InsP3R) forms a calcium channel that resides in the membrane of the endoplasmic reticulum and is activated by inositol trisphosphate (InsP3). InsP3 is a phosphorylated monosaccharide that is generated via hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, and activation of the InsP3R is involved in a broad range of biological processes, including cell division, apoptosis and development. Rahman et al.1,2 reported that exposure to low concentrations of InsP3 induces rapid clustering of InsP3R Ca2+ release channels normally randomly distributed in endoplasmic reticulum/outer nuclear membranes. Importantly, clustered channels gate differently from lone channels. Using similar protocols, we observed InsP3R channel clustering without exposure to InsP3 (Fig. 1a), as we found in other systems3–5 with protocols designed to avoid InsP3 pre-exposure. More significantly, we find that clustering has no effect on InsP3R channel gating. For this reason, we believe that InsP3-induced channel clustering and modification of channel gating by clustering may not be universal phenomena.
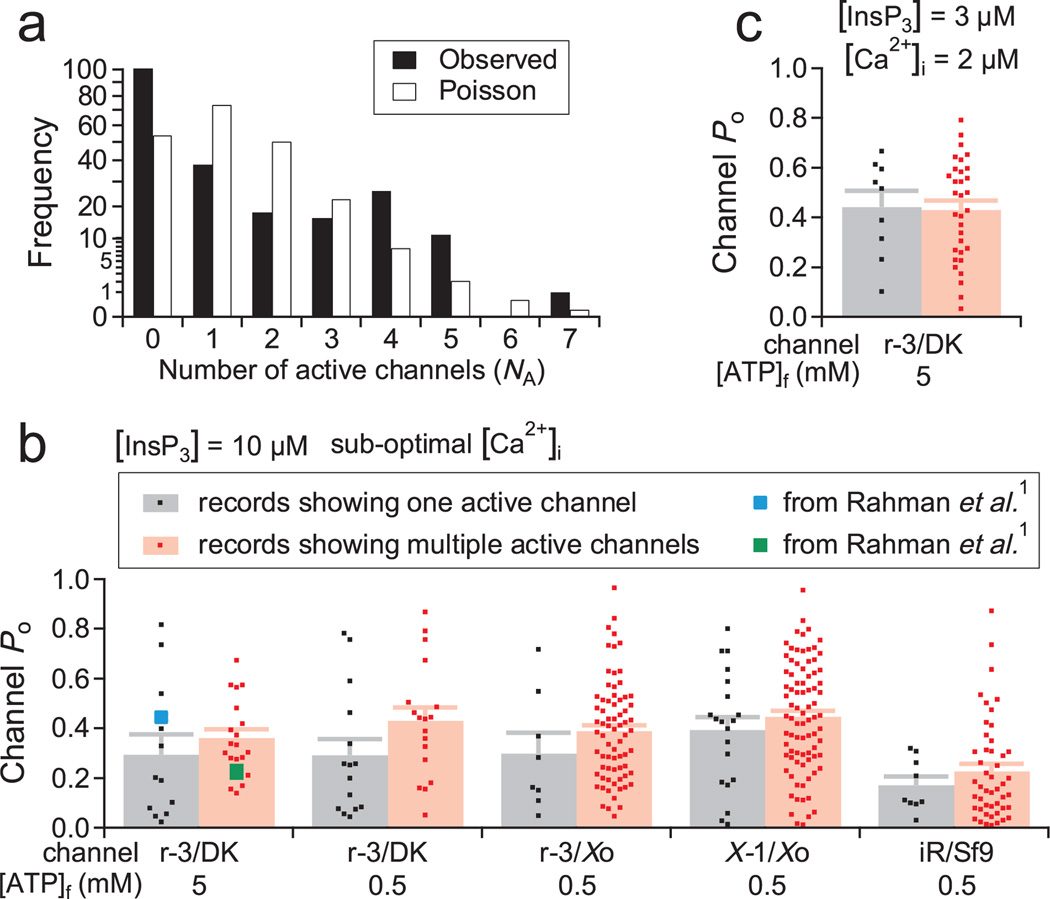
Figure 1. InsP3R channels are clustered before exposure to InsP3, with gating properties unaltered by clustering.
a, NA in nuclear membrane patches with no pre-exposure to InsP3 obtained from InsP3R3 expressing DT40-KO cells. Note nonlinear square-root scale for frequency axis. b, Po observed under saturating [InsP3] and sub-optimal [Ca2+]i in multi- and apparent single-channel current records for recombinant rat InsP3R3 (r-3) channels expressed in DT40-KO (DK) cells or Xenopus oocytes (Xo), endogenous Xenopus InsP3R1 (X-1) channels from Xenopus oocytes (Xo), and endogenous insect InsP3R (iR) channels from Sf9 cells. Concentrations of free ATP4− ([ATP]f) in the pipette solutions used are indicated. Mean Po with s.e.m. (as error bars) and Po for individual current records are shown, together with mean Po from Rahman et al.1 c, Po of r-3 channels in DK cells in optimal [Ca2+]i and sub-saturating [InsP3], ligand conditions not investigated in Rahman et al.1. Same symbols as in b are used.
Rahman et al.1,2 reported that in sub-optimal cytoplasmic free Ca2+ concentrations ([Ca2+]i), clustered recombinant rat type 3 InsP3R (InsP3R3) channels expressed in InsP3R-deficient DT40-KO cells gated identically and independently, but with lower open probability (Po) than lone channels, regardless of cluster size. In contrast, clustered channels had the same Po as lone channels in optimal ligand conditions, but gated with positive cooperativity. If broadly observed, these surprising findings have important implications for understanding InsP3-mediated Ca2+ signals, and for quantitative analyses in single-channel InsP3R electrophysiology.
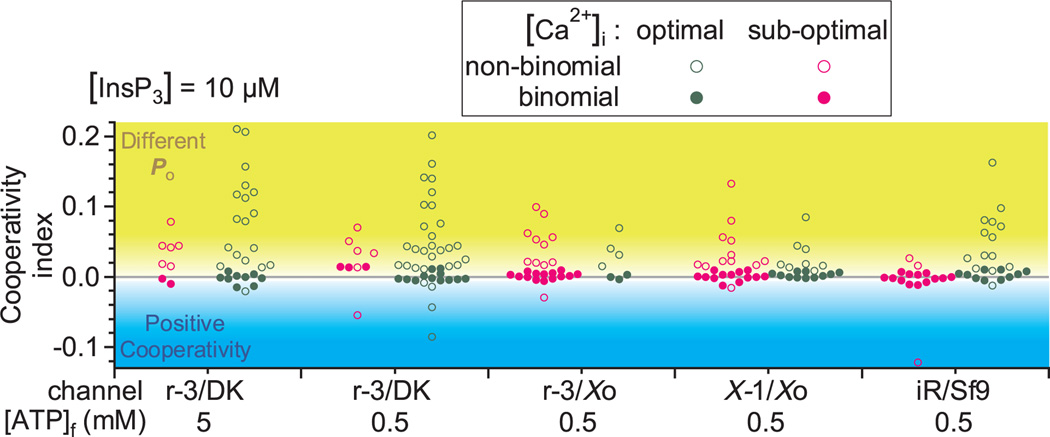
To verify these observations, we examined the same InsP3R3 channels in the same DT40-KO cells using similar protocols and ligand conditions. Specifically, we used 5 mM (same as Rahman et al.1) and 0.5 mM (more physiological) cytoplasmic free [ATP4−] ([ATP]f). Records with ≤4 active channels were analysed with the same algorithm1. In addition, we similarly analysed nuclear patch-clamp records previously acquired under comparable ligand conditions for recombinant rat InsP3R3 expressed in Xenopus oocytes6, endogenous Xenopus type 1 InsP3R (InsP3R1) in oocytes7 and endogenous insect InsP3R in Sf9 cells5. For all channels examined in these various systems, we detected no statistical difference (P > 0.05, t-test) between Po in single- versus multi-channel patches in saturating [InsP3] and sub-optimal [Ca2+]i (Fig. 1b), or in sub-saturating [InsP3] and optimal [Ca2+]i (Fig. 1c). Furthermore, in two-channel records, similar channel gating patterns were detected in all [Ca2+]i (Fig. 2), with only a small fraction exhibiting positive cooperativity. Thus, our extensive data set reveals no effect of clustering on InsP3R channel gating in all ligand conditions.
Figure 2. Distribution of cooperativity index for two-channel current records of different InsP3R channels in various systems in optimal and sub-optimal [Ca2+]i.
Filled and open circles represent records with two channels exhibiting identical and independent, or non-binomial gating, respectively. Non-binomial records with cooperativity index, (P2+P1/2)2−P2, significantly greater than 0 (in yellow shaded region) had two channels gating with different Po, and those with cooperativity index significantly smaller than 0 (in blue shaded region) had two channels gating with positive cooperativity. The cooperativity indices have no correlation with the durations of the current records (data not shown) and therefore are unlikely to be significantly affected by current record durations limited by channel inactivation.
In constant ligand conditions, we consistently observed abrupt, stochastic, irreversible inactivation of InsP3R in on-nucleus or excised luminal-side-out nuclear patches, with mean activity durations of ~40 s for oocyte InsP3R (ref. 3), ~100 s for Sf9 InsP3R (ref. 5) and ~140 s for InsP3R from DT40-KO cells, whereas Rahman et al. reported no such inactivation1. Importantly, we analysed only current records long enough for the number of active channels to be counted with >99% confidence1,5,6,8. Because finite time elapsed between pipettes making contact with the outer nuclear membrane and gigaohm seal formation (< 5 s for oocyte and Sf9 nuclei, ~10 s for DT40 nuclei), apparent single-channel patches possibly included a fraction (~11–26%) that actually contained multiple channels in which all but one channel inactivated before gigaohm seal formation. Yet, the mean number of active channels (NA) we observed for InsP3R3 in DT40 nuclear patches (1.36 ± 0.12 for 211 patches) is similar to that reported in Rahman et al.1, suggesting that inactivation did not substantially impair our ability to count channels in these patches. We did detect larger NA (10.8 ± 1)8 in outside-out nuclear patches, which probably have significantly larger membrane areas and were isolated using a different technique, and therefore are not an appropriate comparison to illustrate either the variability in InsP3R expression level in DT40-KO cells or the effect of inactivation on NA detected.
The Po distributions that we observed in apparent single-channel and true multi-channel patches were similar, with no indication that two populations of channels with different Po exist. Furthermore, mean Po of our true multi-channel patches is comparable to the lone-channel Po observed by Rahman et al.1 (Fig. 1b). Thus, our conclusion that clustering does not affect InsP3R channel gating is not compromised by the irreversible inactivation of InsP3R channels.
We have no clear explanation for the discrepancies between our observations and those reported by Rahman et al.1. However, neither InsP3-induced InsP3R clustering nor its modification of InsP3R gating are consistently observed for InsP3R expressed in DT40-KO and other cells. In contrast, channel clustering before InsP3 exposure was observed in all cell systems investigated without effect on channel gating3–5. Thus, we suggest that InsP3-induced channel clustering and modification of channel gating by clustering may not be universal phenomena.
METHODS SUMMARY
Single-channel Po for a sufficiently long5 current record with NA active channels was evaluated as , where Pi, the probability that i channels were active simultaneously in the record, was determined by the same method as Rahman et al.1. The channel gating pattern for a two-channel current record was determined from Pi. If Pi are similar to the expected binomial values, for i = 0,1,2 (P > 0.05 by χ2-test), the channels gated independently with similar Po. Otherwise, if the cooperativity index, (P2+P1/2)2 − P2, is >0, they gated with different Po, with or without negative cooperativity. If (P2+P1/2)2 − P2 < 0, they gated with positive cooperativity9.
Footnotes
Author contributions All authors contributed to project planning and wrote the paper. H.V. and D.-O.D.M. did data acquisition and analysis.
Competing financial interests: declared none.
References
- 1.Rahman TU, Skupin A, Falcke M, Taylor CW. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+ Nature. 2009;458:655–659. doi: 10.1038/nature07763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman T, Taylor CW. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels (Austin) 2009;3:226–232. doi: 10.4161/chan.3.4.9247. [DOI] [PubMed] [Google Scholar]
- 3.Mak D-OD, Foskett JK. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak D-OD, et al. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 2000;115:241–256. doi: 10.1085/jgp.115.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ionescu L, et al. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J. Physiol. (Lond.) 2006;573:645–662. doi: 10.1113/jphysiol.2006.109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mak D-OD, McBride S, Foskett JK. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels. Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mak D-OD, McBride S, Foskett JK. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vais H et al. Redox-regulated heterogeneous thresholds for ligand recruitment among InsP3R Ca2+ release channels. Biophys. J. 2010;99:407–416. doi: 10.1016/j.bpj.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenyon JL, Bauer RJ. Amplitude histograms can identify positively but not negatively coupled channels. J. Neurosci. Methods. 2000;96:105–111. doi: 10.1016/s0165-0270(99)00189-2. [DOI] [PubMed] [Google Scholar]