Abstract
It is generally believed that during the sexual transmission of HIV-1, the glycan-specific DC-SIGN receptor binds the virus and mediates its transfer to CD4+ cells. The lectins griffithsin (GRFT), cyanovirin-N (CV-N) and scytovirin (SVN) inhibit HIV-1 infection by binding to mannose-rich glycans on gp120. We measured the ability of these lectins to inhibit both the HIV-1 binding to DC-SIGN and the DC-SIGN-mediated HIV-1 infection of CD4+ cells. While GRFT, CV-N and SVN were moderately inhibitory to DC-SIGN binding, they potently inhibited DC-SIGN-transfer of HIV-1. The introduction of the 234 glycosylation site abolished HIV-1 sensitivity to lectin inhibition of binding to DC-SIGN and virus transfer to susceptible cells. However, the addition of the 295 glycosylation site increased the inhibition of transfer. Our data suggest that GRFT, CV-N and SVN can block two important stages of the sexual transmission of HIV-1, DC-SIGN binding and transfer, supporting their further development as microbicides.
Keywords: Griffithsin, Cyanovirin-N, Scytovirin, mannose-rich glycans, HIV-1 gp120, DC-SIGN receptor
INTRODUCTION
HIV-1 is mainly transmitted through sexual intercourse that accounts for ~80% of infections around the world (http://www.unaids.org). In females, transmission begins when viral particles released into the vaginal tract cross the epithelial cell lining and infect target cells (Shattock and Moore, 2003). Some of these viruses bind to intraepithelial or submucosal dendritic cells (DC) via the interaction of mannose-rich glycans on the HIV-1 envelope with carbohydrate binding receptors such DC-SIGN, DC immune receptor (DCIR) and mannose receptors (Hong et al., 2002; Lambert et al., 2008; Li et al., ; Liu et al., 2004; Piguet and Sattentau, 2004; Pohlmann, Baribaud, and Doms, 2001). Similarly, in men, the foreskin of the penis contains DC that express the DC-SIGN receptor and are believed to play a role in female to male transmission (Fischetti et al., 2009; Hussain and Lehner, 1995; McCoombe and Short, 2006; Patterson et al., 2002; Soilleux and Coleman, 2004). The DC-SIGN receptor is also expressed on rectal mucosa mononuclear cells and may mediate infection, as these cells have been shown to transfer HIV-1 to CD4+ T cells in vitro via this receptor (Gurney et al., 2005).
DC are antigen-presenting cells that become activated upon interaction with an invading pathogen (Piguet and Sattentau, 2004). Following this they migrate to the lymph nodes to stimulate naïve T-helper cells. HIV-1 interaction with the DC-SIGN receptor exploits this process by enabling the virus to reach the lymph nodes and infect CD4+ T cells (Banchereau and Steinman, 1998; Lanzavecchia and Sallusto, 2001). Previous studies suggested that HIV-1 binding to this receptor can result in its internalization by DC, the so called Trojan Horse model of trans-infection (Piguet and Sattentau, 2004; Pohlmann, Baribaud, and Doms, 2001). However, more recent studies dispute this and propose that surface-bound viral particles mediate DC transfer of HIV-1 to susceptible cells (Cavrois, Neidleman, and Greene, 2008; Yu, Reuter, and McDonald, 2008). Nonetheless, in addition to HIV-1 infection in trans (virus transfer to target cells), it has been shown that DC-SIGN can also promote the infection in cis (infection of the cell expressing the receptor) of immature DC and macrophages (Burleigh et al., 2006; Pohlmann, Baribaud, and Doms, 2001).
Like the DC-SIGN receptor, carbohydrate binding agents or lectins, bind to mannose-rich glycans found on HIV-1 envelope (Bokesch et al., 2003; Boyd et al., 1997; Leonard et al., 1990; Mori et al., 2005; Ziolkowska et al., 2006). Griffithsin (GRFT), cyanovirin-N (CV-N) and scytovirin (SVN) were isolated from the red algae Griffithsia sp, the cyanobacteria Nostoc ellipsosporum and Scytonema varium, respectively. While CV-N is found in both monomeric and dimeric forms, SVN exists exclusively as a monomer and GRFT as a dimer (Barrientos et al., 2002; Botos and Wlodawer, 2005; Moulaei et al., 2007; Ziolkowska et al., 2006; Ziolkowska and Wlodawer, 2006). Both the native and recombinant forms of these lectins have demonstrated potent and broad anti-viral activity against laboratory adapted strains and primary isolates of HIV-1 (Alexandre et al., 2010; Bolmstedt et al., 2001; Esser et al., 1999; O'Keefe et al., 2009; Xiong et al., 2006). Since these compounds are inhibitors of HIV-1 entry, they are being actively pursued as potential microbicides for the prevention of HIV-1 transmission (Balzarini and Van Damme, 2007; Bokesch et al., 2003; O'Keefe et al., 2009).
Previously we showed that GRFT, CV-N and SVN potently inhibit infection of TZM-bl cells by cell-free viral particles (Alexandre et al., 2010; Alexandre et al., 2011). Studies by others have shown that CV-N can inhibit the DC-SIGN mediated HIV-1 transfer to a cell line expressing the CD4 receptor (Balzarini et al., 2007). In this study, we investigated the ability of GRFT and SVN, in addition to CV-N, to inhibit both HIV-1 binding to the DC-SIGN receptor and the DC-SIGN-mediated transfer to target cells. We found that these lectins are efficient inhibitors of the DC-SIGN-mediated transfer of HIV-1 to both CD4+ TZM-bl cells and PBMC. As such, these lectins may be useful in blocking early events in HIV-1 transmission in mucosal tissues.
RESULTS
GRFT, CV-N and SVN inhibit HIV-1 binding to the DC-SIGN receptor
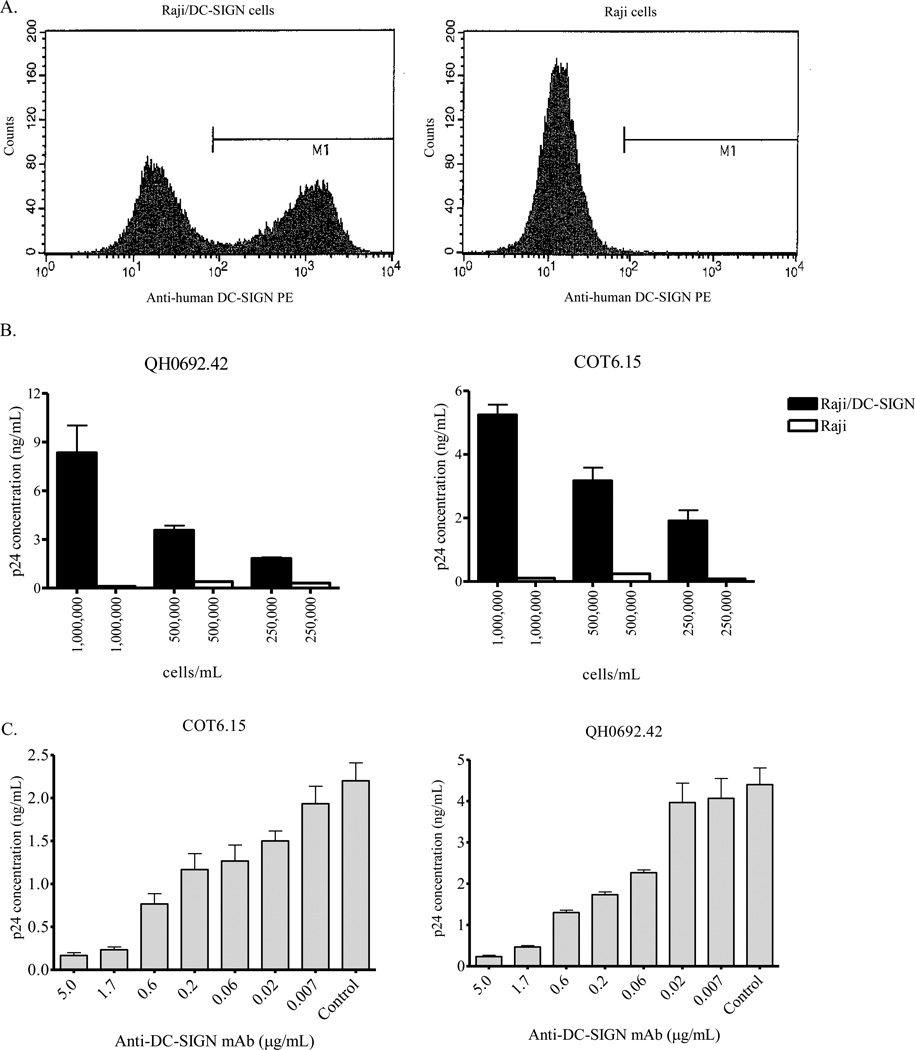
The lectins GRFT, CV-N and SVN interact with mannose-rich glycans on the HIV-1 envelope to inhibit the infection of susceptible cells (Bokesch et al., 2003; Boyd et al., 1997; Mori et al., 2005; Ziolkowska and Wlodawer, 2006). Since the DC-SIGN receptor also binds mannose-rich glycans on the viral envelope (Piquet and Sattenteau, 2004; Pohlmann, Baribaud, and Doms, 2001), we investigated the ability of GRFT, CV-N and SVN to inhibit HIV-1 binding to the DC-SIGN receptor. For this we used Raji/DC-SIGN cells, a Burkitt’s lymphoma cell line that has been engineered to express the DC-SIGN receptor (Wu et al., 2004). Using a DC-SIGN specific antibody, we found that ~55% of Raji/DC-SIGN cells expressed this receptor as measured by flow cytometry while control Raji cells did not (Figure 1A). We first evaluated the ability of HIV-1 to bind to the DC-SIGN receptor by incubating the virus with Raji/DC-SIGN cells and measuring the amount of cell-bound p24 after repeated washing. Using the subtype B QH0692.42 and the subtype C COT6.15, we found that both pseudoviruses bound to Raji/DC-SIGN cells while their binding to control Raji cells was negligible (Figure 1B). In addition, the binding to Raji/DC-SIGN was dependent on the cell concentration used, with 106 cells/mL showing the highest binding. However, for subsequent experiments we chose to use 500,000 cells/mL since the differential binding was in a workable range and minimized the number of cells required. To discount the possibility that the virus interacted with Raji/DC-SIGN cells via a mechanism other than binding to the DC-SIGN receptor, we incubated these cells with mouse anti-human DC-SIGN antibody prior to the addition of the virus. As shown in Figure 1C, the antibody inhibited the binding of two HIV-1 isolates to Raji/DC-SIGN cells confirming the involvement of the DC-SIGN receptor in this interaction.
Figure 1. DC-SIGN receptor expression on Raji/DC-SIGN cells.
(A) Raji/DC-SIGN and Raji cells were stained with phycoerythrin (PE)-labeled mouse anti-human CD209 (DC-SIGN) and analyzed by flow cytometry. DC-SIGN expression is shown beneath the bar labeled M1. (B) HIV-1 subtype B, QH0692.42 and subtype C, COT6.15 were captured by Raji/DC-SIGN and Raji cells at 3 different cell concentrations. The amount of captured virus was measured by p24 ELISA. (C) Inhibition of HIV-1 binding to Raji/DC-SIGN cells by mouse anti-human CD209 antibody. The amount of captured virus was measured by p24 ELISA.
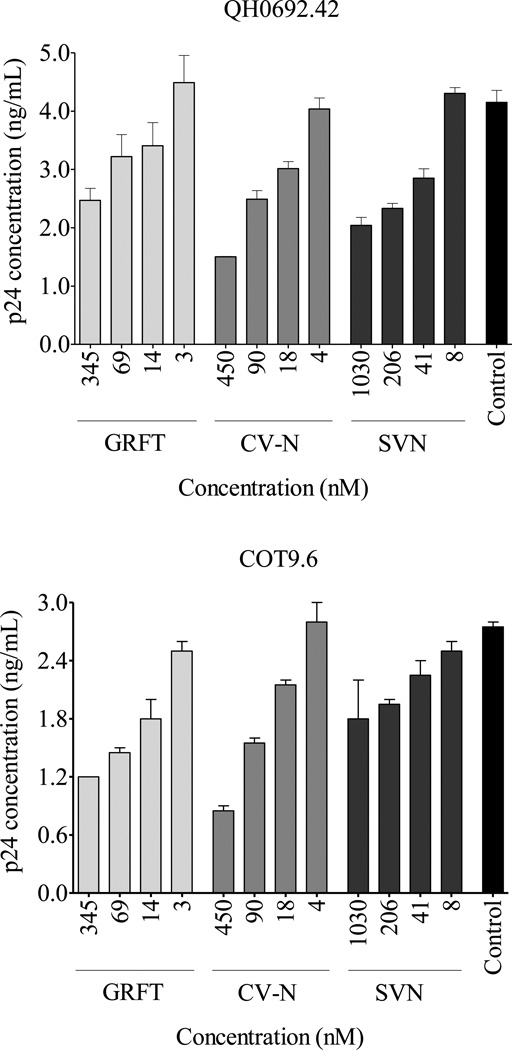
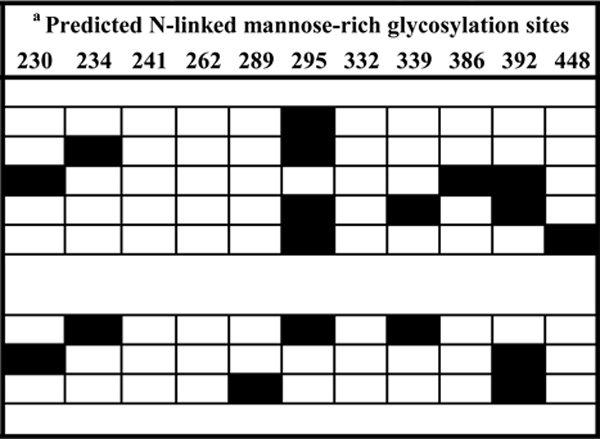
We then tested GRFT, CV-N and SVN inhibition of HIV-1 binding to the DC-SIGN receptor by incubating the virus with the lectins prior to capture with Raji/DC-SIGN cells. Unbound viruses were removed by repeated washing and the amount of captured virus was measured by p24 ELISA. Five subtype C (COT6.15, Du151.2, Du156.12, COT9.6 and CAP63.A9J) and three subtype B (QH0692.42, PVO.4 and CAAN5342.A2) pseudoviruses were used. Figure 2 shows the dose response graphs for two representative viruses that displayed lectin-mediated inhibition of binding to DC-SIGN. All three compounds inhibited in a dose-dependent manner with SVN showing the weakest effect. Table 1 shows the data for all eight viruses where the percentage inhibition was calculated based on the amount of p24 antigen at the highest concentration of the lectin relative to the control. This ranged from 10 to 90% and was highest for CV-N. There was no correlation between the number and pattern of mannose-rich glycosylation sites on gp120 and sensitivity to GRFT, CV-N and SVN (Table 1). For example, COT6.15 (subtype C) and PVO.4 (subtype B) that both lacked two mannose-rich glycans had very different sensitivities to these lectins. Although there was a trend towards better inhibition of subtype B compared to subtype C viruses for GRFT (p = 0.072) and CV-N (p = 0.054), this was only significant for SVN (p = 0.015). The number and position of complex glycans (ie not mannose-rich) also did not affect the sensitivity as expected given that complex glycans do not bind these lectins (data not shown).
Figure 2. Preincubation of HIV-1 with GRFT, CV-N and SVN inhibited binding to the DC-SIGN receptor.
HIV-1 was incubated with GRFT, CV-N and SVN prior to capture on Raji/DC-SIGN cells. Bound virus was lysed and the amount of captured p24 was measured by ELISA. Bars represent mean ± SD of three different experiments. Untreated controls are shown in black.
Table 1.
Inhibition of HIV-1 binding to the DC-SIGN receptor expressed on Raji cells
|
HIV-1 envelope pseudovirus |
 |
b Percentage inhibition | ||
| c GRFT | CV-N | SVN | ||
| Subtype C | ||||
| COT9.6 | 56.4 ± 1.0 | 69.1 ± 1.8 | 34.8 ± 18.9 | |
| CAP63.A9J | 37.9 ± 3.2 | 68.4 ± 2.9 | 39.1 ± 11.9 | |
| Du156.12 | 27.7 ± 5.9 | 34.1 ± 2.0 | 19.7 ± 0.9 | |
| Du151.2 | 16.5 ± 7.6 | 46.6 ± 0.4 | 28.5 ± 6.0 | |
| COT6.15 | 9.8 ± 3.7 | 46.7 ± 22.5 | 43.9 ± 3.2 | |
| Median | 27.7 | 46.7 | 34.8 | |
| Subtype B | ||||
| CAAN5342.A2 | 66.8 ± 0.6 | 89.7 ± 0.3 | 50.3 ± 13.0 | |
| PVO.4 | 64.3 ± 23.2 | 82.3 ± 20.6 | 57.9 ± 26.4 | |
| QH0692.42 | 40.1 ± 11.1 | 63.7 ± 2.6 | 50.8 ± 11.7 | |
| Median | 64.3 | 82.3 | 50.8 | |
Mannose-rich glycosylation sites were identified from the amino acid sequence of each envelope clone (related to HxB2) based on a study using monomeric gp120 (Leonard et al., 1990). Absent glycans are indicated by black boxes.
The percentage inhibition was tested at 345 nM, 450 nM and 1030 nM of GRFT, CV-N and SVN, respectively.
Viruses are ranked according to their sensitivity to GRFT.
Effects of the 234 and 295 glycosylation sites on lectin inhibition of HIV-1 binding to the DC-SIGN receptor
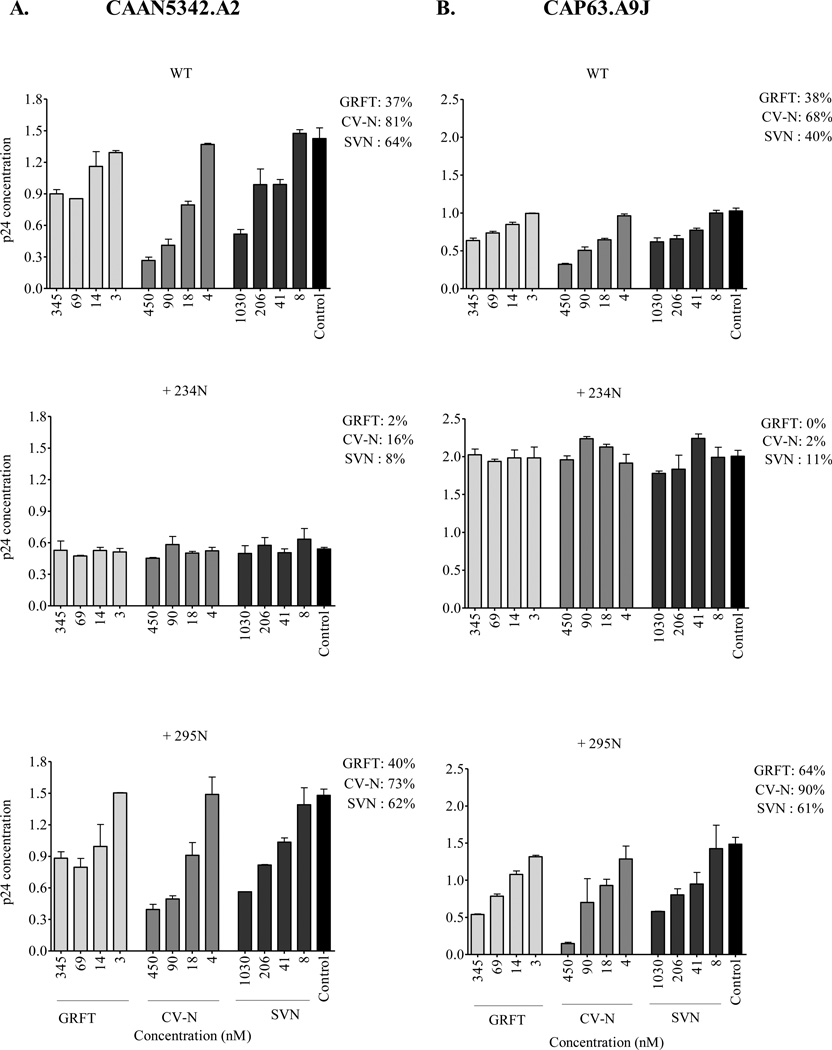
Previously, while investigating the association between mannose-rich glycosylation patterns and HIV-1 sensitivity to GRFT, CV-N and SVN, we observed that glycans at positions 234 and 295 in gp120 increased HIV-1 neutralization sensitivity in TZM-bl cells to all three lectins (Alexandre et al., 2010). Therefore, we investigated whether the addition of these sites also affected the inhibition of HIV-1 binding to the DC-SIGN-receptor. CAAN5342.A2 and CAP63.A9J viruses together with their 234N and 295N mutants were incubated with the lectins prior to capture with Raji/DC-SIGN cells. Contrary to what we expected, the addition of the 234 glycosylation site almost completely abolished lectin inhibition of CAAN5342.A2 and CAP63.A9J while the introduction of the 295 glycosylation site had no effect (Figure 3). Interestingly, when comparing the amount of p24 antigen captured by Raji/DC-SIGN cells in the absence of lectin (control wells – black bars in Figure 3), the addition of the 234 glycosylation site decreased CAAN5342.A2 binding to the DC-SIGN receptor by ~50% while it increased CAP63.A9J binding by ~100%. To further examine this, we also tested COT6.15 which naturally expresses 234N. The deletion of 234N in COT6.15 resulted in a 62% decrease in DC-SIGN binding (the wild-type virus bound 2.1 ng/mL p24 compared to 0.8 ng/mL bound by the COT6-N234Q mutant). Taken together, our data suggest the glycan at position 234 affects the binding of HIV-1 to DC-SIGN and also ablates the lectin’s ability to inhibit this interaction.
Figure 3. Effects of 234 and 295 glycans on GRFT, CV-N and SVN inhibition of HIV-1 binding to the DC-SIGN receptor.
(A) CAAN5342.A2 and (B) CAP63.A9J mutants lacking 234N and 295N were captured with Raji/DC-SIGN cells in the presence of GRFT, CV-N and SVN. The amount of bound virus was measured by p24 ELISA. Bars represent mean ± SD of three different experiments. Untreated controls are shown in black. The highest percentage inhibition obtained with each lectin is show next to the graphs.
Inhibition of the DC-SIGN-mediated HIV-1 transfer with GRFT, CV-N and SVN
Since the DC-SIGN receptor is purported to play a critical role in transferring the virus to susceptible cells during the sexual transmission of HIV-1 (Piguet and Sattentau, 2004; Pohlmann, Baribaud, and Doms, 2001), we tested the ability of GRFT, CV-N and SVN to inhibit DC-SIGN-mediated transfer of HIV-1 to CD4+ TZM-bl cells. We proceeded by using two methods to mimic the possibility that when used as a microbicide, the lectin may interact with the virus prior to or after binding to the DC-SIGN receptor. In the first method, termed the post-DC-SIGN binding method, the virus was preincubated with Raji/DC-SIGN cells followed by the addition of the lectin. The lectins were used at concentrations previously found to be inhibitory to HIV and non-toxic to host cells (Alexandre et al., 2010). SVN was the least potent requiring higher concentrations while GRFT, being the most potent, was used at lower concentrations. Virus-bound Raji/DC-SIGN cells were then co-cultured with TZM-bl cells and the inhibition of transfer was determined after 48 hours. As shown in Table 2, GRFT, CV-N and SVN inhibited HIV-1 transfer with IC50 in the low nanomolar range (Table 2). Similar to our earlier findings in the TZM-bl neutralization assay (Alexandre et al., 2010), GRFT was the most potent of the three compounds followed by CV-N and SVN. It is likely that the inhibition of HIV-1 transfer observed here is related to the neutralization potency of the lectin. In addition and also in agreement with our previous study (Alexandre et al., 2010), we observed no correlation between the number of mannose-rich glycans on gp120 and sensitivity to GRFT, CV-N and SVN (see Table 1 for glycan patterns). Despite the differences in glycosylation patterns (Zhang et al., 2004), HIV-1 subtype B and C showed similar sensitivity to lectin inhibition of DC-SIGN-mediated transfer (Table 2).
Table 2.
Inhibition of HIV-1 transfer to TZM-bl cells via the DC-SIGN receptor
| HIV-1 envelope Pseudovirus |
a Post-DC-SIGN binding method | b Pre-DC-SIGN binding method | ||||
|---|---|---|---|---|---|---|
| GRFT | CV-N | SVN | GRFT | CV-N | SVN | |
| Subtype C | ||||||
| COT9.6 | 4.8 ± 0.5 | 31.5 ± 7.3 | 70.6 ± 14.6 | 19.4 ± 4.2 | 154.1 ± 25.6 | 230.5 ± 9.8 |
| CAP63.A9J | 35.0 ± 5.4 | 24.0 ± 5.6 | 441.3 ± 62.9 | 38.7 ± 5.2 | 33.7 ± 3.7 | 590.0 ± 82.4 |
| Du156.12 | 6.4 ± 0.4 | 69.2 ± 8.3 | 161.9 ± 11.6 | 28.8 ± 8.3 | 136.8 ± 73.3 | 284.0 ± 65.8 |
| Du151.2 | 4.8 ± 0.3 | 30.8 ± 3.2 | 82.8 ± 8.7 | 11.4 ± 1.0 | 131.5 ± 46.0 | 386.5 ± 71.3 |
| COT6.15 | 5.0 ± 0.1 | 31.2 ± 0.6 | 75.9 ± 0.8 | 18.6 ± 4.5 | 151.3 ± 22.7 | 149.7 ± 36.3 |
| Median | 5.0 | 31.2 | 82.8 | 19.4 | 136.8 | 284.0 |
| Subtype B | ||||||
| CAAN5342.A2 | 5.1 ± 0.1 | 18.8 ± 1.7 | 115.0 ± 2.4 | 7.1 ± 0.5 | 24.2 ± 2.1 | 153.0 ± 9.7 |
| PVO.4 | 4.4 ± 0.2 | 35.7 ± 2.1 | 82.4 ± 3.0 | 17.6 ± 2.7 | 162.8 ± 1.1 | 308.6 ± 16.2 |
| QH0692.42 | 5.3 ± 0.1 | 36.9 ± 1.4 | 77.3 ± 5.7 | 21.7 ± 8.3 | 135.4 ± 18.3 | 438.4 ± 58.8 |
| Median | 5.1 | 35.7 | 82.4 | 17.6 | 135.4 | 308.6 |
Results are shown as IC50 (nM) which is the concentration needed to inhibit HIV-1 transfer by 50%.
We also investigated whether GRFT, CV-N and SVN synergized to inhibit the DC-SIGN mediated HIV-1 transfer. Synergism between the three lectins was measured by inhibiting the virus transfer with each lectin alone and in combination and by measuring the combination index (CI). We used two subtype C, COT9.6 and CAP63.A9J, and two subtype B, CAAN5342.A2 and QH0692.42, in this experiment. As shown in Table 3, there was no synergism between GRFT, CV-N and SVN. On the contrary, the three compounds acted antagonistically when used in combination, with CI values of > 1.1 probably due to the fact that they share common binding sites on the viral envelope (Alexandre et al., 2010).
Table 3.
Antagonism between GRFT, CV-N and SVN for the inhibition of DC-SIGN-mediated HIV-1 transfer
| COT9.6 | CAP63.A9J | CAAN5342.A2 | QH0692.42 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post | Pre | Pre + Post | Post | Pre | Pre + Post | Post | Pre | Pre + Post | Post | Pre | Pre + Post | ||
| Single lectin | GRFT | 11.3 ± 2.5 | 37.6 ± 4.3 | 3.5 ± 1.3 | 71.9 ± 5.0 | 33.7 ± 7.2 | 15.6 ± 8.8 | 9.9 ± 1.0 | 8.4 ± 0.6 | 9.0 ± 0.5 | 9.7 ± 2.4 | 19.5 ± 9.2 | 1.4 ± 0.3 |
| CV-N | 46.9 ± 10.1 | 201.8 ± 38.2 | 22.6 ± 4.7 | 48.8 ± 7.9 | 141.7 ± 22.3 | 80.6 ± 42.7 | 43.4 ± 4.9 | 30.1 ± 8.7 | 45.1 ± 7.5 | 35.1 ± 5.5 | 180.3 ± 24.0 | 10.0 ± 5.2 | |
| SVN | 118.2 ± 12.4 | 396.0 ± 72.6 | 67.4 ± 5.7 | 357.9 ± 49.8 | 311.3 ± 32.8 | 185.1 ± 129.1 | 98.6 ± 7.4 | 152.0 ± 11.5 | 103.8 ± 14.7 | 84.8 ± 20.7 | 769.1 ± 67.8 | 67.4 ± 7.4 | |
| Combined lectin | GRFT | 7.5 ± 1.9 | 15.7 ± 2.2 | 4.0 ± 0.3 | 11.1 ± 1.9 | 29.7 ± 4.8 | 15.8 ± 10.4 | 5.8 ± 1.3 | 7.3 ± 1.3 | 7.4 ± 1.4 | 10.4 ± 2.7 | 18.4 ± 3.4 | 1.3 ± 0.3 |
| CV-N | 48.0 ± 12.6 | 203.6 ± 42.2 | 25.6 ± 1.9 | 59.4 ± 6.2 | 185.6 ± 19.8 | 66.8 ± 21.1 | 49.4 ± 7.8 | 47.3 ± 8.6 | 46.4 ± 7.6 | 26.0 ± 5.4 | 117.0 ± 19.7 | 0.3 ± 0.1 | |
| SVN | 110.8 ± 20.7 | 112.8 ± 15.3 | 58.1 ± 4.5 | 318.6 ± 36.1 | 189.7 ± 54.9 | 231.0 ± 160.4 | 94.8 ± 7.0 | 89.3 ± 5.1 | 105.5 ± 17.0 | 55.8 ± 9.0 | 128.7 ± 39.4 | 7.1 ± 0.8 | |
| CI | 2.6 | 1.7 | 3.1 | 2.3 | 2.8 | 3.1 | 2.7 | 3.0 | 2.9 | 2.5 | 1.8 | 1.1 | |
Data are shown as IC50 which is the concentration needed to reduce HIV-1 transfer by 50%.
The CI is the Combination Index: 0.3 to 0.7 indicates synergism, 0.7 to 0.85 indicates moderate synergism, 0.85 to 0.9 indicates slight synergism, 0.9 to 1.1 indicates an additive effect and > 1.1 indicates antagonism.
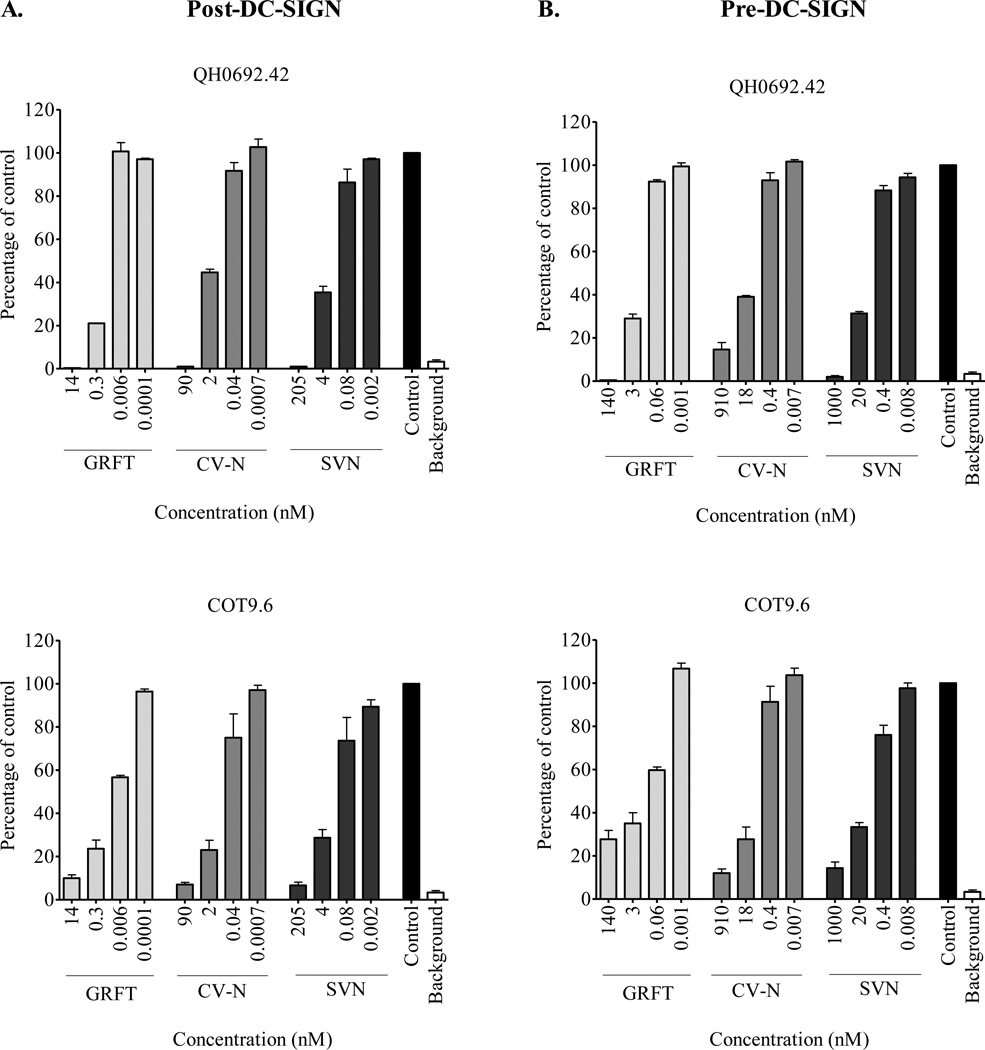
In the second method, termed the pre-DC-SIGN binding method, the virus was first incubated with the lectin before sequentially adding Raji/DC-SIGN cells and TZM-bl cells. Although, GRFT, CV-N and SVN inhibited the DC-SIGN-mediated transfer, the sensitivity of the virus to the three compounds was markedly reduced (Table 2). Similar to the post-DC-SIGN binding method, GRFT was the most potent and there was no difference between subtypes. Dose-response curves for two representative viruses in both formats are shown in Figure 4. Note that the concentrations used in the pre-DC-SIGN method were 5–10 fold higher since the lectins are less potent in this format. We also tested for synergistic interaction between the three lectins when combined using the pre-DC-SIGN binding method and similar to the post-DC-SIGN binding method there was antagonism (Table 3).
Figure 4. Inhibition of DC-SIGN mediated HIV-1 transfer by post and pre-DC-SIGN binding methods.
(A) In the post-DC-SIGN binding method, HIV-1 subtype B QH0692.42 and subtype C COT9.6 were incubated with Raji/DC-SIGN cells prior to the addition of GRFT, CV-N and SVN and co-culture with TZM-bl cells. (B) In the pre-DC-SIGN binding method, the virus was first incubated with GRFT, CV-N and SVN prior to the addition of Raji/DC-SIGN cells and co-culture with TZM-bl cells. The inhibition of transfer for both methods was determined by measuring the RLU. Bars represent mean ± SD of three different experiments. Untreated controls are shown in black.
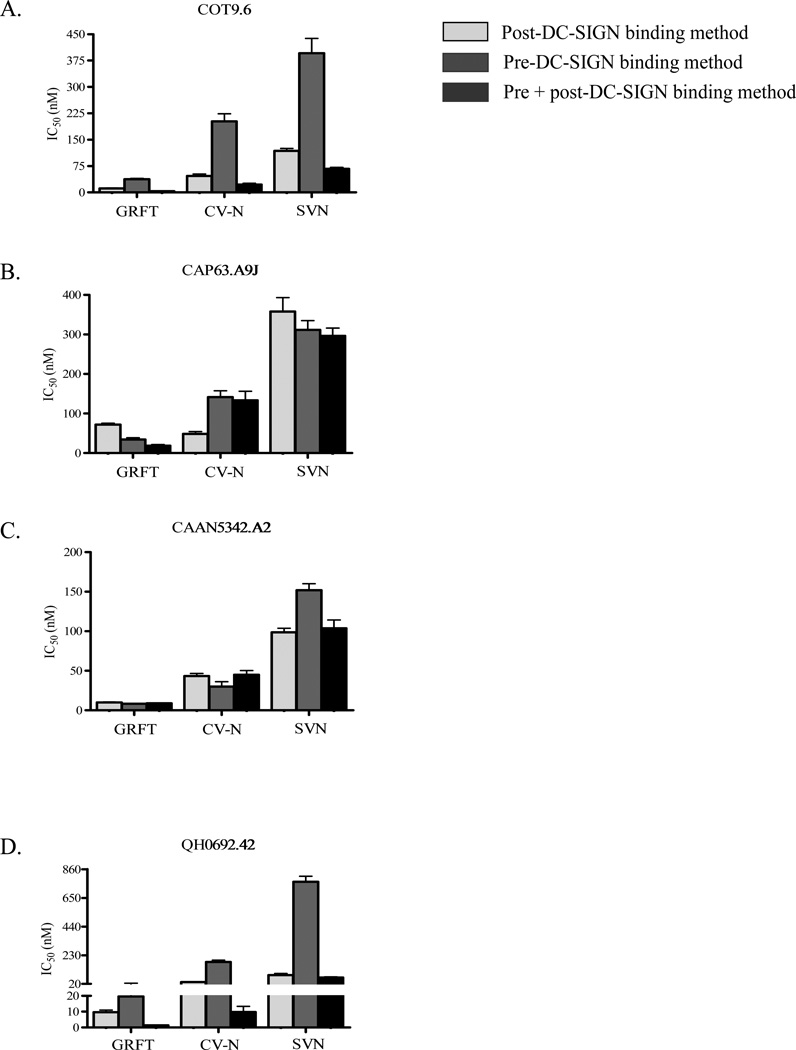
We then asked the question of whether the combination of both the post and pre-DC-SIGN binding methods would increase GRFT, CV-N and SVN inhibition of HIV-1 transfer via the DC-SIGN receptor. This was investigated by first incubating the virus with the lectins followed by the addition of Raji/DC-SIGN cells. After washing off unbound viruses, Raji/DC-SIGN cell bound viruses were again incubated with the lectins before the addition of TZM-bl cells. For this study we used COT9.6, CAP63.A9J, CAAN5342.A2 and QH0692.42. Compared to each method alone, the combination of the pre and post-DC-SGN binding methods resulted in an increased inhibition of transfer for COT9.6 and QH0692.42, while the effect was less clear for CAP63.A9J with CV-N and CAAN5342.A2 with all three lectins (Figure 5). Lastly, similar to the post and pre-DC-SIGN binding methods, we observed antagonism when all three lectins were tested together (Table 3).
Figure 5. Comparison of IC50 values between the post, pre and the combined method using TZM-bl cells.
GRFT, CV-N and SVN inhibition of HIV-1 (A) COT9.6, (B) CAP63.A9J, (C) CAAN5342.A2 and (D) QH0692.42 transfer to TZM-bl cells was tested using the post, pre and combined methods. The IC50 values obtained with each method were then compared. The values shown are average of three different experiments.
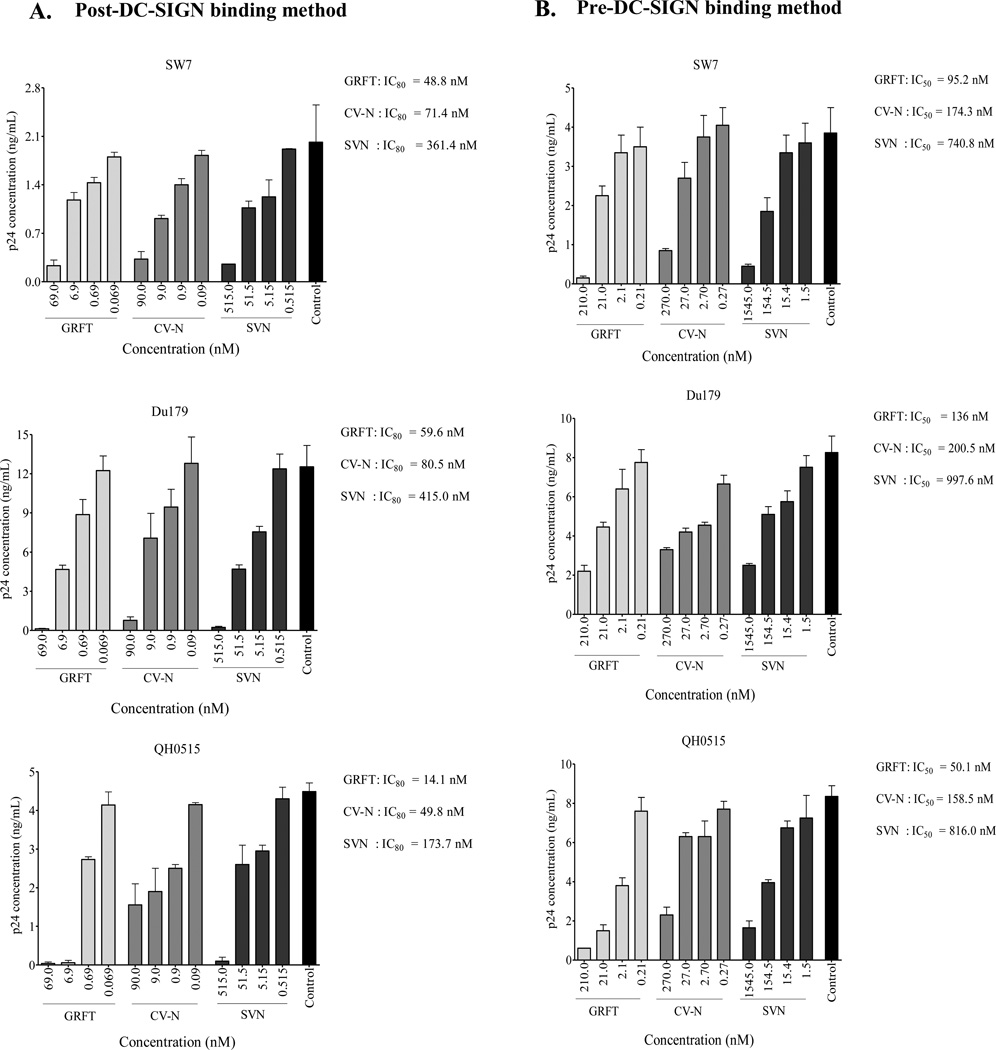
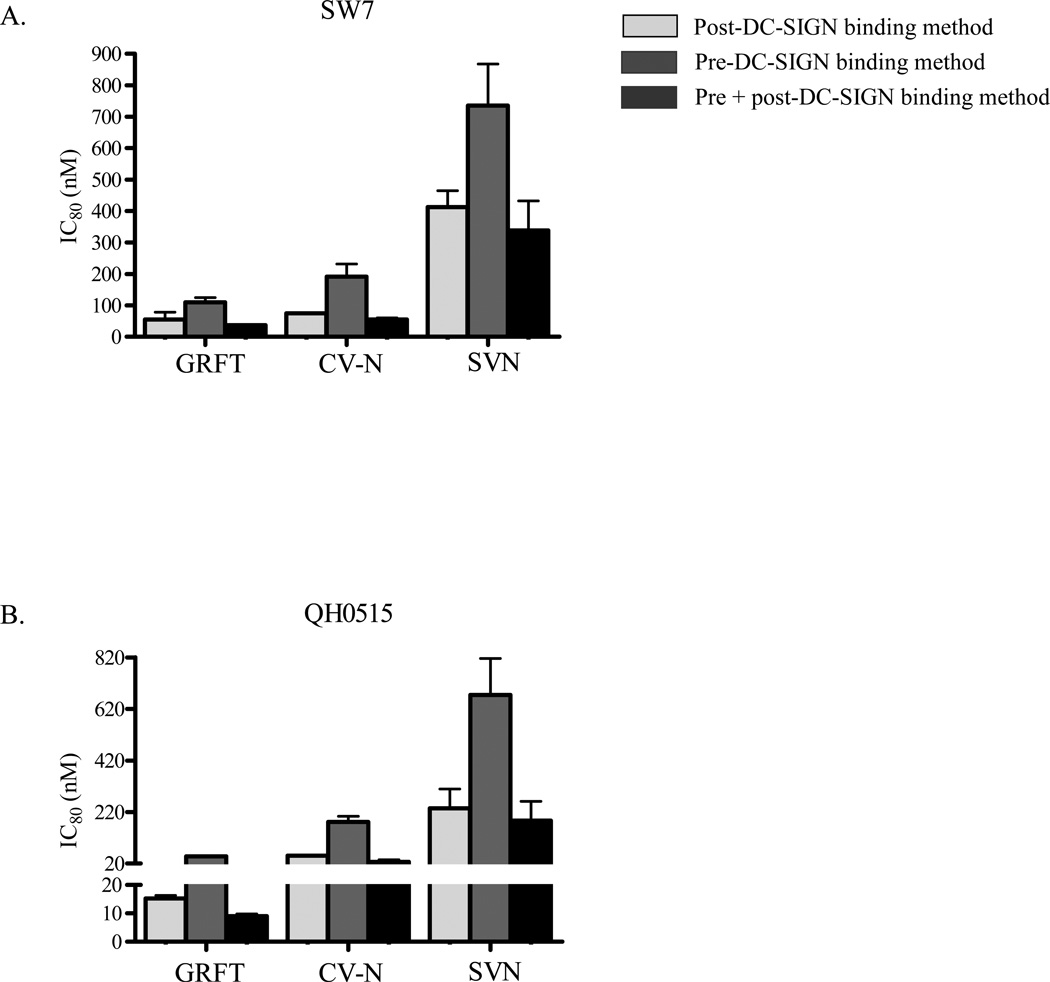
Since PBMC are more relevant than TZM-bl cells as HIV-1 targets, we also tested the inhibition of virus transfer to these primary cells. To achieve this we used infectious replicating isolates SW7 and Du179 (subtype C) and QH0515 (subtype B). Viruses were tested in both the post and pre-DC-SIGN binding methods and similar to what we observed in TZM-bl cells, GRFT, CV-N and SVN were inhibitory to HIV-1 transfer with GRFT being the most potent (Figure 6A and 6B). Furthermore, similar to TZM-bl, viruses were more sensitive to the lectins in the post-DC-SIGN binding method compared to the pre-DC-SIGN binding method. We also tested the combination of the pre and post-DC-SIGN binding methods against SW7 and QH0515 and observed that there was a moderate increase in lectin inhibition of HIV-1 transfer (Figure 7). In conclusion, our data show that GRFT, CV-N and SVN inhibit the DC-SIGN-mediated HIV-1 infection of PBMC, suggesting that these compounds may be able to inhibit the virus transfer to primary CD4+ T cells in vivo.
Figure 6. DC-SIGN-mediated HIV-1 transfer to PBMC is inhibited by GRFT, CV-N and SVN.
HIV-1 subtype C infectious viruses SW7 and Du179 and subtype B QH0515 transfer to PBMC was inhibited with GRFT, CV-N and SVN using (A) the post and (B) pre-DC-SIGN binding methods. The inhibition of transfer was measured by p24 ELISA. Bars represent mean ± SD of three different experiments. Untreated controls are shown in black. Levels of p24 antigen in the absence of virus or lectins were below detection (not shown).
Figure 7. Comparison of IC80 values between the post, pre and combined methods in PBMC.
SW7 (A) and QH0515 (B) transfer to PBMC was inhibited with GRFT, CV-N and SVN using the post, pre and combined methods. This was followed by the comparison of IC80 values obtained with each method. The values shown are average of three different experiments.
Effects of the 234 and 295 glycosylation sites on lectin inhibition of DC-SIGN-mediated HIV-1 infection of TZM-bl cells
We next investigated whether the inhibition of the DC-SIGN mediated HIV-1 transfer was also affected by the 234 and 295 glycosylation sites. To test this, CAAN5342.A2, CAP63.A9J and their respective mutants were incubated with Raji/DC-SIGN cells prior to the sequential addition of the lectin and TZM-bl cells (post-DC-SIGN binding method). Similar to what we observed for the inhibition of binding, the addition of the 234 glycosylation site totally abolished the sensitivity to GRFT, CV-N and SVN (Table 4). However, examination of the virus control (without lectin) showed that the 234N CAAN5342.A2 virus was 30-fold less efficiently transferred (RLU of 4,946 compared to 157,591 for the wild-type virus), while CAP63.A9J-234N was 3-fold more efficiently transferred compared to the wild-type virus (RLU of 16,498 and 5,637). The introduction of the 295 glycosylation site considerably increased CAP63.A9J and CAAN5342.A2 sensitivity to the lectins. Similar data were obtained using the pre-DC-SIGN binding method (Table 4). Note that the 295N mutation had no effect on the efficiency of virus transfer. When put together, these data suggest that the 295 glycan may play a role in GRFT, CV-N and SVN inhibition of HIV-1 transfer via the DC-SIGN receptor while the 234 glycan may interfere with this inhibition.
Table 4.
Effect of HIV-1 glycosylation on DC-SIGN transfer
| Envelope | Genotype | Post-DC-SIGN binding method a IC50 (nM) |
Pre-DC-SIGN binding method IC50 (nM) |
||||
|---|---|---|---|---|---|---|---|
| GRFT | CV-N | SVN | GRFT | CV-N | SVN | ||
| CAAN5342.A2 | b WT | 5.1 ± 0.1 | 18.8 ± 1.7 | 115.0 ± 2.4 | 7.1 ± 0.4 | 24.2 ± 2.1 | 153.0 ± 9.7 |
| 234N | > 50 | > 50 | > 500 | > 50 | > 50 | > 500 | |
| 295N | 1.5 ± 0.2 | 7.3 ± 0.1 | 66.8 ± 3.0 | 3.5 ± 0.3 | 13.4 ± 0.3 | 140.6 ± 9.0 | |
| CAP63.A9J | WT | 35.0 ± 5.4 | 24.0 ± 5.6 | 411.3 ± 62.9 | 38.7 ± 5.2 | 33.7 ± 3.7 | 590.0 ± 82.4 |
| 234N | > 50 | > 50 | > 500 | > 50 | > 50 | > 500 | |
| 295N | 7.9 ± 0.2 | 9.2 ± 0.1 | 228.3 ± 3.0 | 11.5 ± 0.7 | 18.2 ± 1.0 | 357.1 ± 47.3 | |
The concentration needed to inhibit HIV-1 transfer by 50%.
Wild type.
DISCUSSION
In this study we showed that GRFT, CV-N and SVN inhibit both HIV-1 binding to the DC-SIGN receptor and the DC-SIGN mediated transfer of the virus to target cells. However, the inhibition of binding to DC-SIGN was moderate compared to the inhibition of transfer. In addition, HIV-1 was more sensitive to the inhibition of transfer when it bound the lectins after binding to the DC-SIGN receptor. These effects were modulated by glycan changes at positions 234 and 295 in gp120. Despite documented differences in glycosylation patterns, subtype B and subtype C viruses overall did not show significantly different sensitivities to GRFT, CV-N and SVN inhibition of binding and transfer via DC-SIGN. Given the central role of DC-SIGN in HIV transmission, further investigation of these compounds as potential microbicides to prevent the sexual transmission of HIV-1 is warranted.
Lectin inhibition of HIV-1 binding to the DC-SIGN receptor was partial even at concentrations that were more than 3,000-fold higher than required for neutralization (Alexandre et al., 2010). This is likely attributed to the promiscuity of the DC-SIGN receptor which can in addition to mannose-rich also bind complex glycans (Hong et al., 2007; Liao et al., 2011; Lue et al., 2002). Thus while the lectins block virus binding via mannose-rich glycans they would have no effect on virus binding to DC-SIGN via other glycans. It is interesting that CV-N was the most potent of the 3 lectins in inhibiting the binding to DC-SIGN, suggesting that CV-N shares more binding sites with DC-SIGN than GRFT or SVN. These data are in agreement with studies by Spear who reported that CV-N partially inhibited HIV-1 binding to DC-SIGN and Banerjee who reported that GRFT incompletely inhibited monomeric gp120 binding to the DC-SIGN receptor, even at concentrations as high as 10 µM (Banerjee et al., 2011; Spear et al., 2003). A study by Hong showed CV-N did not inhibit HIV-1 JR-CSF gp120 binding to the DC-SIGN receptor even at 1mM (Hong et al., 2002), but given the partial nature of lectin inhibition of binding to DC-SIGN and the range of sensitivities seen with different viruses (Table 1), this is perhaps not too surprising. The degree of inhibition appeared not to be related to the number of mannose-rich or complex glycans. However, it may be possible that the absence or presence of single or combinations of mannose-rich glycans is involved in this differential sensitivity of lectin inhibition of binding to DC-SIGN. This is suggested by our previous studies showing that the position of the missing mannose-rich glycans on HIV-1 envelope was more important than the number in determining sensitivity to GRFT, CV-N and SVN (Alexandre et al., 2010). While the mannose-rich glycosylation patterns differ between subtypes B and C (Kwong et al., 1998; Zhang et al., 2004), this did not have a major impact on lectin sensitivity. Furthermore, recent data from Go and coworkers showing that the glycosylation patterns of transmitted founder viruses from subtypes B and C are similar (Go et al., 2011). Although this is a single study of one virus from each subtype, it nevertheless suggests that a GRFT, CV-N or SVN-based microbicide would be universally effective.
Our data are consistent with that of Balzarini and colleagues who showed that CV-N inhibited the DC-SIGN-mediated transfer of HIV-1 to CD4+ cells (Balzarini et al., 2007). In addition to CV-N, we show here that GRFT and SVN are also inhibitory for HIV-1 transfer with GRFT being the most potent. The DC-SIGN-mediated HIV-1 transfer to target cells can be visualized as a two step process: step 1 is the binding of the virus to the DC-SIGN receptor and step 2 is transfer to target cells for infection. Thus, the partial sensitivity of HIV-1 to GRFT, CV-N and SVN inhibition of binding to the DC-SIGN receptor and its higher sensitivity to the lectin inhibition of the DC-SIGN-mediated transfer suggests that these compounds are more active during the second step of the process. This is consistent with the fact that these three lectins are strong inhibitors of HIV-1 infection of cells (Alexandre et al., 2010; Bokesch et al., 2003; Boyd et al., 1997; Mori et al., 2005; O'Keefe et al., 2009). Indeed, the post-DC-SIGN method measures inhibition of the specific interaction with CD4, as opposed to the rather promiscuous interaction with DC-SIGN via multiple glycans measured in the pre-DC method. Another possible explanation for the higher potency of the post-DC-SIGN method is that HIV-1 binding to DC-SIGN could increase the exposure of mannose-rich glycans on the viral envelope allowing them to bind more of the inhibitory lectin. However, it is clear that in vivo the inhibition of transfer by the post-DC-SIGN binding method will require that the lectins cross the cervico-vaginal mucosa to reach the sub-epithelium where the virus interacts with DCs and CD4+ T cells (Lederman, Offord, and Hartley, 2006; Shattock and Moore, 2003). Since micro-abrasions of the vaginal mucosa are very common during sexual intercourse this may offer a way by which these compounds can breach the mucosal barrier (Norvell, Benrubi, and Thompson, 1984). The combination of the pre and post-DC-SIGN binding methods resulted in increased sensitivity to GRFT, CV-N and SVN for some of the viruses tested. A possible explanation for this is that this combination increased the likelihood of lectins occupying all or most of their binding sites on the virus, thereby, enhancing their potency. The antagonism observed with the combination of the three compounds was somewhat expected since these lectins binding sites overlap on the viral envelope (Alexandre et al., 2010). The finding that these lectins also inhibited DC-mediated transfer in PBMC in the pre- and post-DC-SIGN format, suggests that they will also be effective in inhibiting transfer to CD4+ T cells resident in the cervico-vaginal mucosa (Lederman, Offord, and Hartley, 2006; Shattock and Moore, 2003).
The introduction of the 234 glycosylation site abolished GRFT, CV-N and SVN inhibition of HIV-1 binding to the DC-SIGN receptor and transfer to target cells. Previously we showed that the 234 glycan rendered viruses more sensitive to lectin-mediated neutralization presumably because they bound more lectin. The increased binding to the DC-SIGN receptor for 2 of 3 viruses in which the 234 glycan was present suggested that this glycan may be involved in DC-SIGN binding, similar to complex glycans at positions 158, 276 and 355 and the mannose-rich glycan at position 386 (Hong et al., 2007; Liao et al., 2011; Lue et al., 2002). The loss of lectin inhibition in the presence of 234N could, therefore, be the result of increased interaction of HIV-1 with DC-SIGN that supersedes the sensitivity to the lectins. This is suggested by CAP63.A9J and COT6.15 which showed increased binding to DC-SIGN when 234N was present and a decrease when this glycan was deleted. The loss of inhibition of binding to DC-SIGN in the presence of the 234N was also observed for CAAN5342.A2 although the decrease in DC-SIGN binding in the presence of this glycan was more difficult to understand. Similarly, the 234N mutants differed in their ability to be transferred by DC-SIGN. It is important to note that CAAN5342.A2 differed from CAP63.A9J in that it also lacked the 339 glycan. It is possible that this extra deglycosylation may be involved in the differences observed between these two viruses. The importance of the 234 glycosylation site is suggested by the fact that it is conserved in about 80% of subtype B and C viruses (Zhang et al., 2004), possibly because it participates in interactions with receptors such as DC-SIGN. However, we did not observe a correlation between naturally occurring 234N and binding to DC-SIGN, for example PVO.4 which has 234N bound less well to the DC-SIGN receptor compared to CAP63.A9J and CAAN5342.A2 (data not shown), suggesting this interaction may be contextual.
Similar to 234N we also showed previously that the addition of the 295 glycosylation site increased HIV-1 sensitivity to GRFT, CV-N and SVN (Alexandre et al., 2010). However, we saw no effect of 295N on DC-SIGN binding while there was increased HIV-1 sensitivity to GRFT, CV-N and SVN inhibition of the DC-SIGN-mediated transfer in the presence of 295N. It is, therefore, possible that the effect of the 295 glycosylation site observed here is related to its effect on GRFT, CV-N and SVN neutralization of HIV-1 only.
The difficulties encountered in developing an effective HIV-1 vaccine (Johnston and Fauci, 2007; McElrath and Haynes, 2010) and the fact that the majority of HIV-1 infections are sexually transmitted (http://www.unaids.org) (Stein, 2003) makes the development of microbicides that prevent the sexual transmission of the virus crucial. GRFT, CV-N and SVN block HIV entry into cells and are being actively pursued as potential HIV-1 microbicides (Bokesch et al., 2003; O'Keefe et al., 2009; Shattock and Moore, 2003; Xiong et al., 2006). These compounds have shown little to no toxicity of mammalian cells in vitro while CV-N tested in a gel formulation as a microbicide was able to prevent the vaginal and rectal transmission of SHIV89.6P in macaques (Kouokam et al., 2011; O'Keefe et al., 2009; Tsai et al., 2004; Tsai et al., 2003). In addition, a study conducted in mice showed that CV-N secreted by engineered vaginal lactobacillus did not induce a mucosal immune response (Liu et al., 2006). Except for tenofovir gel, all microbicide candidates that have been tested in human clinical trials have demonstrated little or no efficacy and some have proven to be harmful (Abdool Karim et al., 2010; Cutler and Justman, 2008; Ramjee et al., 2008). The advantage of GRFT, CV-N and SVN is that they selectively target mannose-rich glycan arrays on HIV-1 that are not found on mammalian cells (Balzarini, 2005). These lectins also have the benefit over ARV, such as tenofovir, since they are not being used for routine HIV-1 treatment. Lastly, GRFT has been shown to synergize with tenofovir, maraviroc and enfuvirtide against HIV-1 (Ferir, Palmer, and Schols, 2011), opening up the possibility of combining lectins with other compounds in a single formulation. In conclusion, our data suggest that GRFT, CV-N and SVN could play an important role in preventing the sexual transmission of HIV-1 by inhibiting the interaction of the virus with the DC-SIGN receptor in addition to the CD4 receptor. This supports further investigations on the use of these lectins for HIV-1 prevention.
MATERIALS AND METHODS
Viruses, cell lines and lectins
SW7 and Du179 are HIV-1 subtype C infectious viruses isolated from an AIDS and an acutely infected patient, respectively, while QH0515 is an infectious subtype B from an acutely infected individual (Cilliers et al., 2003; Li et al., 2005; van Harmelen et al., 2001). The subtype C envelope clones, COT9.6 and COT6.15 used to generate pseudoviruses were derived from pediatric isolates (Choge et al., 2006) while Du151.2, Du156.12 and CAP63.A9J were isolated during the acute phase of the infection (Gray et al., 2007; Li et al., 2006). HIV-1 subtype B envelopes CAAN5342.A2, QH0692.42 and PVO.4 were from the clade B reference panel (Li et al., 2005). The 234N and 295N mutants of CAAN5342.A2 and CAP63.A9J were generated by site directed mutagenesis using the QuikChange Site Directed Mutagenesis Kit (Stratagene, LaJolla, CA) (Alexandre et al., 2010). The pSG3Δenv plasmid was obtained from Dr. Beatrice Hahn. HIV-1 pseudoviruses were generated by co-transfection of the Env and pSG3Δenv plasmids (Wei et al., 2003) into 293T cells using the Fugene transfection reagent (Roche Applied Science, Indianapolis, IN). Raji cells (Cat N° 9944), a Burkitt’s lymphoma cell line (Wu et al., 2004), and Raji/DC-SIGN cells that express the DC-SIGN receptor (Cat N° 9945) were provided by the NIH Reference and Reagent Program and were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS). The TZM-bl cell line was obtained from the NIH Reference and Reagent Program (Cat N° 8129) while the 293T cell line came from the American Type Culture Collection. Both cell lines were cultured in DMEM containing 10% fetal bovine serum (FBS). Cell monolayers were disrupted at confluence by treatment with 0.25% trypsin in 1 mM EDTA. Recombinant GRFT, CV-N and SVN were purified from E. coli at the National Cancer Institute, MA, USA (Bokesch et al., 2003; Boyd et al., 1997; Mori et al., 2005).
Expression of the DC-SIGN receptor on Raji/DC-SIGN cells
One million Raji/DC-SIGN or Raji cells were centrifuged at 2,000 rpm for 5 minutes, resuspended in 100 µL of RPMI 1640 containing 10% FBS and stained with 10 µL of phycoerythrin (PE)-labeled mouse anti-human CD206 (DC-SIGN) (BD, San Jose, U.S.A) for 1 hour at 4°C. Cells were then washed by adding 3 mL of phosphate buffered saline (PBS) containing 5% fetal bovine serum (FBS) and centrifuged at 2,000 rpm for 5 minutes. The supernatant was then removed and 300 µL of 1% formaldehyde in PBS (fixer) was added. After 10 minutes incubation at 4°C, cells were analyzed by flow cytometry for the expression of the DC-SIGN receptor.
DC-SIGN receptor binding assay
HIV-1 pseudoviruses in 20% FBS DMEM were centrifuged for 25 minutes at 4,500 rpm and 4°C using the 100,000 MWCO PES vivaspin 20 column (Sartorius Stedim biotech, Aubagne, France), to remove free p24 from the viral samples. This was followed by the determination of the p24 concentration of the supernatant using the Vironostika HIV-1 Antigen Microelisa System (Biomerieux, Boseind, the Netherlands), according to the manufacturer’s instructions. HIV-1 binding to the DC-SIGN receptor was measured by adding 5 ng of p24 to 1.0 × 105, 0.5 × 105 and 0.25 × 105 Raji/DC-SIGN cells in 100 µL of RPMI 1640 containing 10% FBS, in a U bottom 96 well plate. The virus and the cells were incubated at 37°C for 2 hours and then washed three times with RPMI 1640 by centrifuging at 2,000 rpm for 5 minutes. Cell bound viral particles were then lysed with 150 µL of 0.5% Triton-X 100 and the amount of captured p24 was measured by ELISA.
The interaction between HIV-1 and the DC-SIGN receptor on Raji/DC-SIGN cells was confirmed by incubating 105 cells with a serial dilution of mouse anti-human CD209 antibody (BD Biosciences, San Jose, California) for one hour. The cells were then washed three times with RPMI 1640, by centrifuging at 2,000 rpm for 5 minutes, and incubated with 5 ng of p24 for two hours at 37 °C. After the incubation they were again washed three times and bound viruses were lysed with 150 µL of 1% empigen. The amount of captured p24 was measured by ELISA.
GRFT, CV-N and SVN inhibition of HIV-1 binding to the DC-SIGN receptor was measured by preparing a five-fold dilution series of these lectins in 100 µL of RPMI 1640 containing 10% FBS, in a U bottom 96-well plate. This was followed by the addition of 50 µL of 5 ng/mL of HIV-1 pseudovirus-containing supernatant and 1 hour incubation at 37°C. Subsequently 100 µL of 0.5 × 105 Raji/DC-SIGN cells/mL was added and incubated at 37°C for 2 hours. The plate was then washed three times with RPMI 1640 by centrifuging at 2,000 rpm for 5 minutes and virus bound to Raji/DC-SIGN cells was lysed with 150 µL of 0.5% Triton-X 100. The amount of captured p24 was measured by ELISA in the presence and absence of lectins.
DC-SIGN-mediated transfer of HIV-1 infection
The DC-SIGN-mediated HIV-1 transfer to TZM-bl cells was carried out by incubating 7.5 × 104 Raji/DC-SIGN cells/well with HIV-1 pseudovirus in a U bottom 96 well plate at 37°C for 1 hour. Cells were then washed three times with RPMI 1640 by centrifuging at 2,000 rpm for 5 minutes to remove unbound viruses and resuspended in 150 µL of 10% FBS DMEM (growth medium). This was followed by the transfer of 100 µL to the corresponding wells of a flat bottom plate. Subsequently, 3 × 104 TZM-bl cells / 100 µL of growth medium / well were added to the plate that was then placed at 37°C for 48 hours. The Bright Glo™ Reagent (Promega, Madison, WI) was used to measure infection after 48 hours of culture, according to the manufacturer’s instructions. Luminescence was measured in a Wallac 1420 Victor Multilabel Counter (Perkin-Elmer, Norwalk, CT).
GRFT, CV-N and SVN inhibition of DC-SIGN-mediated HIV-1 infection was measured by using two methods. In the first method (post-DC-SIGN binding method) Raji/DC-SIGN cells were incubated with the virus for an hour and after the removal of unbound viruses a five-fold dilution series of GRFT, CV-N and SVN in 150 µL of growth medium was added. This was followed by a 1 hour incubation at 37°C and transfer of 100 µL from each well to the corresponding well of a flat bottom 96 well plate. Subsequently, 3 × 104 TZM-bl cells / 100µL of growth medium/ well were added to the plate that was then placed at 37°C for 48 hours; HIV-1 infection was measured as described above. Raji cells co-cultured with TZM-bl cells, in the absence of the virus, were used here as background controls. In the second method (the pre-DC-SIGN binding method), HIV-1 pseudovirus was first incubated for 1 hour at 37°C with serially diluted GRFT, CV-N and SVN in 150 µL of growth medium before the subsequent addition of Raji/DC-SIGN cells and TZM-bl cells. When the pre and post-DC-SIGN binding methods were combined, the virus was incubated with the lectins for an hour before addition of Raji/DC-SIGN cells for two hours. This was followed by washing and a second incubation with the lectins. The cells were then co-cultured with TZM-bl cells for 48 hours. To determine synergism between GRFT, CV-N and SVN the inhibition of transfer was tested for each lectin alone and in combination. Synergism, additive effect or antagonism were determined by calculating the combination index (CI) using IC50 (Chou and Talalay, 1984; Xu et al., 2001). A CI of 0.3 to 0.7 was deemed indicative of synergism, 0.7 to 0.85 of moderate synergism, 0.85 to 0.9 of slight synergism, 0.9 to 1.1 of an additive effect and > 1.1 of antagonism as previously defined (Chou and Talalay, 1984; Zwick et al., 2001).
For the inhibition of HIV-1 transfer to phytohemagglutin (PHA) / interleukin-2 stimulated peripheral blood mononuclear cells (PBMC), the post and pre-DC-SIGN binding method described above were used with some modifications. With the post-DC-SGN binding method, Raji/DC-SIGN were first allowed to bind to infectious HIV-1 subtype C primary isolates, before incubation with serially diluted GRFT, CV-N and SVN. Subsequently virus-bound Raji/DC-SIGN cells were co-cultured with PHAactivated PBMC (5 × 105 cells / well). The cells were cultured in RPMI 1640 containing 20% FBS and interleukin-2. PBMC cultured with Raji cells in the absence of the virus and lectins were used as negative controls. After 24 hours Raji/DC-SIGN cells and PBMC were washed three times by centrifuging at 1,200 rpm for 5 minutes. All the washing steps were performed with 20% FBS RPMI 1640. Thereon the culture supernatant was collected twice daily and replaced with an equal amount of fresh growth medium. For each harvest the p24 antigen concentration in the virus control wells was measured. The inhibitory activity of the lectins were measured at the time-point that corresponded to the early part of the linear growth period of the virus control (Zhou and Montefiori, 1997). The IC80 were calculated by plotting the lectin concentration vs. the percentage inhibition in a linear regression using GraphPad Prism 4.0. With the pre-DC-SIGN binding method, HIV-1 was first incubated for 1 hour at 37°C with serially diluted GRFT, CV-N and SVN before the subsequent addition of Raji/DC-SIGN cells and PBMC. In the combined method the virus was incubated with the lectins prior and after incubation with Raji/DC-SIGN. Virus bound Raji/DC-SIGN cells were then co-cultured with PBMC and HIV-1 infection was determined as explained above.
Acknowledgement
We thank Dr Nono Mkhize and Dr Penny Moore for helpful suggestions and Mary Phoswa for technical assistance. This work was funded by BioFISA program of NEPAD Agency/SANBio under the microbicide project, the South African AIDS Vaccine Initiative (SAAVI) and a training fellowship to KBA from Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (AITRP grant # D43TW00231). This research was also supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (B. O. & J. M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovirgel an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, Karim SS, McMahon J, O'Keefe B, Chikwamba R, Morris L. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402(1):187–196. doi: 10.1016/j.virol.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre KB, Gray ES, Pantophlet R, Moore PL, McMahon JB, Chakauya E, O'Keefe BR, Chikwamba R, Morris L. Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J Virol. 2011 doi: 10.1128/JVI.02675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5(11):726–31. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–797. doi: 10.1016/S0140-6736(07)60202-5. [DOI] [PubMed] [Google Scholar]
- Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol. 2007;71(1):3–11. doi: 10.1124/mol.106.030155. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Michael E, Eggink D, van Montfort T, Lasnik AB, Palmer KE, Sanders RW, Moore JP, Klasse PJ. Occluding the Mannose Moieties on Human Immunodeficiency Virus Type 1 gp120 with Griffithsin Improves the Antibody Responses to Both Proteins in Mice. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos LG, Louis JM, Botos I, Mori T, Han Z, O'Keefe BR, Boyd MR, Wlodawer A, Gronenborn AM. The domain-swapped dimer of cyanovirin-N is in a metastable folded state: reconciliation of X-ray and NMR structures. Structure. 2002;10(5):673–86. doi: 10.1016/s0969-2126(02)00758-x. [DOI] [PubMed] [Google Scholar]
- Bokesch HR, O'Keefe BR, McKee TC, Pannell LK, Patterson GM, Gardella RS, Sowder RC, 2nd, Turpin J, Watson K, Buckheit RW, Jr, Boyd MR. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42(9):2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- Bolmstedt AJ, O'Keefe BR, Shenoy SR, McMahon JB, Boyd MR. Cyanovirin-N defines a new class of antiviral agent targeting N-linked, high-mannose glycans in an oligosaccharide-specific manner. Mol Pharmacol. 2001;59(5):949–54. doi: 10.1124/mol.59.5.949. [DOI] [PubMed] [Google Scholar]
- Botos I, Wlodawer A. Proteins that bind high-mannose sugars of the HIV envelope. Prog Biophys Mol Biol. 2005;88(2):233–282. doi: 10.1016/j.pbiomolbio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O'Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, 2nd, Buckheit RW, Jr, Nara PL, Pannell LK, Sowder RC, 2nd, Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41(7):1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh L, Lozach PY, Schiffer C, Staropoli I, Pezo V, Porrot F, Canque B, Virelizier JL, Arenzana-Seisdedos F, Amara A. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J Virol. 2006;80(6):2949–2957. doi: 10.1128/JVI.80.6.2949-2957.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois M, Neidleman J, Greene WC. The achilles heel of the trojan horse model of HIV-1 trans-infection. PLoS Pathog. 2008;4(6) doi: 10.1371/journal.ppat.1000051. e1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choge I, Cilliers T, Walker P, Taylor N, Phoswa M, Meyers T, Viljoen J, Violari A, Gray G, Moore PL, Papathanosopoulos M, Morris L. Genotypic and phenotypic characterization of viral isolates from HIV-1 subtype C-infected children with slow and rapid disease progression. AIDS Res Hum Retroviruses. 2006;22(5):458–465. doi: 10.1089/aid.2006.22.458. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cilliers T, Nhlapo J, Coetzer M, Orlovic D, Ketas T, Olson WC, Moore JP, Trkola A, Morris L. The CCR5 and CXCR4 coreceptors are both used by human immunodeficiency virus type 1 primary isolates from subtype C. J Virol. 2003;77(7):4449–4456. doi: 10.1128/JVI.77.7.4449-4456.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler B, Justman J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect Dis. 2008;8(11):685–697. doi: 10.1016/S1473-3099(08)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser MT, Mori T, Mondor I, Sattentau QJ, Dey B, Berger EA, Boyd MR, Lifson JD. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73(5):4360–4371. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferir G, Palmer KE, Schols D. Synergistic activity profile of griffithsin in combination with tenofovir, maraviroc and enfuvirtide against HIV-1 clade C. Virology. 2011;417(2):253–8. doi: 10.1016/j.virol.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Fischetti L, Barry SM, Hope TJ, Shattock RJ. HIV-1 infection of human penile explant tissue and protection by candidate microbicides. Aids. 2009;23(3):319–328. doi: 10.1097/QAD.0b013e328321b778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go EP, Hewawasam G, Liao HX, Chen H, Ping LH, Anderson JA, Hua DC, Haynes BF, Desaire H. Characterization of Glycosylation Profiles of HIV-1 Transmitted/Founder Envelopes by Mass Spectrometry. J Virol. 2011;85(16):8270–8284. doi: 10.1128/JVI.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, Leseka N, Treurnicht F, Mlisana K, Shaw GM, Karim SS, Williamson C, Morris L. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol. 2007;81(12):6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol. 2005;79(9):5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong PW, Flummerfelt KB, de Parseval A, Gurney K, Elder JH, Lee B. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J Virol. 2002;76(24):12855–12865. doi: 10.1128/JVI.76.24.12855-12865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong PW, Nguyen S, Young S, Su SV, Lee B. Identification of the optimal DC-SIGN binding site on human immunodeficiency virus type 1 gp120. J Virol. 2007;81(15):8325–8336. doi: 10.1128/JVI.01765-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain LA, Lehner T. Comparative investigation of Langerhans' cells and potential receptors for HIV in oral, genitourinary and rectal epithelia. Immunology. 1995;85(3):475–484. [PMC free article] [PubMed] [Google Scholar]
- Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007;356(20):2073–2081. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Kouokam JC, Huskens D, Schols D, Johannemann A, Riedell SK, Walter W, Walker JM, Matoba N, O'Keefe BR, Palmer KE. Investigation of griffithsin's interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One. 2011;6(8):e22635. doi: 10.1371/journal.pone.0022635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AA, Gilbert C, Richard M, Beaulieu AD, Tremblay MJ. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood. 2008;112(4):1299–1307. doi: 10.1182/blood-2008-01-136473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106(3):263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6(5):371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem. 1990;265(18):10373–10382. [PubMed] [Google Scholar]
- Li J, Jiang H, Wen W, Zheng J, Xu G. The dendritic cell mannose receptor mediates allergen internalization and maturation involving notch 1 signalling. Clin Exp Immunol. 162(2):251–261. doi: 10.1111/j.1365-2249.2010.04244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J Virol. 2006;80(23):11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CF, Wang SF, Lin YT, Ho DD, Chen YM. Identification of the DC-SIGN-Interactive Domains on the Envelope Glycoprotein of HIV-1 CRF07_BC. AIDS Res Hum Retroviruses. 2011;27(8):831–839. doi: 10.1089/AID.2010.0215. [DOI] [PubMed] [Google Scholar]
- Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, Tsai D, Rao SS, Hamer DH, Parks TP, Lee PP, Xu Q. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50(10):3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78(8):4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue J, Hsu M, Yang D, Marx P, Chen Z, Cheng-Mayer C. Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to human immunodeficiency virus type 1. J Virol. 2002;76(20):10299–10306. doi: 10.1128/JVI.76.20.10299-10306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoombe SG, Short RV. Potential HIV-1 target cells in the human penis. Aids. 2006;20(11):1491–1495. doi: 10.1097/01.aids.0000237364.11123.98. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33(4):542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, O'Keefe BR, Sowder RC, 2nd, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA, Buckheit RW, Jr, McMahon JB, Boyd MR. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280(10):9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- Moulaei T, Botos I, Ziolkowska NE, Bokesch HR, Krumpe LR, McKee TC, O'Keefe BR, Dauter Z, Wlodawer A. Atomic-resolution crystal structure of the antiviral lectin scytovirin. Protein Sci. 2007;16(12):2756–2760. doi: 10.1110/ps.073157507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norvell MK, Benrubi GI, Thompson RJ. Investigation of microtrauma after sexual intercourse. J Reprod Med. 1984;29(4):269–271. [PubMed] [Google Scholar]
- O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A. 2009;106(15):6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson BK, Landay A, Siegel JN, Flener Z, Pessis D, Chaviano A, Bailey RC. Susceptibility to human immunodeficiency virus-1 infection of human foreskin and cervical tissue grown in explant culture. Am J Pathol. 2002;161(3):867–73. doi: 10.1016/S0002-9440(10)64247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114(5):605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquet V, Sattenteau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114(5):605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Doms RW. DC-SIGN and DC-SIGNR: helping hands for HIV. Trends Immunol. 2001;22(12):643–646. doi: 10.1016/s1471-4906(01)02081-6. [DOI] [PubMed] [Google Scholar]
- Ramjee G, Doncel GF, Mehendale S, Tolley EE, Dickson K. Microbicides 2008 conference: from discovery to advocacy. AIDS Res Ther. 2008;5:19. doi: 10.1186/1742-6405-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- Soilleux EJ, Coleman N. Expression of DC-SIGN in human foreskin may facilitate sexual transmission of HIV. J Clin Pathol. 2004;57(1):77–8. doi: 10.1136/jcp.57.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear GT, Zariffard MR, Xin J, Saifuddin M. Inhibition of DC-SIGN-mediated trans infection of T cells by mannose-binding lectin. Immunology. 2003;110(1):80–85. doi: 10.1046/j.1365-2567.2003.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein SEG. Transmission and epidemiology. In: Richman DD, editor. Human immunodeficiency virus. International Medical Press; 2003. pp. 5:1–5:22. [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20(1):11–18. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- Tsai CC, Emau P, Jiang Y, Tian B, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2003;19(7):535–541. doi: 10.1089/088922203322230897. [DOI] [PubMed] [Google Scholar]
- van Harmelen J, Williamson C, Kim B, Morris L, Carr J, Karim SS, McCutchan F. Characterization of full-length HIV type 1 subtype C sequences from South Africa. AIDS Res Hum Retroviruses. 2001;17(16):1527–1531. doi: 10.1089/08892220152644232. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wu L, Martin TD, Carrington M, KewalRamani VN. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology. 2004;318(1):17–23. doi: 10.1016/j.virol.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Xiong C, O'Keefe BR, Botos I, Wlodawer A, McMahon JB. Overexpression and purification of scytovirin, a potent, novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr Purif. 2006;46(2):233–239. doi: 10.1016/j.pep.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Xu W, Smith-Franklin BA, Li PL, Wood C, He J, Du Q, Bhat GJ, Kankasa C, Katinger H, Cavacini LA, Posner MR, Burton DR, Chou TC, Ruprecht RM. Potent neutralization of primary human immunodeficiency virus clade C isolates with a synergistic combination of human monoclonal antibodies raised against clade B. J Hum Virol. 2001;4(2):55–61. [PubMed] [Google Scholar]
- Yu HJ, Reuter MA, McDonald D. HIV traffics through a specialized, surface-accessible intracellular compartment during trans-infection of T cells by mature dendritic cells. PLoS Pathog. 2008;4(8) doi: 10.1371/journal.ppat.1000134. e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Gaschen B, Blay W, Foley B, Haigwood N, Kuiken C, Korber B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology. 2004;14(12):1229–1246. doi: 10.1093/glycob/cwh106. [DOI] [PubMed] [Google Scholar]
- Zhou JY, Montefiori DC. Antibody-mediated neutralization of primary isolates of human immunodeficiency virus type 1 in peripheral blood mononuclear cells is not affected by the initial activation state of the cells. J Virol. 1997;71(3):2512–2517. doi: 10.1128/jvi.71.3.2512-2517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, O'Keefe BR, Mori T, Zhu C, Giomarelli B, Vojdani F, Palmer KE, McMahon JB, Wlodawer A. Domain-swapped structure of the potent antiviral protein griffithsin and its mode of carbohydrate binding. Structure. 2006;14(7):1127–1135. doi: 10.1016/j.str.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowska NE, Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim Pol. 2006;53(4):617–626. [PubMed] [Google Scholar]
- Zwick MB, Wang M, Poignard P, Stiegler G, Katinger H, Burton DR, Parren PW. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J Virol. 2001;75(24):12198–12208. doi: 10.1128/JVI.75.24.12198-12208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]