Abstract
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1E,6E-heptadiene-3,5-dione or diferuloyl methane) is a polyphenol derived from the Curcuma longa plant, commonly known as turmeric. This substance has been used extensively in Ayurvedic medicine for centuries for its anti-oxidant, analgesic, anti-inflammatory and antiseptic activity. More recently curcumin has been found to possess anti-cancer properties linked to its pro-apoptotic and anti-proliferative actions. The underlying mechanisms of these diverse effects are complex, not fully elucidated and subject of intense scientific debate. Despite increasing evidence indicating that different cation channels can be a molecular target for curcumin, very little is known about the effect of curcumin on chloride channels. Since, (i) the molecular structure of curcumin indicates that the substance could potentially interact with chloride channels, (ii) chloride channels play a role during the apoptotic process and regulation of the cell volume, and (iii) apoptosis is a well known effect of curcumin, we set out to investigate whether or not curcumin could (i) exert a modulatory effect (direct or indirect) on the swelling activated chloride current IClswell in a human cell system, therefore (ii) affect cell volume regulation and (iii) ultimately modulate cell survival. The IClswell channels, which are essential for regulating the cell volume after swelling, are also known to be activated under isotonic conditions as an early event in the apoptotic process. Here we show that long-term exposure of a human kidney cell line to extracellular 0.1–10 μM curcumin modulates IClswell in a dose-dependent manner (0.1 μM curcumin is ineffective, 0.5–5.0 μM curcumin increase, while 10 μM curcumin decrease the current), and short-term exposure to micromolar concentrations of curcumin does not affect IClswell neither if applied from the extracellular nor from the intracellular side – therefore, a direct effect of curcumin on IClswell can be ruled out. Furthermore, we show that curcumin exposure induces apoptosis in human kidney cells, and at a concentration of 5.0–10 μM induces the appearance of a sub-population of cells with a dramatically increased volume. In these cells the regulation of the cell volume seems to be impaired, most likely as a consequence of the IClswell blockade. Similarly, 50 μM curcumin induced apoptosis, caused cell cycle arrest in G1-phase and increased the volume of human colorectal adenocarcinoma HT-29 cells. The cell cycle arrest in G1 phase may be the mechanism underlying the volume increase observed in this cell line after exposure to curcumin.
Abbreviations: MEM, minimum essential eagle medium; FBS, fetal bovine serum; IClswell, swelling activated chloride current; EDTA, ethylene diamine tetraacetic acid; DMSO, dimethyl sulfoxide; EGTA, ethylene glycol tetraacetic acid; HEPES, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; FITC, fluorescein isothiocyanate; 7-AAD, 7-AMINO-ACTINOMYCIN D; DAPI, 4′,6-diamidino-2-phenylindole; CFTR, cystic fibrosis transmembrane regulator
Keywords: Curcumin, Apoptosis, Cell volume regulation, IClswell
1. Introduction
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1E,6E-heptadiene-3,5-dione or diferuloyl methane), an orange-yellow component of turmeric (also known as Indian saffron, turmeric yellow or curry powder), is a natural polyphenol product isolated from the Curcuma longa plant rhizome. Curcumin is used in traditional medicinal preparation and as a food-coloring agent (Aggarwal and Sung, 2009), however, research within the past five decades also revealed pharmacological as well as toxicological effects (Cao et al., 2006). Research was focused on, but not limited to, the pro-apoptotic (Shankar et al., 2007; Shankar and Srivastava, 2007a), anti-proliferative (Bachmeier et al., 2010), anticancer (Bar-Sela et al., 2010), antiviral (Rechtman et al., 2010), antiarthritic (Funk et al., 2006), anti-amyloid (Ringman et al., 2005), antioxidant (Glauert et al., 2010), anti-obesity (Alappat and Awad, 2010) and anti-inflammatory (Jurenka, 2009) properties of curcumin. The underlying mechanisms of these diverse effects are only poorly understood, however, they seem to involve the regulation of various molecular targets, including transcription factors (nuclear factor-kB), growth factors (vascular endothelial cell growth factor), inflammatory cytokines (tumor necrosis factor, interleukin 1 and interleukin 6), protein kinases (mammalian target of rapamycin, mitogen-activated protein kinases, and Akt) and other enzymes (cyclooxygenase 2 and 5 lipoxygenase) (Aggarwal and Sung, 2009; Zhou et al., 2011). It is important to note that there are substantial controversies regarding the action of curcumin on HIV as well as inflammatory conditions (Liu et al., 2005; White and Judkins, 2011). Increasing evidence indicates that cation channels also serve as targets for curcumin, i.e. micromolar concentrations of curcumin inhibit Ca2+-release-activated Ca2+ channel (ICRAC) and K+ channels (Kv and SK4) in human T cells (Shin et al., 2011), block the Cav3.2 T-type Ca2+ current in bovine adrenal zona fasciculata (AZF) cells (Enyeart et al., 2009), bTREK-1 K+ channels (Enyeart et al., 2008) and the Kv1.4 voltage-gated K+ channel (Liu et al., 2006) in bovine adrenocortical cells. Curcumin also seems to ameliorate pain hypersensitivity in rats through a selective blockade of transient receptor potential vanilloid 1 (TRPV1) channels (Yeon et al., 2010). In contrast to cation channels, which seem to be inhibited by curcumin, chloride channels seem to be activated by the substance. The open probability of the cystic fibrosis transmembrane regulator (CFTR) chloride channel was reported to be increased by curcumin in excised, inside-out membrane patches (Berger et al., 2005). Accordingly, Wang et al. (Wang et al., 2005, 2007) found that curcumin (0.5–10 μm) stimulated ion currents mediated by both wild-type and ΔF508-CFTR in excised membrane patches. These authors pointed out that the structure of curcumin (two aromatic rings separated by a hydrocarbon spacer) is similar to that of 5-nitro-2-(3 phenylpropylamino)benzoic acid-AM (NPPB-AM), which is an uncharged form of the well-known chloride channel blocker NPPB and acts as a CFTR agonist by increasing the channel opening rate. Interestingly, curcumin was also shown to increase the activity of a CFTR mutant (G551D) with an extremely low open probability despite its normal trafficking to the plasma membrane (Yu et al., 2011). It is important to note that the effect of curcumin on CFTR and its mutants is controversial (Egan et al., 2004; Grubb et al., 2006; Lipecka et al., 2006; Norez et al., 2006). Besides the effect on CFTR, little is known about the effect of curcumin on other chloride channels. Best et al. describe an activation of the swelling-activated chloride current IClswell in rat pancreatic cells by curcumin (Best et al., 2007). IClswell is elicited after hypotonic shock during the homeostatic mechanism regulatory volume decrease (RVD). As a consequence, the exit of osmolytes from the cell drives an osmotic water efflux, allowing the swollen cell to regain its original volume (Furst et al., 2002). Cell volume alterations are involved in numerous cellular events like epithelial transport, metabolic processes, hormone secretion, cell migration, proliferation and apoptosis (Jakab et al., 2002; Lang et al., 2006). Apoptotic stimuli have been reported to rapidly activate Cl− conductances in a large variety of cell types. Cell shrinkage, the so-called apoptotic volume decrease (AVD), is an early event in apoptosis, and the efflux of Cl− contributes to this process. In a variety of cell types (epithelial cells, cardiomyocytes, neurons), the AVD-inducing anion channel was determined to be the volume-sensitive swelling-activated chloride channel, which is usually activated by hypotonicity under non-apoptotic conditions (Lang et al., 2000, 2007; Okada et al., 2006; Pasantes-Morales and Tuz, 2006). Since it is known that substances able to modulate chloride channel activity can also interfere with the apoptotic process (Shimizu et al., 2008), we set out to investigate whether or not curcumin is able to induce apoptosis via the modulation of chloride channels in human embryonic kidney (HEK) cells. Surprisingly, in contrast to Best et al. (2007), we did not find any significant direct effect of 10 or 50 μM curcumin on IClswell; however, we discovered that curcumin indirectly (i) activates IClswell at low concentrations (<5.0 μM), which most likely occurs by inducing apoptosis, and (ii) at higher concentrations (≥5.0 μM) inhibits IClswell and causes an increase of cell volume and cell cycle arrest.
2. Materials and methods
2.1. Cell culture
Human renal HEK293 Phoenix cells (DiCiommo et al., 2004) were cultured in Minimum Essential Eagle Medium (MEM, Sigma, Austria) supplemented with 10% fetal bovine serum (FBS, Cambrex Bio Science), 2 mM l-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin and 1 mM pyruvic acid (sodium salt). Human colorectal adenocarcinoma HT-29 cells were cultured in McCoy's 5a modified medium (Sigma, Austria) supplemented with 10% FBS, 100 μg/ml streptomycin and 100 U/ml penicillin. The cells were maintained at 37 °C, 5% CO2, 95% air and 100% humidity. Subcultures were routinely established every second to third day by seeding the cells into 100 mm diameter Petri dishes following trypsin/EDTA treatment.
2.2. Patch clamp recordings
For patch-clamp experiments, HEK293 Phoenix or HT-29 cells were seeded into 30 mm diameter Petri dishes containing glass cover slips (Ø 10 mm). Single cells were visualized by phase contrast microscopy and voltage-clamped using the whole cell patch clamp technique. Patch clamp micropipettes were obtained by pulling glass capillaries (1BBL W/FIL, OD 1.5 mm, World Precision Instruments, USA) with a model P-97 horizontal puller (Sutter Instrument Co., USA); when necessary, the micropipettes where polished by a model MF-830 microforge (Narishige, Japan). The resistance of the glass pipettes was 3–8 MΩ when filled with the pipette solution (for use with the hypertonic and hypotonic bath solutions the pipette solution was, in mM: CsCl 125, MgCl2 5, EGTA 11, raffinose 50, ATP 2, HEPES 10, 330 mOsm/kg, pH 7.2 (adjusted with CsOH); for use with the isotonic bath solution the pipette solution was, in mM: CsCl 125, MgCl2 5, EGTA 11, raffinose 20, ATP 2, HEPES 10, 308 mOsm/kg, pH 7.2 (adjusted with CsOH)). The hypertonic bath solution was composed of (in mM): NaCl 125, CaCl2 2.5, MgCl2 2.5, HEPES 10, mannitol 100, 360 mOsm/kg, pH 7.4 (adjusted with NaOH). The isotonic bath solution was composed of (in mM): NaCl 125, CaCl2 2.5, MgCl2 2.5, HEPES 10, 308 mOsm/kg, pH 7.4 (osmolarity and pH adjusted with mannitol and NaOH, respectively). Fast exchange of the hypertonic bath solution with a hypotonic bath solution (in mM: NaCl 125, CaCl2 2.5, MgCl2 2.5, HEPES 10, 260 mOsm/kg, pH 7.4) was obtained using a perfusion system with a flow rate of 5 ml/min and a bath volume of ∼300 μl. For the experiments where the intracellular effect of curcumin was tested, 50 μM curcumin was added to the pipette filling (intracellular) solution. For the control experiments, an adequate volume of dimethyl sulfoxide (DMSO) as the vehicle was added to the pipette filling solution; the final concentration of DMSO was 0.5%. For the experiments where the extracellular, short-term effect of curcumin was tested, curcumin was added to the extracellular hypotonic (10 or 50 μM) or isotonic (10 μM) solution; for control experiments, an adequate volume of DMSO was added to the extracellular hypotonic or isotonic solution; the final concentration of DMSO was 0.1% or 0.5% for 10 or 50 μM curcumin, respectively. NPPB (Sigma, Austria) was used to discriminate between chloride currents and leakage currents. For the experiments where the extracellular, long-term effect of curcumin was tested, curcumin was added to the cell culture medium 1 h after seeding to the final concentrations of 0.1, 0.5, 1.0, 5.0 or 10 μM. For the control experiments, an adequate volume of DMSO as the vehicle was added to the medium of the cells; the final concentration of DMSO was 0.05%. Electrophysiology measurements were performed 16–24 h after cell seeding. All patch clamp experiments were carried out at room temperature. For data acquisition, an EPC8 amplifier (HEKA Elektronik, Germany) controlled via an ITC-16 computer interface by a Macintosh computer running Patch Master (HEKA Elektronik, Germany) software was used. Access resistance as well as fast and slow capacitance were compensated and monitored throughout the recordings. All current measurements were filtered at 2.9 kHz and digitized at 2 kHz. The cells were held at 0 mV and step pulses of 400 ms duration were applied from 0 mV to +40 mV every 20 s to monitor the activation of the swelling activated chloride current (IClswell). To establish the current to voltage (IV) relationship of the current, step pulses of 500 ms duration were applied every 10 min from −120 to +100 mV in 20 mV increments from a holding potential of 0 mV. For data analysis, Fit Master (HEKA Elektronik, Germany) and EXCEL (Microsoft, USA) software were used. Current values were normalized to the membrane capacity to obtain the current density.
2.3. Assessment of apoptosis and cell size by flow cytometry
For assessment of apoptosis and cell size analysis, cells were seeded in 100 mm diameter Petri dishes at a density of 100,000/ml (HEK29 Phoenix cells) or 120,000/ml (HT-29 cells) and grown for 19 h (HEK29 Phoenix cells) or 22 h (HT-29 cells) in the presence of 0.1, 0.5, 1.0, 5.0, or 10 μM curcumin (HEK29 Phoenix cells), 5.0, 10, 20 or 50 μM curcumin (HT-29 cells) or 0.05% DMSO as the vehicle. Cells treated with 20 μM staurosporine (Sigma, Austria) for 4 h served as positive controls for apoptosis. Cells were harvested using accutase (Sigma, USA), centrifuged and washed twice in binding buffer (in mM: NaCl 140, CaCl2 2.5, HEPES 10, pH 7.4). 1 × 106–2 × 106 cells/sample were stained with FITC-conjugated Annexin-V (ImmunoTools, Germany) for 20 min. After two washes with binding buffer, 5 μl of 7-AAD (7-AMINI-ACTINOMYCIN D) viability stain solution (BioLegend, Inc.) was added to each sample (final volume 0.5 ml). After 15 min, cells were used for flow cytometry. All preparation steps were performed at room temperature in the dark. Fluorescence emissions of FITC-Annexin-V on FL-1 (525 nm band pass filter) and 7-AAD on FL-3 (670 nm long pass filter) were measured upon excitation with a 488-nm argon laser using a Cell Lab Quanta™ SC flow cytometer (Beckman-Coulter). Unstained and single-stained samples were used for setting the electronic volume (EV) gain, FL-1 and FL-3 PMT-voltages and for compensation of FITC-spill over into the 7-AAD channel. Debris (particles diameter < 7 μm) and cell aggregates (>20 μm) were excluded from analysis. 20,000–30,000 single cells (diameter 7–20 μm) were analyzed in each sample and depicted in FL-3 versus FL-1 dot plots. Quadrant regions were set to segregate cells in four different populations: 7-AAD–/Annexin-V – cells were considered as non-apoptotic, 7-AAD−/Annexin-V+ cells as early apoptotic, 7-AAD+/Annexin-V+ cells as late apoptotic, and 7-AAD+/Annexin-V – cells as post-late apoptotic/necrotic. Cell diameter/volume was directly measured with the Cell Lab Quanta™ employing the Coulter principle for volume assessment, which is based on measuring changes in electrical resistance produced by nonconductive particles suspended in an electrolyte solution. The electronic volume channel was calibrated using 10 μm Flow-Check fluorospheres (Beckman-Coulter) by positioning this size bead in channel 200 on the volume scale. Data were graphed as side-scatter versus electronic volume (EV) dot plots.
2.4. Cell cycle measurements
For assessing the cell cycle distribution, HT-29 cells were seeded in 100 mm Petri dishes at a density of 120,000/ml and grown for 22 h at 37 °C, 5% CO2 and 95% air in the presence of 5.0, 10, or 20 μM curcumin, or 0.05% DMSO (solvent control). Cells were detached by accutase treatment, centrifuged and washed twice with phosphate buffered saline (PBS; in mM: NaCl 136.9, KCl 2.69, Na2HPO4 3.21, K2HPO4 1.47). 106–2 × 106 cells/sample were incubated in nuclear isolation and staining medium containing 4′,6-diamidino-2-phenylindole (DAPI, NPE systems) for 10 min at room temperature. Isolated nuclei were filtered through a 40-μm nylon mesh and analyzed on a Cell Lab Quanta™ SC flow cytometer. The excitation light from the mercury arc lamp was passed through a 355/37 nm band-pass filter. The emission light was directed towards the photomultiplier tube by a dichroic mirror (cut-off 550 nm) and passed through a 465/30 nm band-pass filter. 20,000–40,000 single nuclei were analyzed per sample.
2.5. Salts, chemicals and drugs
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1E,6E-heptadiene-3,5-dione, MW = 368.4, CAS Registry No.: 458-37-7, Cat. No.: 81025, Lot No.: 191793-2) was purchased from Cayman Chemical Company, Ann Arbor, MI, USA. All salts and chemicals used were of “pro analysis” grade.
2.6. Statistical analysis
All data are expressed as arithmetic means ± S.E.M. For statistical analysis, GraphPad Prism software (version 4.00 for Windows, GraphPad Software, San Diego, CA, USA) was used. Significant differences between means were tested by paired, unpaired Student's t-test or one way ANOVA with Dunnet's post-test as appropriate. Statistically significant differences were assumed at p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001); (n) corresponds to the number of cells tested (patch clamp) or to the number of independent experiments (flow cytometry). When indicated, the current density-to-time and current density-to-voltage relationships were fitted with second order polynomials (Y = A + BX + CX2). For detecting significant differences between those data, the extra-sum of squares F test was applied. Statistically significant differences were assumed at p < 0.05.
3. Results
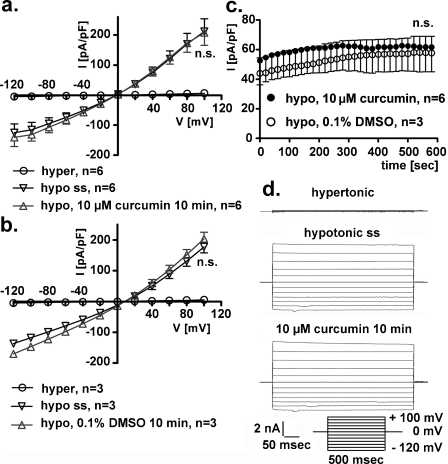
3.1. Short-term, extracellular exposure to 10 μM curcumin does not affect IClswell
In HEK293 Phoenix cells, a seal was established and the whole cell configuration was obtained in extracellular hypertonic solution. Subsequently, IClswell was activated following reduction of the extracellular osmolarity by the omission of mannitol (see Section 2). As previously reported in HEK293 Phoenix cells (Gandini et al., 2008), hypotonic shock induced the activation of a large chloride current with the biophysical fingerprints of IClswell (outward rectification, slow voltage and time dependent inactivation at positive potentials, reversal potential close to the equilibrium potential of chloride in our experimental conditions, Fig. 1a, b and d). After the current reached its maximal activation (i.e. at steady state; hypo ss), 10 μM curcumin (Fig. 1a) or 0.1% DMSO (Fig. 1b) was added to the extracellular hypotonic solution for 10 min. However, no significant difference in IClswell was detected in cells exposed to either 10 μM curcumin or 0.1% DMSO (paired Student's t-test). Fig. 1c shows the current density-to-time relation in hypotonic solution and in the presence of curcumin or DMSO; accordingly, no effect of curcumin was detected (paired Student's t-test). Similar results were obtained after the addition of curcumin to cells kept in extracellular isotonic solution, demonstrating that curcumin is unable to directly stimulate IClswell in HEK293 Phoenix cells under these conditions (paired Student's t-test, data not shown).
Fig. 1.
Short-term exposure to extracellular 10 μM curcumin does not affect IClswell. IClswell was activated in human renal cells (HEK293 Phoenix) by hypotonic shock and measured by the patch clamp technique in whole cell configuration. (a) Current density-to-voltage relations measured in extracellular hypertonic solution (hyper), at the maximal activation of the current in extracellular hypotonic solution (or steady state (hypo ss)), and after a 10 min exposure to an extracellular hypotonic solution containing 10 μM curcumin. (b) Current density-to-voltage relations measured in extracellular hypertonic solution (hyper), at the maximal activation of the current in extracellular hypotonic solution (or steady state (hypo ss)) and after a 10 min exposure to an extracellular hypotonic solution containing 0.1% DMSO as the vehicle. The current is not affected by 10 min exposure to curcumin or DMSO (paired Student's t-test). (c) Current density-to-time graph showing the current in extracellular hypotonic solution containing 10 μM curcumin or 0.1% DMSO; the current is not affected by 10 min exposure to curcumin or DMSO alone (paired Student's t-test). (d) Original recordings obtained in hypertonic solution (upper trace), in hypotonic conditions after the current reached its maximal activation or steady state (ss, middle trace) and 10 min after exposure to an extracellular hypotonic solution containing 10 μM curcumin (lower trace). Currents where elicited with voltage increments of 20 mV from −120 to +100 mV applied from a holding potential of 0 mV (lower right inset).
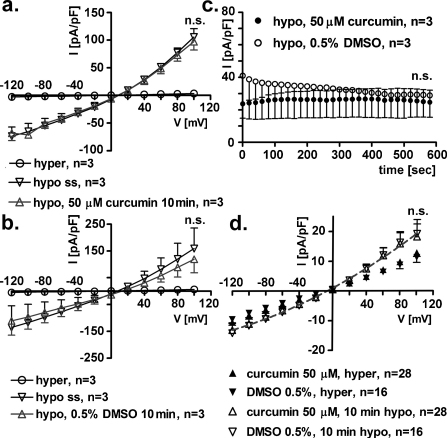
3.2. Short-term, extracellular or intracellular exposure to 50 μM curcumin does not affect IClswell
The same experimental design described above was employed with 50 μM curcumin or 0.5% DMSO (vehicle). Current density-to-voltage relations show that a 10 min extracellular exposure to neither curcumin (Fig. 2a) nor DMSO (Fig. 2b) following hypotonic shock had an effect on IClswell (paired Student's t-test). Fig. 2c shows the time course of the current elicited in hypotonic solution in the presence of curcumin or DMSO; accordingly, no effect of curcumin or DMSO was detected (paired Student's t-test). Similar experiments were performed after adding 50 μM curcumin or 0.5% DMSO to the pipette filling solution (Fig. 2d); after establishing the whole cell configuration, the substances dissolved in the pipette filling solution have access to the intracellular space. The current density-to-voltage relations were measured in hypertonic extracellular solution and after 10 min of hypotonic shock; no differences were detected between IClswell measured in the presence of intracellular 50 μM curcumin or 0.5% DMSO (F test).
Fig. 2.
Short-term exposure to extracellular or intracellular 50 μM curcumin does not affect IClswell. IClswell was activated in human renal cells (HEK293 Phoenix) by hypotonic shock and measured by the patch clamp technique in whole cell configuration. (a) Current density-to-voltage relations measured in extracellular hypertonic solution (hyper), at the maximal activation of the current in extracellular hypotonic solution (or steady state (hypo ss)), and after a 10 min exposure to an extracellular hypotonic solution containing 50 μM curcumin. (b) Current density-to-voltage relations measured in extracellular hypertonic solution (hyper), at the maximal activation of the current in extracellular hypotonic solution (or steady state (hypo ss)) and after a 10 min exposure to an extracellular hypotonic solution containing 0.5% DMSO as the vehicle. The current is not affected by a 10 min exposure to curcumin or DMSO (paired Student's t-test). (c) Current density-to-time graph showing the current in extracellular hypotonic solution containing 50 μM curcumin or 0.5% DMSO; the current is not affected by a 10 min exposure to curcumin or DMSO (paired Student's t-test). (d) Current density-to-voltage relation measured in extracellular hypertonic solution (hyper) and after a 10 min exposure to extracellular hypotonic solution (10 min hypo) in cells where 50 μM curcumin or 0.5% DMSO was added to the pipette filling (intracellular) solution. Data were fitted with second order polynomials (dotted line), following application of the extra-sum of squares F test and showed no effect of intracellular, short-term incubation with 50 μM curcumin.
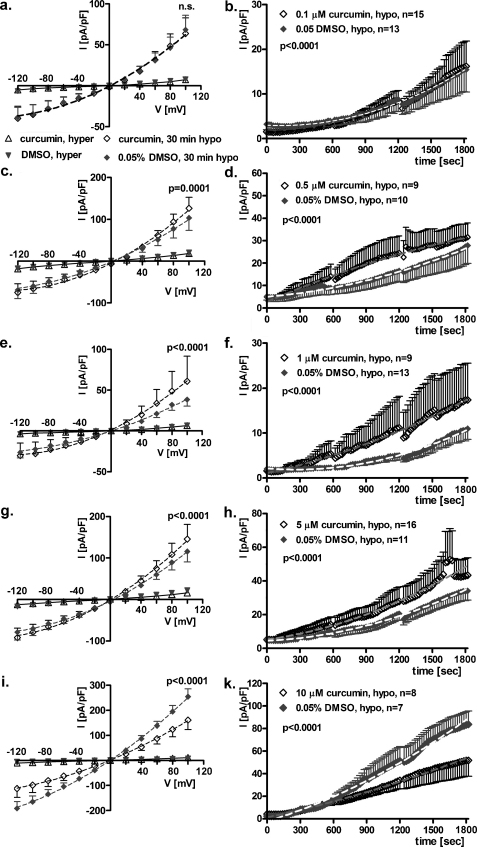
3.3. Long-term, extracellular exposure to 0.1–10 μM curcumin modulates IClswell
Fig. 3a–k show the results of patch clamp experiments obtained from HEK293 Phoenix cells after a long-term exposure (15–23 h in the medium used for cell growth) to 0.1–10 μM curcumin or 0.05% DMSO (vehicle). In contrast to the experiments described above, curcumin or DMSO were not present in the extracellular solutions during current recordings. After establishing the seal, IClswell was activated as mentioned above. The current density-to-voltage relations (Fig. 3a, c, e, g and i) were determined in extracellular hypertonic solution and every 10 min for 30 min in extracellular hypotonic solution. Long-term exposure to 0.1 μM curcumin (Fig. 3a) did not affect IClswell (F test); in contrast, 0.5, 1.0 and 5.0 μM curcumin (Fig. 3c, e and g) significantly up-regulated IClswell (curcumin vs DMSO: p = 0.0001, p < 0.0001 and p < 0.0001 respectively, F test). Surprisingly, a further increase in curcumin concentration led to the opposite effect. As shown in Fig. 3i, long-term exposure to 10 μM curcumin significantly impaired IClswell activation with respect to DMSO (p < 0.0001, F test). The respective current density-to-time relations determined for 30 min in extracellular hypotonic solution (Fig. 3b, d, f, h and k) showed similar results.
Fig. 3.
Long-term exposure to extracellular 0.1–10 μM curcumin modulates IClswell. IClswell was activated in human renal cells (HEK293 Phoenix) by hypotonic shock and measured by the patch clamp technique in whole cell configuration. Current density-to-voltage relations measured in extracellular hypertonic solution (hyper), and after a 30 min exposure to an extracellular hypotonic solution (30 min hypo) in cells incubated 15–23 h in a complete medium containing (a) 0.1, (c) 0.5, (e) 1.0, (g) 5.0, (i) and 10 μM curcumin or 0.05% DMSO. Data were fitted with second order polynomials (dotted lines), following application of the extra-sum of squares F test, and showed that 0.1 μM curcumin had no effect, 0.5–5.0 μM improved and 10 μM impaired the activation of IClswell. The respective current density-to-time relations (b, d, f, h and k) measured in extracellular hypotonic solution showed similar results.
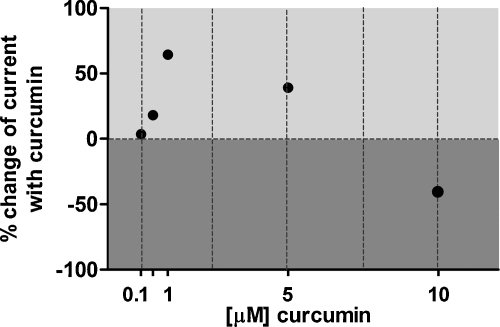
The effects on IClswell induced by the long-term exposure of curcumin are summarized in Fig. 4. The % change of the current determined 30 min following hypotonic shock in cells incubated with curcumin with respect to DMSO is shown. The data clearly indicate that increasing the concentration of curcumin from 0.1 to 1.0 μM increased IClswell. Upregulation of the current reached its maximum (∼64%) with 1.0 μM curcumin. Further increases in curcumin concentration did not lead to a further increase in IClswell; in contrast, the effect of 5.0 μM curcumin became weaker compared to 1 μM, and with 10 μM curcumin, the effect on IClswell was reversed (an inhibition of ∼40% was observed).
Fig. 4.
% change of IClswell following long-term exposure to extracellular 0.1–10 μM curcumin. The % change in current was calculated from the current density-to-time relations represented in Fig. 3, i.e. after a 30 min exposure to an extracellular hypotonic solution in cells incubated 15–23 h in a complete medium containing 0.1, 0.5, 1.0, 5.0 and 10 μM curcumin respectively or 0.05% DMSO. 0.5–5.0 μM curcumin activated the current (∼64% with 1.0 μM curcumin), while 10 μM curcumin suppressed the current (∼40%).
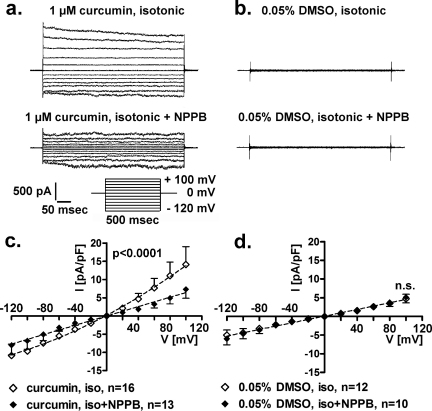
3.4. Long-term, extracellular exposure to 1 μM curcumin in isotonic conditions activates IClswell
Fig. 5 shows the results of patch clamp experiments obtained in isotonic conditions from HEK293 Phoenix cells following long-term exposure (15–23 h in the medium used for cell growth) to 1.0 μM curcumin or 0.05% DMSO (vehicle). The chloride current was measured in the whole-cell configuration after a time frame suitable to allow the dialysis of the intracellular components; curcumin or DMSO were not added to the solutions during current recordings. Long-term exposure to 1.0 μM curcumin (Fig. 5a and c) activated a chloride current showing the biophysical fingerprints of IClswell (i.e. outward rectification, time and voltage dependent inactivation at potentials more positive than +40 mV). This current was significantly blunted (∼50%) by the chloride channel inhibitor NPPB (Fig. 5a, c, p < 0.0001, F test). In contrast, no chloride current was detected under isotonic conditions in cells after a long-term incubation with 0.05% DMSO as a control. Accordingly, NPPB did not show an effect (Fig. 5b and d, n.s., F test).
Fig. 5.
Long-term exposure to extracellular 1 μM curcumin activates a chloride current resembling IClswell in isotonic conditions. The chloride current was measured in human renal cells (HEK293 Phoenix) by the patch clamp technique in whole cell configuration. Original recordings obtained in isotonic solution (upper trace) and isotonic solution after addition of 75 μM NPPB (lower trace) in cells incubated 15–23 h in a complete medium containing (a) 1.0 μM curcumin or (b) 0.05% DMSO as a control are shown. Currents were elicited with voltage increments of 20 mV from −120 to +100 mV applied from a holding potential of 0 mV (a, lower right inset). Respective current density-to-voltage relations obtained in isotonic solution (iso) and isotonic solution after addition of NPPB (iso + NPPB) in cells incubated with 1.0 μM curcumin or 0.05% DMSO as a control are shown in (c) and (d), respectively. NPPB inhibited the chloride current by ∼50% in c (p < 0.0001, F test) and was ineffective in d (n.s., F test), indicating that a chloride current was present after incubation with curcumin (c) but not with DMSO (d).
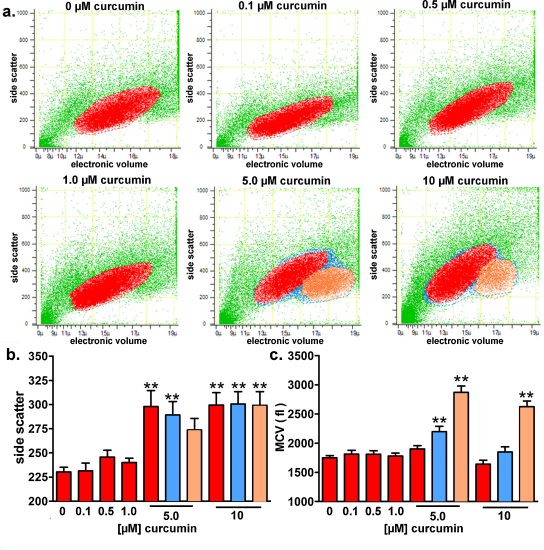
3.5. Long-term exposure to curcumin induces apoptosis in HEK293 Phoenix cells and the appearance of a new sub-population of cells with a nearly doubled volume compared to the main population
We wondered if the stimulating effect of curcumin on IClswell in isotonic conditions might be triggered by the mechanisms orchestrating apoptosis. Flow cytometry was used to investigate the possible pro-apoptotic effect of long-term exposure (19 h in the medium used for cell growth) of cells to 0.1–10 μM curcumin. This technique allows for the detection of morphological signs of apoptosis; i.e. increased cell granularity (in terms of an increased side scatter signal), as well as cell shrinkage (apoptotic volume decrease). As expected, 4 h incubation with 20 μM staurosporine, a well-known apoptosis inducer (Tamaoki et al., 1986), led to a significant increase in side scatter and decrease in cell volume (data not shown). Exposure to 5.0 and 10 μM curcumin significantly increased the side scatter signal (Fig. 6b, red bars) of the main population of cells (depicted in red in Fig. 6a), indicating an increase in cell granularity, which is a hallmark of apoptosis (Bertho et al., 2000). Interestingly, exposure to 5.0 and 10 μM curcumin led to the appearance of a sub-population of cells (depicted in orange in Fig. 6a) with a nearly doubled volume (Fig. 6c, orange bars) as compared to the main population. The increased side scatter of this “swollen” cell population indicates that they are also in the apoptotic state.
Fig. 6.
Long-term exposure to curcumin induces apoptosis in HEK293 Phoenix cells and the appearance of a new sub-population of cells with a nearly doubled volume. The effect of long-term exposure (19 h in a complete medium) to 0.1–10 μM curcumin on the side scatter and cell volume was investigated on HEK293 Phoenix cells by flow cytometry. Control cells were incubated with 0.05% DMSO. (a) Original dot plots of the side scatter (expressed in arbitrary units) versus cell diameter (μm; electronic cell volume); red, orange and blue colors indicate the main population of cell with normal volume, the new cell population with an increased volume and the sum of the two populations, respectively. Bar diagrams of (b) the mean side scatter and (c) the mean cell volume (MCV, fl); red, orange and blue bars refer to the main population of cells with normal volume, the new cell population with an increased volume and the sum of the two populations, respectively. **p < 0.01, one way ANOVA with Dunnet's post-test. Data were collected from 6 independent experiments.
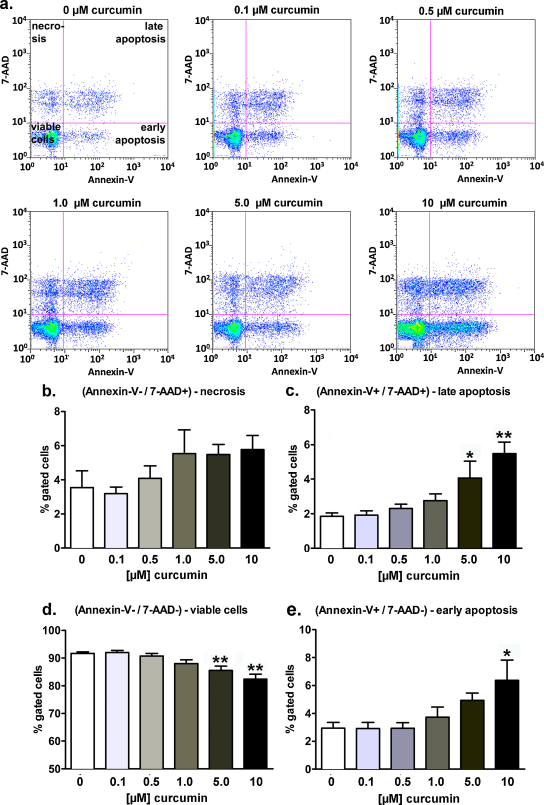
3.6. Long-term exposure to curcumin decreases viability of HEK293 Phoenix cells and induces apoptosis in a dose-dependent manner
The pro-apoptotic effect of long-term exposure (19 h in the medium used for cell growth) to 0.1-10 μM curcumin in the main population of cells (depicted in red in Fig. 6a) was further investigated by flow cytometry (Fig. 7). Quadrant regions (Fig. 7a) were set to segregate cells into four different populations: 7-AAD negative/Annexin-V negative cells were considered as non-apoptotic, non-necrotic (viable), 7-AAD negative/Annexin-V positive cells as early apoptotic, 7-AAD positive/Annexin-V positive cells as late apoptotic, and 7-AAD positive/Annexin-V negative cells as post-late apoptotic/necrotic. As expected, 4 hours incubation with 20 μM staurosporine led to a significant increase of the percentage of cells in the early and late apoptosis, paralleled by a respective significant decrease of the percentage of viable (non apoptotic, non-necrotic) cells (data not shown). Exposure to 10 μM curcumin significantly increased the percentage of cells in the early apoptosis state (Fig. 7e). The percentage of late apoptotic cells was significantly increased after treatment with both 5.0 and 10 μM curcumin (Fig. 7c). Accordingly, after incubation with 5.0 and 10 μM curcumin, the number of viable (non-apoptotic, non-necrotic) cells was significantly decreased (Fig. 7d), whereas the percentage of necrotic cells was not significantly affected (Fig. 7b).
Fig. 7.
Long-term exposure to curcumin decreases cell viability and induces apoptosis in HEK293 Phoenix cells in a dose-dependent manner. The pro-apoptotic effect of long-term exposure (19 h in a complete medium) to 0.1–10 μM curcumin was investigated on HEK293 Phoenix cells by flow cytometry. Control cells were incubated with 0.05% DMSO. (a) Original dot plots of the 7-AAD versus the Annexin-V/FITC fluorescence intensities (logarithmic scales). 7-AAD negative/Annexin-V negative cells were considered as non-apoptotic, non-necrotic (lower left quadrant, viable cells), 7-AAD negative/Annexin-V positive cells as early apoptotic (lower right quadrant), 7-AAD positive/Annexin-V positive cells as late apoptotic (upper right quadrant), and 7-AAD positive/Annexin-V negative cells as post-late apoptotic/necrotic (upper left quadrant). Bar diagrams show the percentages of (b) necrotic, (c) late apoptotic, (d) non-apoptotic, non-necrotic (viable), and (e) early apoptotic cells. *p < 0.05, **p < 0.01, one way ANOVA with Dunnet's post-test. Data were collected from 6 independent experiments.
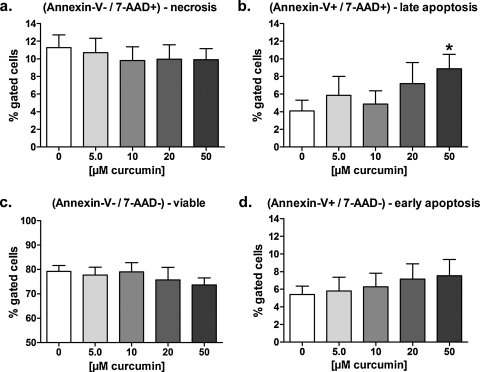
3.7. Long-term exposure to curcumin induces apoptosis and volume increase in HT-29 cells
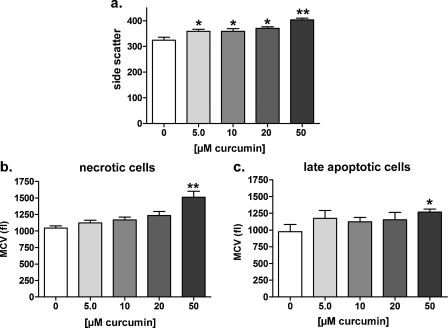
To verify if the effects induced by long-term exposure to curcumin in HEK293 Phoenix cells are restricted to this particular cell line, flow cytometry was used to investigate the possible pro-apoptotic effect of long-term exposure (22 h in the medium used for cell growth) on human colorectal adenocarcinoma HT-29 cells to 5.0–50 μM curcumin. Exposure to 50 μM curcumin significantly increased the percentage of 7-AAD positive/Annexin-V positive cells (Fig. 8b), clearly indicating a pro-apoptotic effect. Accordingly, a significant increase in the side scatter signal was observed (Fig. 9a). Surprisingly, curcumin-induced cell death in these cells was paralleled by a significant increase in the volume of necrotic (Fig. 9b) and late apoptotic (Fig. 9c) cells.
Fig. 8.
Long-term exposure to curcumin induces apoptosis in HT-29 cells in a dose-dependent manner. The pro-apoptotic effect of long-term exposure (22 h in a complete medium) to 5.0–50 μM curcumin was investigated on HT-29 cells by flow cytometry. Control cells were incubated with 0.05% DMSO. 7-AAD negative/Annexin-V negative cells were considered as non-apoptotic, non-necrotic (viable), 7-AAD negative/Annexin-V positive cells as early apoptotic, 7-AAD positive/Annexin-V positive cells as late apoptotic and 7-AAD positive/Annexin-V negative cells as post-late apoptotic/necrotic. Bar diagrams show the percentages of (a) necrotic, (b) late apoptotic, (c) non-apoptotic, non-necrotic (viable), and (d) early apoptotic cells. *p < 0.05, unpaired Student's t-test. Data were collected from at least four independent experiments.
Fig. 9.
Long-term exposure to curcumin increases the side scatter and volume of HT-29 cells in a dose-dependent manner. The effect of long-term exposure (22 h in a complete medium) to 5.0–50 μM curcumin on the side scatter and cell volume was investigated in HT-29 cells by flow cytometry. Control cells were incubated with 0.05% DMSO. Bar diagrams of (a) the mean side scatter of the whole population of cells and the mean cell volume (MCV, fl) of (b), necrotic and (c) late apoptotic cells. *p < 0.05, **p < 0.01, one way ANOVA with Dunnet's post-test or unpaired Student's t-test. Data were collected from at least four independent experiments.
3.8. Long-term exposure to curcumin induces G1-phase arrest and S-phase depression in HT-29 cells
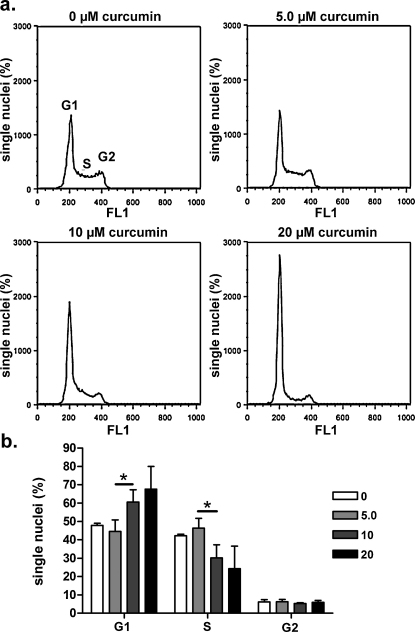
To gain further insights about the mechanisms of the curcumin-induced cell volume increase, the cell cycle distribution of HT-29 cells after exposure to curcumin was assessed. Isolated nuclei were stained with DAPI and analyzed by flow cytometry. Long-term exposure (22 h in the medium used for cell growth) to 0.5–20 μM curcumin significantly increased the percentage of cells in G1-phase and decreased the percentage of cells in S-phase (Fig. 10a and b), thereby suggesting a cell cycle arrest in G1-phase.
Fig. 10.
Long-term exposure to curcumin induces G1-phase arrest and S-phase depression in HT-29 cells. The cell cycle distribution of HT-29 cells after long-term (22 hours) exposure to 5.0–20 μM curcumin added to the cell culture medium was assessed by analyzing isolated nuclei stained with DAPI using flow cytometry. Control cells were incubated with 0.05% DMSO. (a) Histograms showing the distribution of single nuclei in the G1, S and G2 phase in absence and presence of 5.0, 10, or 20 μM curcumin. (b) Bar diagrams show the percentages of single nuclei in the G1, S and G2 phase. *p < 0.05, unpaired Student's t-test. Data were collected from at least four independent experiments.
4. Discussion
Curcumin is an active compound of turmeric for which anticancer, antioxidative and antiinflammatory properties have been described. Typically, clinical trials show negligible free curcumin plasma levels with oral dosing (Anand et al., 2007; Baum et al., 2008; Lao et al., 2006). However, curcumin is a highly hydrophobic molecule, and it could accumulate in some body compartments (such as adipose tissue and brain) after repetitive exposure. Indeed, Begum et al. (2008) demonstrated that micromolar concentrations of curcumin accumulates in rat brain after chronic administration. Increasing curcumin solubility with phosphatidyl choline, olive oil, stearic acid or lipid-rich diet, increases the amount of curcumin in plasma and brain (Begum et al., 2008). In this work, we set out to investigate the short and long-term effect of 0–50 μM curcumin in a human renal and intestinal cell line. Indeed, kidney cells may be involved in excretion of curcumin and/or its metabolites and intestinal cells may be exposed to relatively high curcumin concentrations after ingestion of a meal containing this spice.
Considerable effort has been devoted in the last years in identifying the molecular targets responsible for curcumin related effects. Increasing evidence indicates that cationic channels (selective for calcium or potassium and unselective cation channels (Enyeart et al., 2008, 2009; Liu et al., 2006; Shin et al., 2011; Yeon et al., 2010)) can be blocked by extracellular curcumin. In contrast to cationic channels, chloride channels seem to be activated by curcumin. This was shown for CFTR and two of its mutants found in patients suffering from cystic fibrosis, i.e. G551D (Yu et al., 2011) and ΔF508-CFTR (Berger et al., 2005; Wang et al., 2005, 2007). Wang et al. pointed out that the structure of curcumin (two aromatic rings separated by a hydrocarbon spacer) is similar to that of NPPB-AM, an uncharged NPPB derivative that activates CFTR. It is important to note that the effect of curcumin on CFTR and its mutants is controversial (Egan et al., 2004; Grubb et al., 2006; Lipecka et al., 2006; Norez et al., 2006). Besides the possible effect of curcumin on CFTR, little is known about a possible effect of curcumin on other chloride channels. Best et al. (2007) describe an activation of IClswell in rat pancreatic cells by curcumin. Curcumin was shown to have pro-apoptotic activity (Shankar et al., 2007; Shankar and Srivastava, 2007a), and might therefore be a candidate for cancer treatment (Kelkel et al., 2010). Since the molecular structure of curcumin is reminiscent of a substance that could interact with a chloride channel (Wang et al., 2005), and IClswell is activated during RVD following hypotonic stress and as an early event in apoptosis (in isotonic conditions), we set out to investigate the link between curcumin, IClswell and apoptosis. In contrast to Best et al. (2007), we did not detect a direct stimulatory effect of curcumin on IClswell in isotonic conditions. It is important to note, however, that we used a human kidney cell line as opposed to the Best et al. study, which used murine (rat) pancreatic β-cell primary cultures. Interestingly, however, is our finding that long-term exposure (15–23 h in the medium used for cell growth) to 0.1–10 μM extracellular curcumin modulates IClswell in a dose-dependent manner in a human epithelial cell model. Particularly, 0.5–5.0 μM curcumin up-regulates IClswell, while 10 μM curcumin down-regulates IClswell current (Figs. 3 and 4). The current up-regulation reached its maximal extent with 1.0 μM curcumin. This effect could not be ascribed to a direct action of curcumin on the channel since short-term exposure with similar concentrations of curcumin applied to either the extracellular or intracellular side did not affect IClswell (Figs. 1 and 2). In agreement with previous reports, long-term exposure to curcumin induced apoptosis in the HEK293 Phoenix cells (Figs. 6b and 7). As it is known that IClswell activation is an early event in apoptosis and a key step in apoptotic volume decrease (Okada et al., 2006), we hypothesized that the observed IClswell up-regulation by curcumin is the consequence of the induction of apoptosis. Indeed, the swelling-activated chloride channel and the chloride channel triggering the apoptotic volume decrease are likely the same molecular entity (Okada et al., 2006; Pasantes-Morales and Tuz, 2006). In agreement with this hypothesis, long-term exposure to curcumin also induces the activation of a chloride current resembling IClswell in the absence of hypotonic shock (Fig. 5). Interestingly, we showed for the first time that a long-term exposure to 5.0–10 μM curcumin resulted in the appearance of a sub-population of cells with a volume nearly double that of the main cell population (Fig. 6a and c). In these “swollen” cells, volume regulation appears to be impaired and underscores the principle that basal cell volume is slightly smaller than the equilibrium would predict (Cao et al., 2011), most likely by active IClswell. We hypothesize that derangement of cell volume regulation is a possible consequence of the IClswell blockade that was observed with higher curcumin concentrations (Figs. 3i and k and 4). Accordingly, Light et al. showed that 20 μM curcumin could inhibit cell volume regulation in mudpuppy red blood cells; although this effect was attributed to inhibition of the 5-lipoxygenase pathway (Light et al., 1997).
The curcumin-induced derangement of cell viability and cell volume is not restricted to renal HEK293 Phoenix cells. Indeed, 5.0–50 μM curcumin induced apoptosis in intestinal HT-29 cells, evidenced as an increase of Annexin-V binding (Fig. 8b) and side scatter (Fig. 9a). Surprisingly, cell death in these cells was not accompanied by the typical apoptotic cell shrinkage. Indeed, the volume of necrotic (Fig. 9b) and late apoptotic (Fig. 9c) cells was significantly increased. Interestingly, a cell cycle arrest in G1 phase is often observed following exposure to a variety of substances (such as hydrogen peroxide, vitamin D and prostaglandin E2) (Artaza et al., 2010; Oyama et al., 2011; Qian et al., 2009) that can induce increased cell size and hypertrophy. These considerations prompted us to verify if the progression of the cell cycle in curcumin-treated HT-29 cells was deranged. Indeed, long-term exposure to 5.0–20 μM curcumin induced G1-phase arrest and S-phase depression (Fig. 10) in HT-29 cells. While the cell cycle arrest may explain the increased volume observed in curcumin-treated HT-29 cells, the underlying mechanisms leading to the deranged progression of cell cycle in these cells need to be elucidated. It is worth to note however, that an arrest of cell growth in the G0/G1 phase is often associated with a significant decrease in IClswell (He et al., 2011; Klausen et al., 2007; Shen et al., 2000).
Curcumin induces apoptosis through a wide variety of mechanisms (Reuter et al., 2008). These mechanisms include the activation of the mitochondrial pathway via activation of Bax/Bak (Shankar and Srivastava, 2007b) or BID (Anto et al., 2002). Moreover, evidence exists that curcumin activated caspase 3 and 8 with no activation of caspase 9, raising the hypothesis of an activation of a death receptor-dependent (non-mitochondrial) pathway via FasL-independent aggregation of Fas receptors (Bush et al., 2001). In addition, activation by curcumin of novel apoptosis-like pathways, independent of mitochondria and caspases, was described (Piwocka et al., 1999). Therefore, it is likely that curcumin could induce apoptosis also when the mitochondrial pathway is blocked.
5. Conclusion
From the presented data we conclude that curcumin is able to affect cell survival and cell volume in a dose-dependent manner. At lower concentrations (<5.0 μM), curcumin indirectly activates IClswell, which is most likely the result of apoptosis induction. Higher curcumin concentrations (≥5.0 μM) indirectly lead to an inhibition of IClswell, an arrest of cell cycle in G1-phase and hence to cell swelling.
Funding
Charity Nofziger is supported by the Lise Meitner stipend of the Fonds zur Förderung der Wissenschaftlichen Forschung (FWF) (M11108-B11). The experimental work was further supported by the FWF and the FP-7 to M.P. (P18608; PIRSES-GA-2008-230661).
Conflict of interest statement
None.
Acknowledgements
We greatly appreciated the helpful discussion with M. Ritter. The authors acknowledge the expert secretarial assistance of Elisabeth Mooslechner.
Contributor Information
Silvia Dossena, Email: silvia.dossena@pmu.ac.at.
Markus Paulmichl, Email: markus.paulmichl@pmu.ac.at.
References
- Aggarwal B.B., Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol. Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Alappat L., Awad A.B. Curcumin and obesity: evidence and mechanisms. Nutr. Rev. 2010;68:729–738. doi: 10.1111/j.1753-4887.2010.00341.x. [DOI] [PubMed] [Google Scholar]
- Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Anto R.J., Mukhopadhyay A., Denning K., Aggarwal B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: its suppression by ectopic expression of Bcl-2 and Bcl-xl. Carcinogenesis. 2002;23:143–150. doi: 10.1093/carcin/23.1.143. [DOI] [PubMed] [Google Scholar]
- Artaza J.N., Sirad F., Ferrini M.G., Norris K.C. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J. Steroid Biochem. Mol. Biol. 2010;119:73–83. doi: 10.1016/j.jsbmb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier B.E., Killian P., Pfeffer U., Nerlich A.G. Novel aspects for the application of Curcumin in chemoprevention of various cancers. Front. Biosci. (Schol. Ed) 2010;2:697–717. doi: 10.2741/s95. [DOI] [PubMed] [Google Scholar]
- Bar-Sela G., Epelbaum R., Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr. Med. Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- Baum L., Lam C.W., Cheung S.K., Kwok T., Lui V., Tsoh J., Lam L., Leung V., Hui E., Ng C., Woo J., Chiu H.F., Goggins W.B., Zee B.C., Cheng K.F., Fong C.Y., Wong A., Mok H., Chow M.S., Ho P.C., Ip S.P., Ho C.S., Yu X.W., Lai C.Y., Chan M.H., Szeto S., Chan I.H., Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Begum A.N., Jones M.R., Lim G.P., Morihara T., Kim P., Heath D.D., Rock C.L., Pruitt M.A., Yang F., Hudspeth B., Hu S., Faull K.F., Teter B., Cole G.M., Frautschy S.A. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer's disease. J. Pharmacol. Exp. Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A.L., Randak C.O., Ostedgaard L.S., Karp P.H., Vermeer D.W., Welsh M.J. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. J. Biol. Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- Bertho A.L., Santiago M.A., Coutinho S.G. Flow cytometry in the study of cell death. Membr. Inst. Oswaldo Cruz. 2000;95:429–433. doi: 10.1590/s0074-02762000000300020. [DOI] [PubMed] [Google Scholar]
- Best L., Elliott A.C., Brown P.D. Curcumin induces electrical activity in rat pancreatic beta-cells by activating the volume-regulated anion channel. Biochem. Pharmacol. 2007;73:1768–1775. doi: 10.1016/j.bcp.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bush J.A., Cheung K.J., Jr., Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp. Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- Cao G., Zuo W., Fan A., Zhang H., Yang L., Zhu L., Ye W., Wang L., Chen L. Volume-sensitive chloride channels are involved in maintenance of basal cell volume in human acute lymphoblastic leukemia cells. J. Membr. Biol. 2011;240:111–119. doi: 10.1007/s00232-011-9349-7. [DOI] [PubMed] [Google Scholar]
- Cao J., Jia L., Zhou H.M., Liu Y., Zhong L.F. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol. Sci. 2006;91:476–483. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- DiCiommo D.P., Duckett A., Burcescu I., Bremner R., Gallie B.L. Retinoblastoma protein purification and transduction of retina and retinoblastoma cells using improved alphavirus vectors. Invest. Ophthalmol. Vis. Sci. 2004;45:3320–3329. doi: 10.1167/iovs.04-0140. [DOI] [PubMed] [Google Scholar]
- Egan M.E., Pearson M., Weiner S.A., Rajendran V., Rubin D., Glockner-Pagel J., Canny S., Du K., Lukacs G.L., Caplan M.J. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- Enyeart J.A., Liu H., Enyeart J.J. Curcumin inhibits bTREK-1 K+ channels and stimulates cortisol secretion from adrenocortical cells. Biochem. Biophys. Res. Commun. 2008;370:623–628. doi: 10.1016/j.bbrc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyeart J.A., Liu H., Enyeart J.J. Curcumin inhibits ACTH- and angiotensin II-stimulated cortisol secretion and Ca(v)3.2 current. J. Nat. Prod. 2009;72:1533–1537. doi: 10.1021/np900227x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk J.L., Oyarzo J.N., Frye J.B., Chen G., Lantz R.C., Jolad S.D., Solyom A.M., Timmermann B.N. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J. Nat. Prod. 2006;69:351–355. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst J., Gschwentner M., Ritter M., Botta G., Jakab M., Mayer M., Garavaglia L., Bazzini C., Rodighiero S., Meyer G., Eichmuller S., Woll E., Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 2002;444:1–25. doi: 10.1007/s00424-002-0805-1. [DOI] [PubMed] [Google Scholar]
- Gandini R., Dossena S., Vezzoli V., Tamplenizza M., Salvioni E., Ritter M., Paulmichl M., Furst J. LSm4 associates with the plasma membrane and acts as a co-factor in cell volume regulation. Cell Physiol. Biochem. 2008;22:579–590. doi: 10.1159/000185542. [DOI] [PubMed] [Google Scholar]
- Glauert H.P., Calfee-Mason K., Stemm D.N., Tharappel J.C., Spear B.T. Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Mol. Nutr. Food Res. 2010;54:875–896. doi: 10.1002/mnfr.200900482. [DOI] [PubMed] [Google Scholar]
- Grubb B.R., Gabriel S.E., Mengos A., Gentzsch M., Randell S.H., Van Heeckeren A.M., Knowles M.R., Drumm M.L., Riordan J.R., Boucher R.C. SERCA pump inhibitors do not correct biosynthetic arrest of deltaF508 CFTR in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2006;34:355–363. doi: 10.1165/rcmb.2005-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Luo X., Wei W., Xie M., Wang W., Yu Z. DCPIB, a specific inhibitor of volume-regulated anion channels (VRACs), inhibits astrocyte proliferation and cell cycle progression Via G1/S Arrest. J. Mol. Neurosci. 2011 doi: 10.1007/s12031-011-9524-4. [DOI] [PubMed] [Google Scholar]
- Jakab M., Furst J., Gschwentner M., Botta G., Garavaglia M.L., Bazzini C., Rodighiero S., Meyer G., Eichmueller S., Woll E., Chwatal S., Ritter M., Paulmichl M. Mechanisms sensing and modulating signals arising from cell swelling. Cell Physiol. Biochem. 2002;12:235–258. doi: 10.1159/000067895. [DOI] [PubMed] [Google Scholar]
- Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- Kelkel M., Jacob C., Dicato M., Diederich M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules. 2010;15:7035–7074. doi: 10.3390/molecules15107035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausen T.K., Bergdahl A., Hougaard C., Christophersen P., Pedersen S.F., Hoffmann E.K. Cell cycle-dependent activity of the volume- and Ca2+-activated anion currents in Ehrlich lettre ascites cells. J. Cell. Physiol. 2007;210:831–842. doi: 10.1002/jcp.20918. [DOI] [PubMed] [Google Scholar]
- Lang F., Shumilina E., Ritter M., Gulbins E., Vereninov A., Huber S.M. Ion channels and cell volume in regulation of cell proliferation and apoptotic cell death. Contrib. Nephrol. 2006;152:142–160. doi: 10.1159/000096321. [DOI] [PubMed] [Google Scholar]
- Lang F., Ritter M., Gamper N., Huber S., Fillon S., Tanneur V., Lepple-Wienhues A., Szabo I., Gulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol. Biochem. 2000;10:417–428. doi: 10.1159/000016367. [DOI] [PubMed] [Google Scholar]
- Lang F., Foller M., Lang K., Lang P., Ritter M., Vereninov A., Szabo I., Huber S.M., Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol. 2007;428:209–225. doi: 10.1016/S0076-6879(07)28011-5. [DOI] [PubMed] [Google Scholar]
- Lao C.D., Ruffin M.T., 4th., Normolle D., Heath D.D., Murray S.I., Bailey J.M., Boggs M.E., Crowell J., Rock C.L., Brenner D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D.B., Mertins T.M., Belongia J.A., Witt C.A. 5-Lipoxygenase metabolites of arachidonic acid regulate volume decrease by mudpuppy red blood cells. J. Membr. Biol. 1997;158:229–239. doi: 10.1007/s002329900260. [DOI] [PubMed] [Google Scholar]
- Lipecka J., Norez C., Bensalem N., Baudouin-Legros M., Planelles G., Becq F., Edelman A., Davezac N. Rescue of DeltaF508-CFTR (cystic fibrosis transmembrane conductance regulator) by curcumin: involvement of the keratin 18 network. J. Pharmacol. Exp. Ther. 2006;317:500–505. doi: 10.1124/jpet.105.097667. [DOI] [PubMed] [Google Scholar]
- Liu H., Danthi S.J., Enyeart J.J. Curcumin potently blocks Kv1.4 potassium channels. Biochem. Biophys. Res. Commun. 2006;344:1161–1165. doi: 10.1016/j.bbrc.2006.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.P., Manheimer E., Yang M. Herbal medicines for treating HIV infection and AIDS. Cochrane Database Syst. Rev. 2005 doi: 10.1002/14651858.CD003937.pub2. CD003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norez C., Antigny F., Becq F., Vandebrouck C. Maintaining low Ca2+ level in the endoplasmic reticulum restores abnormal endogenous F508del-CFTR trafficking in airway epithelial cells. Traffic. 2006;7:562–573. doi: 10.1111/j.1600-0854.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Shimizu T., Maeno E., Tanabe S., Wang X., Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J. Membr. Biol. 2006;209:21–29. doi: 10.1007/s00232-005-0836-6. [DOI] [PubMed] [Google Scholar]
- Oyama K., Takahashi K., Sakurai K. Hydrogen peroxide induces cell cycle arrest in cardiomyoblast H9c2 cells, which is related to hypertrophy. Biol. Pharm. Bull. 2011;34:501–506. doi: 10.1248/bpb.34.501. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Tuz K. Volume changes in neurons: hyperexcitability and neuronal death. Contrib. Nephrol. 2006;152:221–240. doi: 10.1159/000096326. [DOI] [PubMed] [Google Scholar]
- Piwocka K., Zablocki K., Wieckowski M.R., Skierski J., Feiga I., Szopa J., Drela N., Wojtczak L., Sikora E. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp. Cell Res. 1999;249:299–307. doi: 10.1006/excr.1999.4480. [DOI] [PubMed] [Google Scholar]
- Qian Q., Kassem K.M., Beierwaltes W.H., Harding P. PGE2 causes mesangial cell hypertrophy and decreases expression of cyclin D3. Nephron. Physiol. 2009;113:7–14. doi: 10.1159/000232399. [DOI] [PubMed] [Google Scholar]
- Rechtman M.M., Har-Noy O., Bar-Yishay I., Fishman S., Adamovich Y., Shaul Y., Halpern Z., Shlomai A. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1alpha. FEBS Lett. 2010;584:2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Reuter S., Eifes S., Dicato M., Aggarwal B.B., Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem. Pharmacol. 2008;76:1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Ringman J.M., Frautschy S.A., Cole G.M., Masterman D.L., Cummings J.L. A potential role of the curry spice curcumin in Alzheimer's disease. Curr. Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar S., Srivastava R.K. Involvement of Bcl-2 family members, phosphatidylinositol 3’-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int. J. Oncol. 2007;30:905–918. [PubMed] [Google Scholar]
- Shankar S., Srivastava R.K. Bax and Bak genes are essential for maximum apoptotic response by curcumin, a polyphenolic compound and cancer chemopreventive agent derived from turmeric, Curcuma longa. Carcinogenesis. 2007;28:1277–1286. doi: 10.1093/carcin/bgm024. [DOI] [PubMed] [Google Scholar]
- Shankar S., Chen Q., Sarva K., Siddiqui I., Srivastava R.K. Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J. Mol. Signal. 2007;2:10. doi: 10.1186/1750-2187-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M.R., Droogmans G., Eggermont J., Voets T., Ellory J.C., Nilius B. Differential expression of volume-regulated anion channels during cell cycle progression of human cervical cancer cells. The Journal of physiology. 2000;529(Pt 2):385–394. doi: 10.1111/j.1469-7793.2000.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Lee E.L., Ise T., Okada Y. Volume-sensitive Cl(−) channel as a regulator of acquired cisplatin resistance. Anticancer Res. 2008;28:75–83. [PubMed] [Google Scholar]
- Shin D.H., Seo E.Y., Pang B., Nam J.H., Kim H.S., Kim W.K., Kim S.J. Inhibition of Ca2+-release-activated Ca2+ channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. J. Pharmacol. Sci. 2011;115:144–154. doi: 10.1254/jphs.10209FP. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Wang W., Li G., Clancy J.P., Kirk K.L. Activating cystic fibrosis transmembrane conductance regulator channels with pore blocker analogs. J. Biol. Chem. 2005;280:23622–23630. doi: 10.1074/jbc.M503118200. [DOI] [PubMed] [Google Scholar]
- Wang W., Bernard K., Li G., Kirk K.L. Curcumin opens cystic fibrosis transmembrane conductance regulator channels by a novel mechanism that requires neither ATP binding nor dimerization of the nucleotide-binding domains. J. Biol. Chem. 2007;282:4533–4544. doi: 10.1074/jbc.M609942200. [DOI] [PubMed] [Google Scholar]
- White B., Judkins D.Z. Clinical inquiry does turmeric relieve inflammatory conditions? J. Fam. Pract. 2011;60:155–156. [PubMed] [Google Scholar]
- Yeon K.Y., Kim S.A., Kim Y.H., Lee M.K., Ahn D.K., Kim H.J., Kim J.S., Jung S.J., Oh S.B. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J. Dent. Res. 2010;89:170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- Yu Y.C., Miki H., Nakamura Y., Hanyuda A., Matsuzaki Y., Abe Y., Yasui M., Tanaka K., Hwang T.C., Bompadre S.G., Sohma Y. Curcumin and genistein additively potentiate G551D-CFTR. J. Cyst. Fibros. 2011;10:243–252. doi: 10.1016/j.jcf.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Beevers C.S., Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12:332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]