Abstract
OBJECTIVES
The pancreas is vulnerable to injury at the time of shock wave lithotripsy (SWL) as evidenced by case studies; thus, concern exists for the development of diabetes mellitus following SWL. Since previous studies may have been limited by referral and detection biases, the current study was completed in a population-based cohort.
METHODS
The Rochester Epidemiologic Project (REP) was used to identify all Olmsted County, Minnesota residents diagnosed with urolithiasis from 1985 to 2008. New onset diabetes was identified by diagnostic codes and treatment with SWL by surgical codes. Cox proportional hazards models were used to determine the risk of diabetes following SWL therapy.
RESULTS
There were 5,287 incident stone formers without pre-existing diabetes and with at least 3 months of follow-up. After an average follow-up of 8.7 years, 423 patients (8%) were treated with SWL and new onset diabetes developed in 743 (12%). The diagnosis of diabetes followed SWL in 77 patients. However, there was no evident association between SWL and the development of diabetes before (HR=0.98, 95% CI: 0.76 to 1.26) or after (HR 0.92, 95% CI: 0.71 to 1.18) controlling for age, gender, and obesity.
CONCLUSION
In this large, population-based cohort, the long-term risk for developing diabetes was not increased in persons who received SWL to treat their kidney stones.
Keywords: lithotripsy, urolithiasis, diabetes mellitus
Introduction
Since its inception and introduction to clinical urologic practice in the 1980’s, shock wave lithotripsy (SWL) has become a common first line modality for the treatment of urolithiasis.1 In fact, SWL has evolved to become the most frequently-performed procedure for ureteral and renal caluli.2 Although initially perceived as harmless to the surrounding kidney and adjacent organs, multiple studies have demonstrated that the use of repetitive shock waves for successful stone fragmentation can occasionally induce acute injury to kidney tissue and other surrounding structures including the pancreas, colon, liver, spleen, pleura and large vessels. 3, 4
Even though the pancreas is potentially vulnerable to injury at the time of SWL, as evidenced by multiple case reports of acute pancreatitis occurring immediately following SWL, the relationship of SWL and new onset diabetes has not been firmly established.5–7 A prior retrospective study from our institution found an increased risk of diabetes after SWL, while another long term study failed to demonstrate this relationship.8,9 Previous investigations have focused on stone formers referred to urology practices and relied heavily on patient self-reporting. 8,9 Study designs and control groups have also been potentially problematic. In one major study patients undergoing SWL for ureteral calculi were used as a control population for those undergoing the procedure for renal calculi, assuming a significant differential effect on adjacent structures. 9 Another surveyed patients that had undergone SWL for associated complications while this information was obtained for controls treated with conservative management by chart review.8 SWL has been employed for the treatment of nephrolithiasis at Mayo Clinic since 1985. Therefore, a cohort of Olmsted County patients could be studied that had received SWL with up to 25 years of follow-up and compared to those Olmsted County stone formers who did not receive SWL. The purpose of this study was to determine the long-term risk of new-onset diabetes in those kidney stone formers who had or had not received SWL therapy.
Materials and Methods
After gaining approval from Mayo Clinic’s Institutional Review Board, a cohort of stone formers was identified using the Rochester Epidemiology Project (REP). The REP is a unique, unified electronic medical record system from all care providers in Olmsted County from 1935 onwards that indexes and links diagnostic codes based on the final diagnoses contained in clinical notes.10 Olmsted County residents with the diagnosis of urolithiasis between 1985 and 2010 were identified using the appropriate codes employed by the REP over the time-frame of this study, including International Classification of Diseases (ICD)-9 codes 592, 594, 274.11, Hospital International Classification of Diseases Adapted (HICDA) codes 05920, 05921, 05940, 05949 and Berkson codes 14675, 15672, 15673, and 15674. The “index stone date” was defined as the date of the first stone occurrence in Olmsted County between 1985 and 2010.
The following groups of patients were excluded: non-residents of Olmsted County or residents who did not have Minnesota research authorization; patients with a stone diagnosis prior to the index stone date; patients who had less than 90 days of follow-up; patients with diabetes prior to their index stone date were excluded; and patients diagnosed with diabetes within 90 days after their 1st stone episode, since they may have had pre-existing diabetes and there is increased detection of comorbidities with the medical care surrounding a stone episode.
The final study sample was followed forward in time for SWL treatments and the development of diabetes. Dates of SWL were identified using surgical codes. Date of first diabetes diagnosis was identified using ICD-9 codes 357.2, 362.0, 366.4, 648.8 and 790.2. Potential confounders that may affect choice of SWL as a treatment modality and the risk of diabetes were captured including age, gender, and obesity (ICD-9 codes 259.9, 278.0 and 278.01). Follow-up was terminated at last care provider contact, death, or diabetes development.
Statistical Analysis
Kaplan-Meier analysis, log-rank tests and proportional hazards models were used to determine the risk of diabetes after SWL. Follow-up was terminated at last known date of residency in Olmsted County, death or the diagnosis of diabetes. SWL may have been used to treat the incident stone or as treatment for recurrent urolithiasis; therefore, it was studied as a time-dependent covariate in a Cox proportional hazards model, with the estimation of hazard ratios and 95% confidence intervals for the effect of SWL on the subsequent development of diabetes. Obesity, age and gender were adjusted using a multivariable model. The effect of prior SWL (time dependent covariate) on the development of diabetes was studied using the Landmark method, stratifying on the use of SWL in the first two-years after stone diagnosis, and then plotting (Kaplan-Meier curve) rates of diabetes after year two.11 All tests were two-sided with significance level 0.05, and performed using SAS 9.1 (SAS, NC).
Results
Over the time period of study (January 1, 1985 to December 31, 2008), 7,202 stone formers with Minnesota research authorization were identified in the REP. Of these 6,498 had not been previously diagnosed with renal or ureteral calculi, leaving a final study cohort of 6,077 that had follow-up greater than 90 days (Figure 1).
Figure 1. Venn Diagram of Nephrolithiasis patients based on diagnoses codes.
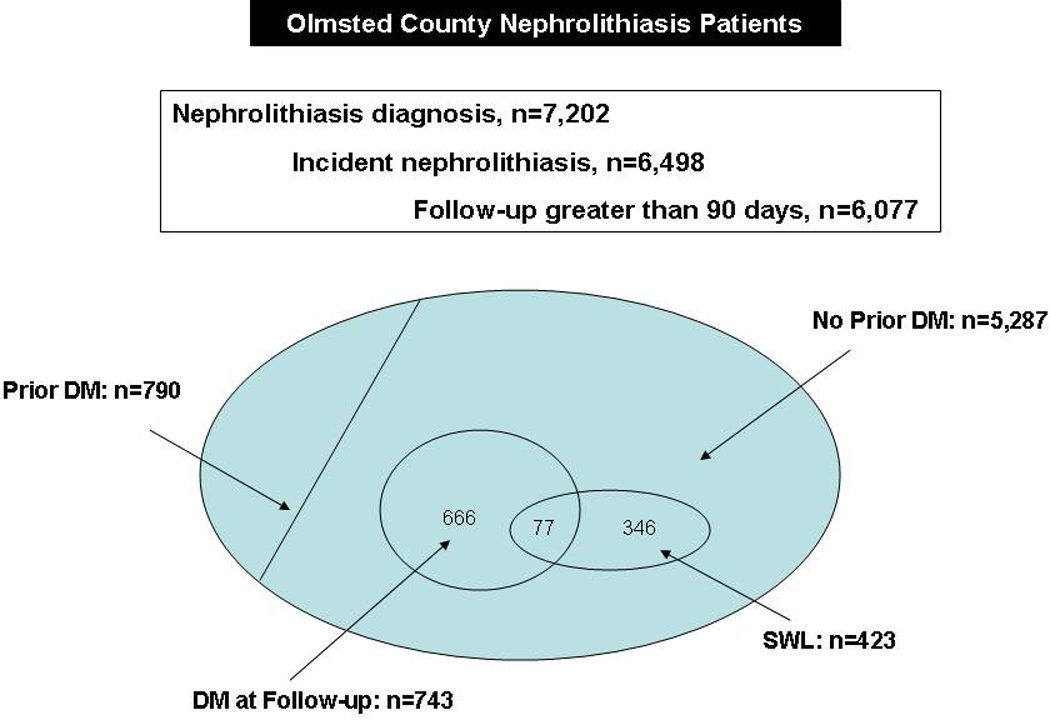
Incident stone formers with greater than 90 days of follow-up were included in the study cohort and patients with prevalent diabetes mellitus were excluded. Of the incident nephrolithiasis patients without diabetes, 423 were treated with shock wave lithotripsy (SWL), and 743 went on to develop diabetes. Among the 77 with both ESWL and diabetes, 68 had diabetes after ESWL.
Mean age (±SD) at diagnosis of urolithiasis was 45.4 ±17.5 years and 56.4% of patients were male. During a mean follow-up of 8.7 years (range 3 to 23 years), 478 (7.9%) incident stone formers underwent SWL. Of the 6,077 patients in the study group, 790 (13.0%) had prevalent diabetes (diagnosed before the index stone date plus 90 days), leaving 5,287 patients without prevalent diabetes. Among the non-diabetic stone formers, 743 (14.1%) eventually developed diabetes greater than 90 days following their index stone, at a median of 6.6 years (Table 1).
Table 1.
Temporal relationship of SWL and diabetes diagnosis after index stone date
| Diagnosis | Number (%) |
|---|---|
| Neither diabetes or SWL | 4198 (79.4) |
| SWL only | 346 (6.5) |
| Diabetes only | 666 (12.6) |
| SWL then diabetes | 68 (1.3) |
| Diabetes then SWL | 9 (0.2) |
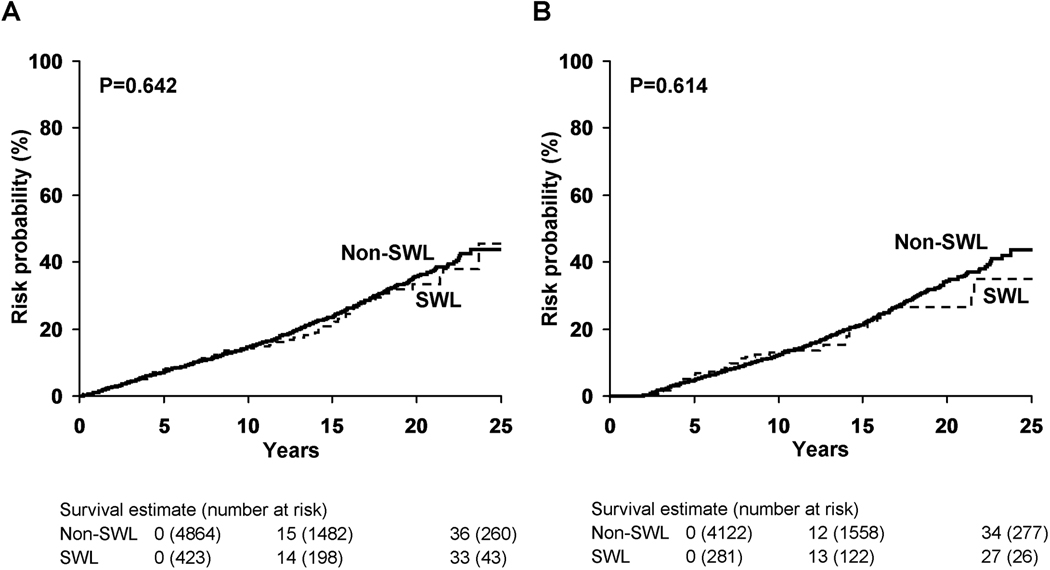
Among the stone formers without pre-existing diabetes, 423 (8%) received one (357), two (49), or three or more (17) SWL treatments, with 60 (1.1%) receiving SWL therapy for their index stone. Of the 423 patients treated with SWL, 305 (72%) had SWL performed within 2 years of the index stone diagnosis. The diagnosis of diabetes was eventually made in 77 (18.2%) of the patients treated with SWL (Table 1). The median time to development of DM in the SWL group was 7.3 years compared to 6.4 years in the control (non-SWL) population. After 20 years of follow-up, the risk of developing diabetes was similar in the SWL (33%) and non-SWL (36%) groups (p=0.642, Figure 2a). Among patients who underwent SWL within 2 years of index stone, the 20-year risk of diabetes was 27% for the SWL group and 34% for the matched controls that did not receive SWL (Landmark method, p= 0.614, Figure 2b).
Figure 2. Risk of the development of diabetes in subsets of patients treated with and without SWL.
A) Compares risk of diabetes over 20 years from incident stone date among patients treated with and without SWL. B) Compares risk of diabetes over 20 years in the same subgroups, starting at 2 years following index stone, stratified on SWL in first 2 years among those still at risk (Landmark method).
The temporal relationship of SWL and the development of diabetes were further evaluated in time-dependent models. Even when the timing of SWL was taken into account, we found no increased risk for new-onset diabetes following SWL (Hazard ratio [95% CI] 0.98 [0.76, 1.26], p=0.87). A multivariable model adjusting for age, gender and baseline obesity also demonstrated no increased risk for the subsequent diagnosis of diabetes after SWL (Hazard ratio [95% CI] 0.92 [0.71, 1.18], p=0.51, Table 2). In addition, the risk of diabetes after SWL was not increased among persons older than the median age (men >44 years and women >38 years), even though older adults are at increased risk for diabetes (Table 2).
Table 2.
Multivariate and subgroup analysis assessing the association between SWL and incident diabetes in nephrolithiasis patients.
| Adjustment | SWL HR* | 95% CI | p Value |
|---|---|---|---|
| No adjustment | 0.98 | 0.76, 1.26 | 0.8706 |
| Age, gender | 0.95 | 0.74, 1.22 | 0.6685 |
| Age, gender, baseline obesity | 0.92 | 0.71, 1.18 | 0.5112 |
| Subgroup | |||
| Male, younger than median of 44 yrs (n=1,473) | 1.07 | 0.63, 1.83 | 0.8009 |
| Male, median of 44 yrs or older (n=1,517) | 0.81 | 0.55, 1.19 | 0.2843 |
| Female, younger than median of 38 yrs (n=1,174) | 1.29 | 0.66, 2.49 | 0.4565 |
| Female, median of 38 yrs or older (n=1,123) | 0.92 | 0.62, 1.52 | 0.7686 |
SWL HR for the development of diabetes was determined using a Cox Model, with SWL analyzed as a time dependent covariate.
Comment
SWL has become a widely used treatment for ureteral and renal pelvic calculi since its’ introduction in the early 1980s.1,12 SWL’s minimally-invasive nature has made it a mainstay therapy for symptomatic urolithiasis, and the majority of urinary calculi are considered to be amenable to SWL.13 However, it has been recognized that SWL can also cause acute injury to the kidney and other vulnerable adjacent structures.3,4 Therefore, concern exists as to the long term effects of SWL on the pancreas, and the subsequent risk for diabetes.5–9
Multiple case reports describe acute pancreatitis after both left and right-sided SWL. 5–7 Kirkali and colleagues also detected increased serum amylase, serum lipase, and urinary amylase that were sustained for up to 1 week post-SWL for renal and proximal ureteral calculi, without the overt development of pancreatitis.14 Furthermore, pancreatic hematomas and microvascular changes have been noted after SWL in patients who are asymptomatic.15
Although multiple studies have demonstrated that the pancreas is potentially vulnerable at the time of SWL, there has been conflicting data regarding the risk of diabetes following SWL. A retrospective review at our institution performed in 2006 investigated the long-term effects of SWL among 288 patients treated in 1985 with the HM3 lithotripter for renal pelvic and proximal ureteral calculi. This study demonstrated an increased incidence of diabetes after 19 years follow-up among patients treated with SWL when compared to those treated conservatively, even when controlling for BMI. This risk appeared to directly correlate with the number of shocks administered and the treatment intensity.8 However, the detection of diabetes was based on self-reporting using a survey sent to all SWL patients, with a concomitant Mayo Clinic medical record review to collect this data in the non-SWL patients. This may have introduced a differential detection bias, particularly since controls may have been diagnosed with diabetes at other institutions. Subsequently, Sato and associates investigated the long-term sequelae of SWL, comparing 772 patients undergoing SWL for renal calculi to 505 patients undergoing treatment for ureteral calculi. They found no appreciable difference in the risk for development of diabetes between these two groups at 17 years of follow-up.9 However, it is not known for certain that SWL to the ureter cannot affect the pancreas.
Therefore, we investigated the potential association of SWL and the subsequent development of diabetes in a population-based group of stone formers using the same medical records to minimize the potential for referral and detection bias. Among 6,077 persons with incident kidney stones between 1985 to 2008, over 14% developed new-onset diabetes. However, previous SWL did not increase the risk of diabetes in a univariate analysis, or multivariate analysis that accounted for age, gender and obesity. The results of our current study are significantly different from those observed in the 2006 study at our institution described above. One important distinction is that the current study employed a cohort of community stone formers as opposed to a referral population. Patients referred to high volume, academic centers generally have a more complicated medical history than patients evaluated and treated in the community setting, and are therefore at a potentially increased risk for the development of chronic medical conditions, such as diabetes. Therefore, the current study decreased the potential confounding effects of referral bias. In addition, unlike the previous study, diabetes was detected using the same approach (diagnostic codes) in both the SWL and non-SWL patients in order to avoid a detection bias.
Although our study group was large and comprised 6,077 patients, with the majority not carrying a previous diagnosis of diabetes, only a small proportion underwent SWL therapy. Nevertheless, this still represents one of the larger urolithiasis patient populations studied to address this question. Significantly, during the time frame studied, most SWL at Mayo Clinic Rochester was performed with an HM3 lithotripter. This is both a strength and limitation. SWL treatments over this time frame at Mayo Clinic were relatively uniform, however the shock waves administered by the HM3 are less focused than those from newer models. Although one might argue that the less focused nature of the HM3 shock waves might be more likely to damage adjacent organs like the pancreas, and therefore patients treated with this model might be those at highest risk for subsequent diabetes, findings from this study could differ from those obtained using other lithotripter models or at other institutions.
In addition, our current study did not differentiate the risk of developing DM based on calculus size, location, lithotripter used or energy delivered. However, at our institution the standard protocol was a maximum of 2500 shocks (renal calculi) or 3000 shocks (ureteral calculi), so that variation in shock wave exposure was limited. It is still possible that tissue injury to adjacent organs could correlate with the side of treatment or calculus size, as well as treatment intensity and number of shocks delivered, so that stratification of DM risk in relation to these factors could be considered in cohorts where significant variation is likely to be present.
Our current study does have other potential limitations. Although the mean follow-up in this study was 8.7 years, this still may not have been long enough for diabetes to develop among those treated with SWL. Notably, the study that found an increased risk of diabetes with SWL had a mean follow-up that was nearly twice as long (19 years).8 However, the Kaplan-Meier plots in our current study do not demonstrate a difference in the development of DM between the two groups at any time during follow-up. The 2010 United States Census reported that the population of Olmsted County is approximately 90% white, so results may not accurately reflect risks in other racial groups. However, nephrolithiasis is more common in Caucasians than other ethnic groups and results can still therefore be extrapolated to the majority of stone formers in the U.S.16 Also, as urologists ultimately decide which patients to offer SWL or other therapies to, a selection bias may have occurred that could mask the true risk of diabetes. Finally, we employed diagnostic codes rather than chart reviews to identify stone formers. However, review of a random subset of 1097 Olmsted County stone former charts has validated this approach, since 89% of the charts reviewed had evidence in the medical record supporting the diagnosis of urolithiasis.
Conclusion
Our study in a large, community-based cohort of stone formers does not suggest that receiving SWL using an HM3 lithotriptor increased the overall subsequent risk of diabetes. Further population-based studies with longer follow-up or different lithotriptors would be helpful to confirm these findings, but the existing best evidence does not suggest diabetes is a common complication of SWL.
Acknowledgment
We would like to acknowledge the contributions of Timothy Roth, M.D. to the study design. The National Institute of Health provided funds for this study through the Mayo Clinic O’Brien Urology Research Center (DK83007) and the Rochester Epidemiology Project (R01 AG034676).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaussy C, Schniedt E, Jocham D, et al. First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol. 1982;127:417–420. doi: 10.1016/s0022-5347(17)53841-0. [DOI] [PubMed] [Google Scholar]
- 2.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: Urolithiasis. J Urol. 2005;173:848–857. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 3.Chaussy C, Fuchs G. Extracorporeal lithotripsy in the treatment of renal lithiasis. 5 years’ experience. J Urol. 1986;92:339–343. [PubMed] [Google Scholar]
- 4.McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008;28:200–213. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassana I, Zietlow SP. Acute pancreatitis after extracorporeal shock wave lithotripsy for a renal calculus. Urol. 2002;60:111iii–1111v. doi: 10.1016/s0090-4295(02)01984-2. [DOI] [PubMed] [Google Scholar]
- 6.Abe H, Nisimura T, Osawa S, et al. Acute pancreatitis caused by extracorporeal shock wave lithotripsy for bilateral renal pelvic calculi. Int J Urol. 2000;7:65–68. doi: 10.1046/j.1442-2042.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 7.Karakayali F, Sevmis S, Ayvaz I, et al. Acute necrotizing pancreatitis as a rare complication of extracorporeal shock wave lithotripsy. Int J Urol. 2006;13:613–615. doi: 10.1111/j.1442-2042.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- 8.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742–1747. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 9.Sato Y, Tanda H, Ohnishi S, et al. Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urol. 2008;71:586–591. doi: 10.1016/j.urology.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 12.Galvin DJ, Pearle MS. The contemporary management of renal and ureteric calculi. BJU Int. 2006;98:1283–1288. doi: 10.1111/j.1464-410X.2006.06514.x. [DOI] [PubMed] [Google Scholar]
- 13.Lingeman JE, Woods JR, Toth PD. Blood pressure changes following extracorporeal shock wave lithotripsy and other forms of treatment for nephrolithiasis. JAMA. 1990;263:1789–1794. [PubMed] [Google Scholar]
- 14.Kirkali Z, Kirkali G, Tanci S, et al. The effect of extracorporeal shock wave lithotripsy on pancreatic enzymes. Int Urol Nephrol. 1994;26:405–408. doi: 10.1007/BF02768009. [DOI] [PubMed] [Google Scholar]
- 15.Hung SY, Chen HM, Jan YY. Common bile duct and pancreatic injury after extracorporeal shock wave lithotripsy for renal stone. Hepatogast. 2000;47:1162–1163. [PubMed] [Google Scholar]
- 16.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]