Abstract
The natural products combretastatin A-4 (CA4) and combretastatin A-1 (CA1) are potent cancer vascular disrupting agents (VDAs) and inhibitors of tubulin assembly (IC50 = 1–2 μM). The phosphorylated prodrugs CA4P and CA1P are undergoing human clinical trials against cancer. CA1 is unique due to its incorporation of a vicinal phenol, which has afforded the opportunity to prepare both diphosphate and regioisomeric monophosphate derivatives. Here, we describe the first synthetic routes suitable for the regiospecific preparation of the CA1-monophosphates, CA1MPA (8a/b) and CA1MPB (4a/b). The essential regiochemistry necessary to distinguish between the two vicinal phenolic groups was accomplished with a tosyl protecting group strategy. Each of the four monophosphate analogues (including Z and E isomers) demonstrated in vitro cytotoxicity against selected human cancer cell lines comparable to their corresponding diphosphate congeners. Furthermore, Z-CA1MPA (8a) and Z-CA1MPB (4a) were inactive as inhibitors of tubulin assembly (IC50 > 40 μM), as anticipated in this pure protein assay.
Introduction
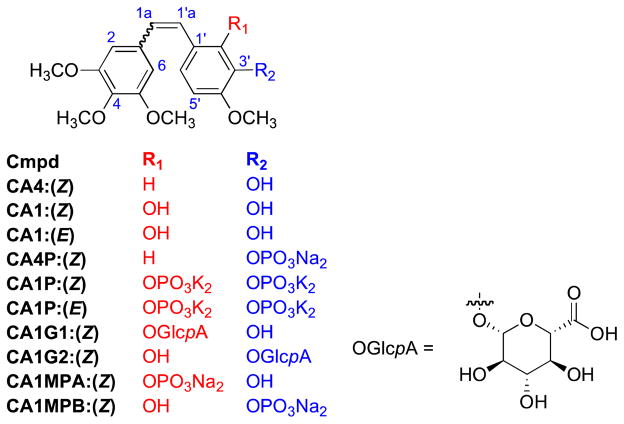
Combretastatin A-1 (CA1)1 and combretastatin A-4 (CA4)2–4 are both Z-stilbenoid natural products originally isolated from the African bush willow tree Combretum caffrum Kuntze (Combretaceae). CA1 and CA4 are remarkably potent against human cancer cell lines (in vitro)5 and are strongly inhibitory against the polymerization of tubulin into microtubules.6,7 Formulated as phosphate prodrugs [(CA1P, OXi4503)8,9 and (CA4P, Zybrestat™, fosbretabulin)10] to increase aqueous solubility, these compounds are currently under investigation in human clinical trials as anticancer drugs.11–21 CA1P and CA4P fall into a relatively new grouping of compounds collectively referred to as vascular disrupting agents (VDAs).14,22–24 Mechanistically distinct from antiangiogenic agents, VDAs are characterized by their ability to selectively damage existing microvasculature25 feeding a tumor, thus starving that tumor of oxygen and required nutrients.26–30 Interestingly, while CA4 (Z) is generally more potent than CA1 (Z) against human cancer cell lines (in vitro), the corresponding prodrug CA4P (Z) is somewhat less active than CA1P (Z) in certain in vivo preclinical tumor growth delay studies carried out in Severe Combined Immunodeficiency (SCID) mice.31,32 CA1 (Z) showed more consistent results against murine P388 leukemia in vitro, and CA1 (Z), in preclinical development, showed higher vascular disruption and antitumor activity than CA4 (Z).31 The increased effectiveness of CA1 (Z), compared to CA4 (Z), may be attributed to the presence of the second hydroxy substituent, which facilitates formation of the highly reactive ortho quinone analogue, obtainable through biological oxidation of the 1,2-diol functionality present in CA1 (Z).33,34
Recent studies by Kirwan et al. have shown that the additional phosphate group present in CA1P, as compared to CA4P, results in the formation of numerous metabolites of CA1, several of which have been identified as monophosphates, monoglucuronides, and a bis-glucuronide.35 Partial enzymatic dephosphorylation of CA1P may lead to two regioisomeric CA1 monophosphates [combretastatin A-1 monophosphate A (CA1MPA) and combretastatin A-1 monophosphate B (CA1MPB), Figure 1] in addition to formation of the active drug CA1.35 Each of the monophosphates (CA1MPA 8a(Z), 8b(E) and CA1MPB 4a(Z), 4b(E) has distinct chemical properties.36 Since the regioisomeric monophosphate salts are structurally distinct from both CA1P and CA1, it is anticipated that they may have different activity profiles in biological systems.
Figure 1.
Combretastatin A-1 (CA1) and combretastatin A-4 (CA4) derivatives.
Results and Discussion
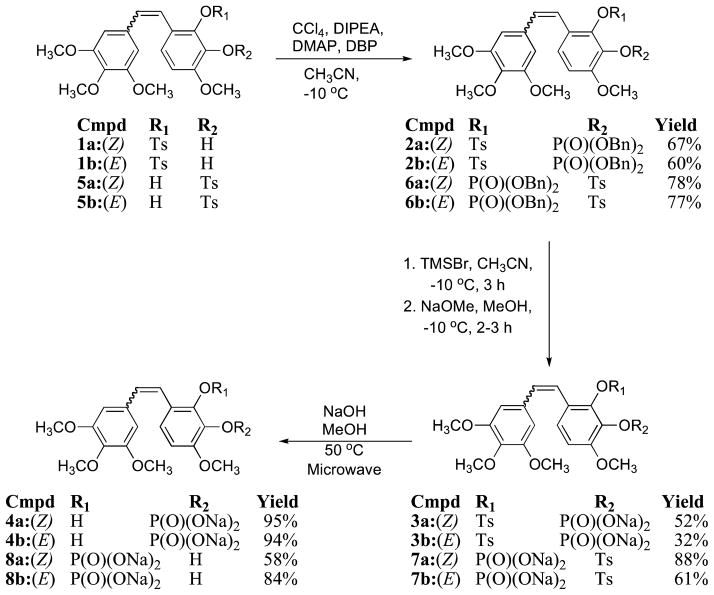
The synthesis of the stilbenoid core of CA4 and CA1, along with numerous derivatives, has been efficiently achieved using the Wittig approach.4,32,33 The regiospecificity necessary for the synthesis of the CA1 monophosphates (Scheme 1) was accomplished by distinguishing between the two vicinal phenolic functional groups at C-2′ and C-3′ of stilbenes 1a/b and 5a/b with a selective tosyl protecting group strategy.37 The phosphorylation of monophenols 1a/b and 5a/b was achieved with dibenzylphosphite as the reagent of choice due to its high reactivity, which resulted in good yields. Deprotection of the resultant benzyloxy phosphate esters 2a/b, 6a/b with TMSBr followed by treatment with MeONa provided the corresponding CA1-disodium phosphate salts 3a/b and 7a/b. Removal of the tosyl group was achieved using NaOH (2 M soln.) in a microwave reactor at a moderate temperature (50°C) to afford CA1MPA 8a(Z), CA1MPA 8b(E), CA1MPB 4a(Z), or CA1MPB 8b(E). In addition to characterization of the intermediates and final compounds by 1H and 13C NMR and HRMS data, the phosphorous-containing compounds were further characterized by their 31P NMR data. Compounds 4a, 4b, 8a, and 8b each showed a singlet in their respective 31P NMR spectrum at ~ −6.2 ppm, a characteristic of phosphoric acid triesters. On conversion of these phosphoric acid triesters to their respective sodium salts, each of them showed a downfield shift of ~ 9 ppm in their 31P NMR spectrum. HPLC studies were carried out on each of the Z-monophosphates (4a, 8a) to provide further confirmation of their chemical purity. Under these HPLC conditions neither dephosphorylation, Z/E isomerization, nor intramolecular phosphate migration was observed (see Supporting Information).
Scheme 1.
Synthesis of Z/E-CA1-2′-Monophosphates and Z/E-CA1-3′-Monophosphates.
Biological Evaluation
The cytotoxicity of the four CA1-monophosphates CA1-MPA (8a,b) and CA1-MPB (4a,b) was evaluated using a panel of three human cancer cell lines: prostate (DU-145), ovarian (SK-OV-3), and lung (NCI-H460), with doxorubicin as a reference compound. This procedure was based on the standard sulforhodamine B (SRB) assay.38,39 The GI50 values are shown in Table 1. The Z-series CA1 monophosphates (4a and 8a) showed enhanced cytotoxicity compared to the corresponding E-series regioisomers (4b and 8b). This reflects the increased cytotoxicity of Z-CA1 compared to E-CA1. The Z-series CA1 monophosphates (4a and 8a) were evaluated for their ability to inhibit tubulin assembly and were found to be inactive (IC50 > 40 μM), which is consistent with the results obtained with CA1P.
Table 1.
Cytotoxicity of CA1-monophosphate Analogues and Related Compounds Against Human Cancer Cell Lines SK-OV-3, NCI-H460, and DU-145 and Inhibition of Tubulin Polymerization.
| Cmpd | GI50 (μM) SRB assaya | Inhibition of tubulin polymerization IC50 (μM) | ||
|---|---|---|---|---|
| SK-OV-3 | NCI-H460 | DU-145 | ||
| CA1 (Z)b | 0.0384±0.0242 | 0.0153±0.0158 | 0.0326±0.0173 | 1.9c |
| CA1 (E)d | 2.27 | 1.32 | 2.14 | 11 ± 0.9 |
| 8a (Z) | 0.00164±0.000700 | 0.00356±0.000267 | 0.00277±0.000884 | >40 |
| 8b (E) | 0.465±0.0597 | 1.55±1.30 | 2.13±1.37 | nde |
| 4a (Z) | 0.00260±0.0000403 | 0.0334±0.00206 | 0.0155±0.00836 | >40 |
| 4b (E) | 0.681±0.177 | 0.336±0.0856 | 0.520±0.302 | nde |
| CA1P (Z)f | 0.00103 | 0.0133 | 0.00287 | >40g |
Experimental Section
General Experimental Procedures
Dichloromethane, acetonitrile, and tetrahydrofuran (THF) were used in their anhydrous forms, as obtained from the chemical suppliers. Reactions were performed under an inert atmosphere using nitrogen gas, unless specified. Thin-layer chromatography (TLC) plates (pre-coated glass plates with silica gel 60 F254, 0.25 mm thickness) were used to monitor reactions. Purification of intermediates and products was carried out with a flash purification system using silica gel (200–400 mesh, 60 Å) or RP-18 prepacked columns. Intermediates and products synthesized were characterized on the basis of their 1H NMR (500 MHz), 13C NMR (125 MHz), 31P NMR (202 MHz), gHSQC, and gHMBC spectroscopic data. TMS was used as an internal standard for spectra recorded in CDCl3. For spectra recorded in D2O: δ 1H 4.80. All the chemical shifts are expressed in ppm (δ), coupling constants (J) are presented in Hz, and peak patterns are reported as broad (br), singlet (s), doublet (d), triplet (t), quartet (q), septet (sept), and multiplet (m). HRESIMS were obtained using (+ve or −ve) electrospray ionization (ESI) techniques. Purity of the final compounds was further analyzed at 25 °C using a Agilent 1200 HPLC system with a diode-array detector (λ= 190–400 nm), a Zorbax XDB-C18 HPLC column (4.6 mm × 150 mm, 5 μm), and a Zorbax reliance cartridge guard-column; eluents, solvent A, 0.1% TFA in water, solvent B, 0.08% TFA in acetonitrile:water (80:20 (v/v) ratio); gradient, 80% A/20% B over 0 to 5 min; 80% A/20% B →5% A/95% B over 5 to 35 min; 5% A/95% B over 35 to 45 min; post-time 15 min; flow rate 1.0 mL/min; injection volume 20 μL; monitored at wavelengths of 254, 264, 280 and 300 nm.
(Z)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(para-toluenesulfonyl)oxy]-3″-[([bis-[(benzyl)oxy]]phosphoryl)oxy]-4″-methoxyphenyl] ethene (2a)
Phenol 1a37 (0.120 g, 0.247 mmol) was dissolved in acetonitrile (10 mL) under nitrogen and cooled to −10 °C. Carbon tetrachloride (0.20 mL, 2.1 mmol) was added, and the reaction mixture was stirred for 5 min. Diisopropylethylamine (0.40 mL, 2.3 mmol) and 4-dimethylaminopyridine (0.065 g, 0.532 mmol) were added, and the reaction mixture was stirred for an additional 10 min. Dibenzylphosphite (0.30 mL, 1.4 mmol) was added slowly to the reaction mixture, which was then stirred for 45 min and monitored by TLC. On completion, potassium dihydrogen phosphate (25 mL) was added, and the reaction mixture was allowed to return to ambient temperature. Water (25 mL) was added to the reaction mixture, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (3 × 10 mL), and the combined organic phases were dried with sodium sulfate, filtered, and concentrated under reduced pressure. Flash chromatography of the crude product using a pre-packed 10 g silica column [eluents; solvent A, EtOAc, solvent B, hexanes; gradient, 40% A/60% B over 1.15 min (1 CV), 40% A/60% B →10% A/90% B over 12.30 min (10 CV), 10% A/90% B over 3.07 min (2.5 CV); flow rate 12.0 mL/min; monitored at 254 and 280 nm] afforded 2a (0.12 g, 0.59 mmol, 67%) as a yellow oil: 1H NMR (CDCl3, 500 MHz) δ 7.77 (2H, d, J = 8.4 Hz, H-2″, H-6″), 7.40-7.30 (10H, m, Ph), 7.16 (2H, d, J = 8.4 Hz, H-3″, H-5″), 7.00 (1H, d, J = 8.8 Hz, H-6′), 6.71 (1H, d, J = 8.8 Hz, H-5′), 6.47 (2H, s, H-2, H-6), 6.36 (1H, d, J = 16.2 Hz, H-1a), 6.30 (1H, d, J = 16.2 Hz, H-1′a), 5.12 (4H, m, OCH2Ph), 3.81 (3H, s, OCH3-4), 3.75 (3H, s, OCH3-4′), 3.68 (6H, s, OCH3-3, -5), 2.33 (3H, s, CH3-4″); 13C NMR (CDCl3, 125 MHz): δ 152.8 (C, C-3, C-5), 151.5 (C, C-4′), 145.2 (C, C-4″), 140.4 (C, C-2′), 137.2 (C, C-4), 135.9 (C, Ph), 134.2 (C, C-3′), 133.7 (C, C-1″), 132.0 (C, C-1), 131.5 (CH, C-1a), 129.6 (CH, C-3″, C-5″), 128.5 (CH, C-2″, C-6″), 128.4 (4CH, Ph), 128.3 (2CH, Ph), 127.7 (4CH, Ph), 127.0 (CH, C-6′), 126.0 (C, C-1′), 123.9 (CH, C-1′a), 110.8 (CH, C-5′), 106.1 (CH, C-2, C-6), 69.7 (2CH2, OCH2Ph), 60.9 (CH3, OCH3-4), 56.5 (CH3, OCH3-4′), 56.0 (CH3, OCH3-3, -5), 21.6 (CH3, CH3-4″); 31P NMR (CDCl3, 202 MHz) δ −6.16; HRESIMS m/z 746.2017 [M + 1]+ (calcd for C39H40O11PS+, 746.2024).
(Z)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(para-toluenesulfonyl)oxy]-3″-[(disodium)phosphate]-4″-methoxyphenyl] ethene (3a)
Dibenzylphosphate 2a (0.31g, 0.41 mmol) was dissolved in acetonitrile (25 mL) cooled to −10 °C. Freshly distilled TMSBr (0.27 mL, 2.1 mmol) was added, and the reaction mixture was stirred for 3 h at −10 °C. Next the reaction mixture was added dropwise to a suspension of NaOMe (0.111g, 2.06 mmol) in MeOH (10 mL) cooled to −10 °C. The reaction mixture was stirred for 3 h and then allowed to slowly return to ambient temperature. On completion, MeOH was removed in vacuo. Flash chromatographic separation of the crude product using a pre-packed 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 0 to 1.19 min (1 CV), 100% A/0% B →60% A/40% B over 18.28 min (14 CV), 0% A/100% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] afforded sodium phosphate 3a (0.13 g, 52%) as an off-white solid: 1H NMR (CD3OD, 500 MHz) δ 7.93 (2H, d, J = 8.1 Hz, H-2″, H-6″), 7.28 (2H, d, J = 8.1 Hz, H-3″, H-5″), 6.78 (1H, d, J = 8.6 Hz, H-6′), 6.75 (1H, dd, J = 8.6 Hz, H-5′), 6.53 (2H, s, H-2, H-6), 6.04 (1H, d, J = 12.2 Hz, H-1′a), 6.01 (1H, d, J = 12.2 Hz, H-1a), 3.86 (3H, s, OCH3-4′), 3.74 (3H, s, OCH3-4), 3.64 (6H, s, OCH3-3, -5), 2.38 (3H, s, CH3-4″); 13C NMR (CD3OD, 125 MHz) δ 154.9 (C, C-4′), 154.1 (C, C-3, C-5), 146.8 (C, C-4″), 143.4 (C, C-2′), 140.5 (C, C-3′), 138.4 (C, C-4), 135.7 (C, C-1″), 133.9 (C, C-1), 131.5 (CH, C-1a), 130.8 (CH, C-3″, C-5″), 129.8 (CH, C-2″, C-6″), 126.1 (CH, C-1′a), 124.8 (CH, C-6′), 124.5 (C, C-1′), 112.2 (CH, C-5′), 107.9 (CH, C-2, C-6), 61.3 (CH3, OCH3-4), 57.2 (CH3, OCH3-4′), 56.6 (CH3, OCH3-3, -5), 21.8 (CH3, CH3-4″); 31P NMR (CD3OD, 122 MHz) δ 2.73; HRESIMS m/z 611.0723 [M + 1]+ (calcd for C25H26Na2O11PS+, 611.0723).
(Z)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[hydroxy]-3″-[(disodium)phosphate]-4″-methoxyphenyl] ethene (4a)
A solution of sulfonate ester 3a (0.089 g, 0.146 mmol) and NaOH (3 mL, 2M) in MeOH (3 mL) in a 5 mL microwave safe sealed vial was heated to 50 °C for 30 min. Reverse phase TLC (30:70 acetonitrile-water) was used to monitor the reaction. On completion, aqueous solvents were evaporated under reduced pressure. The crude product was subjected to flash chromatography using a pre-packed 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.19 min (1 CV), 100% A/0% B →60% A/40% B over 13.12 min (10 CV), 0% A/100% B over 3.57 min (2 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] affording sodium phosphate 4a (0.063 g, 0.14 mmol, 95%) as an off-white solid: 1H NMR (D2O, 500 MHz) δ 6.82 (1H, d, J = 8.6 Hz, H-6′), 6.69 (1H, d, J = 12 Hz, H-1′a), 6.65 (2H, s, H-2, H-6), 6.60 (1H, d, J = 12 Hz, H-1a), 6.53 (1H, d, J = 8.6 Hz, H-5′), 3.83 (3H, s, OCH3-4′), 3.75 (3H, s, OCH3-4), 3.69 (6H, s, OCH3-3, -5); 13C NMR (D2O, 125 MHz): δ 152.0 (C, C-3, C-5), 151.6 (C, C-4′), 147.3 (C, C-2′), 135.6 (C, C-4), 133.7 (C, C-1), 131.3 (C, C-3′), 129.5 (CH, C-1a), 126.3 (CH, C-1′a), 123.8 (CH, C-6′), 119.7 (C, C-1′), 106.4 (CH, C-2, C-6), 104.4 (CH, C-5′), 60.8 (CH3, OCH3-4), 56.0 (CH3, OCH3-4′), 55.8 (CH3, OCH3-3, -5); 31P NMR (D2O, 122 MHz) δ 3.68; HRESIMS m/z 457.0633 [M + H]+ (calcd for C18H20Na2O9P+, 457.0635).
(Z)-1-[3′,4′,5′-Trimethoxy]-2-[2″-[(benzyl)oxy]]phosphoryl)oxy]-3″-[(para-toluenesulfonyl)oxy]-4″-methoxyphenyl] ethene (6a)
Phenol 5a37 (0.77 g, 1.8 mmol) was dissolved in acetonitrile (15 mL) cooled to −10 °C. Carbon tetrachloride (2.00 mL, 20.7 mmol) was added, and the reaction mixture was stirred for 5 min. Diisopropylethylamine (0.7 mL, 4 mmol) and 4-dimethylaminopyridine (0.151 g, 1.23 mmol) were added, and the reaction mixture was stirred for an additional 10 min. Dibenzylphosphite (0.50 mL, 2.3 mmol) was added slowly to the reaction mixture, which was then stirred for 1 h and monitored by TLC. On completion, saturated potassium dihydrogen phosphate solution (25 mL) was added, and the reaction mixture was allowed to return to ambient temperature. Water (25 mL) was added to the reaction mixture, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (2 × 20 mL), and the combined organic phases were dried over sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was subjected to flash chromatography using a pre-packed 100 g silica column [eluents; solvent A, EtOAc, solvent B, hexanes; gradient, 30% A/70% B over 3.18 min (1 CV), 30% A/70% B →80% A/20% B over 33.00 min (10 CV), 80% A/20% B over 6.36 min (2 CV); flow rate 40.0 mL/min; monitored at 254 and 280 nm], affording 6a (0.91 g, 1.2 mmol, 78%) as a yellow oil: 1H NMR (CDCl3, 500 MHz) δ 7.75 (2H, d, J = 8.4 Hz, H-2″, H-6″), 7.35-7.25 (10H, m, Ph), 7.21 (2H, d, J = 8.4 Hz, H-3″, H-5″), 7.04 (1H, d, J = 8.8 Hz, H-6′), 6.63 (1H, d, J = 11.9 Hz, H-1′a), 6.62 (1H, d, J = 8.8 Hz, H-5′), 6.51 (1H, d, J = 11.9 Hz, H-1a), 6.42 (2H, s, H-2, H-6), 5.06 (4H, m, OCH2Ph), 3.82 (3H, s, OCH3-4), 3.64 (6H, s, OCH3-3, -5), 3.60 (3H, s, OCH3-4′), 2.36 (3H, s, CH3-4″); 13C NMR (CDCl3, 125 MHz) δ 152.8 (C, C-3, C-5), 152.7 (C, C-4′), 144.9 (C, C-4″), 142.8 (C, C-2′), 137.2 (C, C-4), 135.7 (2C, Ph-C1), 134.3 (C, C-1″), 132.1 (C, C-1a), 132.0 (C, C-1), 131.6 (C, C-3′), 129.3 (CH, C-3″, -5″), 129.0 (CH, C-6′), 128.5 (CH, C-2″, C-6″), 128.43 (4CH, Ph), 128.36 (2CH, Ph), 127.8 (4CH, Ph), 124.7 (C, C-1′), 124.3 (CH, C-1′a), 109.1 (CH, C-5′), 106.2 (CH, C-2, C-6), 69.9 (2CH2, OCH2Ph), 60.9 (CH3, OCH3-4), 56.1 (CH3, OCH3-4′), 55.9 (CH3, OCH3-3, -5), 21.6 (CH3, CH3-4″); 31P NMR (CDCl3, 202 MHz) δ −6.16; HRESIMS m/z 747.2024 [M]+ (calcd for C39H40O11PS+, 747.2023); anal. C 62.92, H 5.29%, calcd for C39H39O11PS, C 62.73, H 5.26%.
(Z)-1-[3′,4′,5′-Trimethoxy]-2-[2″-[(disodium)phosphate]-3″-[(para-toluenesulfonyl)oxy]-4″-methoxyphenyl] ethene (7a)
Compound 6a (0.60 g, 0.80 mmol) was dissolved in acetonitrile (12 mL) cooled to −10 °C under nitrogen. Freshly distilled TMSBr (0.3 mL, 2.3 mmol) was added dropwise, and the mixture was stirred for 1 h at −10 °C. At that point the mixture was added to a suspension of NaOMe (0.45 g, 8.3 mmol) in MeOH (25 mL) cooled to −10 °C. The mixture was then stirred for 2 h and allowed to slowly return to ambient temperature. On completion, MeOH was evaporated at 50 °C in vacuo, and flash chromatographic separation of the crude product using a pre-packed 12 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.15 min (1 CV), 100% A/0% B →45% A/55% B over 17.30 min (14 CV), 0% A/100% B over 3.45min (3 CV); flow rate 12.0 mL/min; monitored at 254 and 280 nm] led to sodium phosphate 7a (0.43 g, 0.71 mmol, 88 %): 1H NMR (D2O, 500 MHz) δ 7.71 (2H, d, J = 8.4 Hz, H-2″, -6″), 7.39 (2H, d, J = 8.1 Hz, H-3″, -5″), 7.01 (1H, d, J = 8.7 Hz, H-6′), 7.00 (1H, d, J = 12.1 Hz, H-1′a), 6.60 (1H, d, J = 12.1 Hz, H-1a), 6.59 (1H, d, J = 8.8 Hz, H-2, -6), 6.49 (2H, s, H-5′), 3.73 (3H, s, OCH3-4), 3.70 (6H, s, OCH3-3, -5), 3.29 (3H, s, OCH3-4′), 2.41 (3H, s, CH3-4″); 13C NMR (D2O, 125 MHz) δ 151.9 (C, C-3, C-5), 151.3 (C, C-4′), 146.6 (C, C-2′), 146.5 (C, C-4″), 135.6 (C, C-4), 133.7 (C, C-1), 131.8 (C, C-1″), 131.5 (C, C-3′), 129.5 (CH, C-1a), 129.5 (CH, C-3″, C-5″), 128.8 (CH, C-6′), 128.2 (CH, C-2″, C-6″), 127.2 (CH, C-1′a), 125.3 (C, C-1′), 106.9 (CH, C-5′), 106.6 (CH, C-2, C-6), 60.8 (CH3, OCH3-4), 55.8 (CH3, OCH3-3, -5), 55.5 (CH3, OCH3-4′), 20.7 (CH3, CH3-4″); 31P NMR (D2O, 202 MHz) δ −3.19; HRESIMS m/z 611.0716 [M + 1]+ (calcd for C25H26Na2O11PS+, 611.0723).
(Z)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(disodium)phosphate]-3″-[hydroxy]-4″-methoxyphenyl] ethene (8a)
Sulfonate ester 7a (0.107 g, 0.175 mmol) was dissolved in MeOH (3 mL) in a 5 mL microwave safe vial with a stir bar. To this solution NaOH (2 mL, 2 M) was added, the vial was capped, and the reaction mixture was pre-stirred for 5 min. The reaction mixture was heated at 50 °C in a microwave reactor for 30 min. Temperatures higher than 50 °C may lead to isomerization of the compound. On completion, the solvents were evaporated in vacuo and the crude product was subjected to flash chromatographic separation using a prepacked 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 0 to 1.19 min (1 CV), 100% A/0% B →60% A/40% B over 13.12 min (10 CV), 60% A/40% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] affording 8a (0.046 g, 0.100 mmol, 58%): 1H NMR (500 MHz, D2O) δ 6.82 (1H, d, J = 12 Hz, H-1′a), 6.67 (1H, d, J = 8.7 Hz, H-6′), 6.66 (2H, s, H-2, -6), 6.64 (1H, d, J = 8.7 Hz, H-5′), 6.62 (1H, d, J = 12 Hz, H-1a), 3.83 (3H, s, OCH3-4′), 3.76 (3H, s, OCH3-4), 3.69 (6H, s, OCH3-3, -5); 13C NMR (125 MHz, D2O) δ 151.9 (C, C-3, C-5), 148.6 (C, C-4′), 140.4 (C, C-2′), 138.5 (C, C-3′), 135.5 (C, C-4), 133.9 (C, C-1), 129.1 (CH, C-1a), 127.2 (CH, C-1′a), 124.2 (CH, C-1′), 120.2 (C, C-6′), 107.3 (CH, C-5′), 106.5 (CH, C-2, C-6), 60.9 (CH3, OCH3-4), 56.0 (CH3, OCH3-4′), 55.8 (CH3, OCH3-3, -5); 31P NMR (D2O, 202 MHz) δ 3.21. HRESIMS m/z 457.0632 [M + H]+ (calcd for C18H20Na2O9P+, 457.0635).
(E)-1-[3′,4′,5′-Trimethoxy]-2-[2″-[(benzyl)oxy]]phosphoryl)oxy]-3″-[(para-toluenesulfonyl)oxy]-4″-methoxyphenyl] ethene (6b)
Phenol 5b37 (0.47 g, 0.96 mmol) was dissolved in acetonitrile (10 mL) cooled to −10 °C, carbon tetrachloride (1 mL, 10 mmol) was added, and the reaction mixture was stirred for 5 min. Diisopropylethylamine (0.3 mL, 1.7 mmol) and 4-dimethylaminopyridine (0.1 g, 0.8 mmol) were added, and the reaction mixture was stirred for an additional 10 min. Dibenzylphosphite (0.25 mL, 1.1 mmol) was added slowly to the mixture, and stirring continued for 45 min (monitored by TLC). On completion, saturated potassium dihydrogen phosphate solution (50 mL) was added, and the mixture was allowed to return to ambient temperature. Water (50 mL) was added, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (3 × 10 mL), and the combined organic phase was dried over sodium sulfate. The solution was filtered and concentrated under reduced pressure. Flash chromatographic separation of the crude product was performed using a prepacked 100 g silica column [eluents; solvent A, EtOAc, solvent B, hexanes; gradient, 40% A/60% B over 1.15 min (1 CV), 40% A/60% B →54% A/46% B over 3.22 min (2.7 CV), 54% A/46% B →100% A/0% B over 11.15 min (9 CV), 100% A/0% B over 3.07 min (2.5 CV); flow rate 12.0 mL/min; monitored at 254 and 280 nm] and yielded a pure yellow oil 6b (0.55 g, 0.74 mmol, 77%): 1H NMR (CDCl3, 500 MHz) δ 7.84 (2H, d, J = 8.4 Hz, H-2″, H-6″), 7.50 (1H, d, J = 9.0 Hz, H-6′), 7.34 (1H, d, J = 16.3 Hz, H-1′a), 7.28 (2H, d, J = 8.4 Hz, H-3″, H-5″), 7.26-7.22 (10H, m, Ph), 6.88 (1H, d, J = 16.3 Hz, H-1a), 6.76 (1H, dd, J = 9.0, 0.8 Hz, H-5′), 6.67 (2H, s, H-2, H-6), 5.08 (4H, m, OCH2Ph), 3.86 (3H, s, OCH3-4), 3.76 (6H, s, OCH3-3, -5), 3.58 (3H, s, OCH3-4′), 2.41 (3H, s, CH3-4″); 13C NMR (CDCl3, 125 MHz) δ 153.3 (C, C-3, C-5), 152.6 (C, C-4′), 144.9 (C, C-4″), 142.4 (C, C-2′), 137.9 (C, C-4), 135.5 (2C, Ph), 134.2 (C, C-1″), 133.0 (C, C-1), 131.5 (C, C-3′), 129.5 (CH, C-1a), 129.3 (CH, C-3″, C-5″), 128.6 (CH, C-2″, C-6″), 128.42 (4CH, Ph), 128.40 (2CH, Ph), 127.9 (4CH, Ph), 124.5 (C, C-1′), 124.2 (CH, C-6′), 121.5 (CH, C-1′a), 109.6 (CH, C-5′), 103.6 (CH, C-2, C-6), 70.0 (2CH2, OCH2Ph), 60.9 (CH3, OCH3-4), 56.0 (CH3, OCH3-3, -5), 55.9 (CH3, OCH3-4′), 21.6 (CH3, CH3-4″); 31P NMR (CDCl3, 202 MHz) δ −5.84; HRESIMS m/z 747.2019 [M]+ (calcd for C39H40O11PS+, 747. 2023); anal. C 62.46, H 5.23%, calcd for C39H39O11PS, C 62.73, H 5.26%.
(E)-1-[3′,4′,5′-Trimethoxy]-2-[2″-[(disodium)phosphate]-3″-[(para-toluenesulfonyl)oxy]-4″-methoxyphenyl] ethene (7b)
Dibenzylphosphate 6b (0.32 g, 0.43 mmol) was dissolved in acetonitrile (5 mL) cooled to −10 °C under nitrogen. Freshly distilled TMSBr (0.25 mL, 1.9 mmol) was added, and the reaction mixture was stirred for 3 h at −10 °C. The initial mixture was added dropwise to a suspension of NaOMe (0.22 g, 4.1 mmol) in MeOH (15 mL) cooled to −10 °C. The reaction was stirred for 3 h and allowed to return to ambient temperature. On completion, solvents were evaporated in vacuo (<50 °C to prevent isomerization), and the crude product was subjected to flash chromatographic separation using a prepacked 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.19 min (1 CV), 100% A/0% B →61% A/39% B over 12.52 min (9.7 CV), 61% A/39% B→45% A/55% B over 5.16 min (4 CV), 0% A/100% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] affording 7b (0.16 g, 2.6 mmol, 61 %) as an off-white solid: 1H NMR (D2O, 500 MHz) δ 7.59 (2H, d, J = 8.4 Hz, H-2″, H-6″), 7.36 (1H, d, J = 16.3 Hz, H-1′a), 7.28 (1H, d, J = 9.5 Hz, H-6′), 7.22 (2H, d, J = 8.4 Hz, H-3″, H-5″), 6.69 (2H, s, H-2, H-6), 6.63 (1H, d, J = 16.5 Hz, H-1a), 6.58 (1H, dd, J = 8.5 Hz, H-5′), 3.82 (6H, s, OCH3-3, -5), 3.70 (3H, s, OCH3-4), 3.33 (3H, s, OCH3-4′), 2.28 (3H, s, CH3-4″); 13C NMR (D2O, 125 MHz) δ 152.3 (C, C-3, C-5), 151.6 (C, C-4′), 146.4 (C, C-4″), 144.5 (C, C-2′), 136.1 (C, C-4), 134.0 (C, C-1), 131.6 (C, C-1″), 131.0 (C, C-3′), 129.5 (CH, C-3″, C-5″), 128.3 (CH, C-2″, C-6″), 128.0 (CH, C-1a), 124.5 (C, C-1′), 124.3 (CH, C-6′), 122.2 (CH, C-1′a), 108.7 (CH, C-5′), 103.7 (CH, C-2, C-6), 60.8 (CH3, OCH3-4), 55.9 (CH3, OCH3-3, -5), 55.6 (CH3, OCH3-4′), 20.8 (CH3, CH3-4″); 31P NMR (CDCl3, 202 MHz) δ −4.25; HRESIMS m/z 611.0716 [M + H]+ (calcd for C25H26Na2O11PS+, 611.0723); anal. C 48.51, H 4.62%, calcd for C25H25Na2O11PS·0.5H2O, C 48.47, H 4.23%.
(E)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(disodium)phosphate]-3″-[hydroxy]-4″-methoxyphenyl] ethene (8b)
Sulfonate ester 7b (0.100 g, 0.164 mmol) was dissolved in MeOH (17 mL) in a 20 mL microwave safe vial. To this solution NaOH (3 mL, 2M) was added, the vial was capped, and the reaction was pre-stirred for 2 min. The reaction mixture was heated at 50 °C in a microwave reactor for 30 min. As noted above, temperatures higher than 50 °C can lead to isomerization. On completion, the solvents were evaporated in vacuo, and the crude product was subjected to flash chromatographic separation using a prepacked 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.19 min (1 CV), 100% A/0% B →60% A/40% B over 13.12 min (10 CV), 60% A/40% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] providing sodium phosphate 8b (0.063 g, 0.138 mmol, 84%): 1H NMR (D2O, 500 MHz) δ 7.46 (1H, d, J = 16.55 Hz, H-1′a), 7.25 (1H, d, J = 8.75 Hz, H-6′), 7.07 (1H, d, J = 16.55 Hz, H-1a), 7.03 (2H, s, H-2, H-6), 6.86 (1H, d, J = 8.75 Hz, H-5′), 3.94 (6H, s, OCH3-3, -5), 3.89 (3H, s, OCH3-4′), 3.81 (3H, s, OCH3-4); 13C NMR (D2O, 125 MHz): δ 152.6 (C, C-3, C-5), 148.9 (C, C-4′), 140.2 (C, C-2′), 138.3 (C, C-3′), 136.1 (C, C-4), 134.6 (C, C-1), 127.5 (CH, C-1a), 124.2 (CH, C-1′), 123.9 (C, C-1′a), 116.5 (CH, C-6′), 107.9 (CH, C-5′), 104.1 (CH, C-2, C-6), 60.9 (CH3, OCH3-4), 56.1 (CH3, OCH3-3, -5), 56.0 (CH3, OCH3-4′); 31P NMR (D2O, 202 MHz) δ 3.54; HRESIMS m/z 457.0632 [M + H]+ (calcd for C18H20Na2O9P+, 457.0635).
(E)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(para-toluenesulfonyl)oxy]-3″-[([bis-[(benzyl)oxy]]phosphoryl)oxy]-4″-methoxyphenyl] ethene (2b)
To a solution of phenol 1b37 (0.499 g, 1.03 mmol) in acetonitrile (10 mL) cooled to −10 °C under nitrogen was added CCl4 (0.20 mL, 2.07 mmol), and the reaction mixture was stirred for 5 min. Diisopropylethylamine (0.7 mL, 4.0 mmol) and 4-dimethylaminopyridine (0.06 g, 0.49 mmol) were added, and the mixture was stirred for an additional 10 min. Next, dibenzylphosphite (0.5 mL, 2.26 mmol) was slowly added to the mixture. After stirring for 45 min (monitored by TLC), potassium dihydrogen phosphate (10 mL) was added, and the mixture was allowed to return to ambient temperature. Water (20 mL) was then added, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (3 × 10 mL), and the combined organic phase was dried (sodium sulfate) and the solution was filtered and concentrated in vacuo. The crude product was separated by flash chromatography using a prepacked 25 g silica column [eluents; solvent A, EtOAc, solvent B, hexanes; gradient, 20% A/80% B over 1.19 min (1 CV), 20% A/80% B →70% A/30% B over 13.51 min (10.5 CV), 70% A/30% B over 1.27 min (1.1 CV), 100% A/0% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] to yield 2b (0.46 g, 0.61 mmol, 60%) as a yellow oil: 1H NMR (CDCl3, 500 MHz) δ 7.73 (2H, d, J = 8.1 Hz, H-2″, H-6″), 7.40 (1H, d, J = 9.0 Hz, H-6′), 7.38-7.32 (10H, m, Ph), 7.06 (2H, d, J = 8.1 Hz, H-3″, H-5″), 6.90 (1H, d, J = 9.0 Hz, H-5′), 6.80 (1H, d, J = 16.2 Hz, H-1′a), 6.71 (1H, d, J = 16.2 Hz, H-1a), 6.53 (2H, s, H-2, H-6), 5.21 (4H, m, OCH2Ph), 3.89 (6H, s, OCH3-3, -5), 3.88 (3H, s, OCH3-4), 3.80 (3H, s, OCH3-4′), 2.16 (3H, s, CH3-4″); 13C NMR (CDCl3, 125 MHz): δ 153.2 (C, C-3, C-5), 151.7 (C, C-4′), 145.6 (C, C-4″), 140.1 (C, C-2′), 137.9 (C, C-4), 136.0 (C, Ph), 134.5 (C, C-3′), 133.2 (C, C-1″), 132.8 (C, C-1), 129.6 (CH, C-3″, C-5″), 129.0 (CH, C-1a), 128.6 (CH, C-2″, C-6″), 128.4 (4CH, Ph), 128.3 (2CH, Ph), 127.8 (4CH, Ph), 125.6 (C, C-1′), 122.1 (CH, C-6′), 121.4 (CH, C-1′a), 111.5 (CH, C-5′), 103.7 (CH, C-2, C-6), 69.8 (2CH2, OCH2Ph), 61.0 (CH3, OCH3-4), 56.4 (CH3, OCH3-4′), 56.1 (CH3, OCH3-3, -5), 21.5 (CH3, CH3-4″); 31P NMR (CDCl3, 202 MHz) δ −6.23; HRESIMS m/z 747.2020 [M + 1]+ (calcd for C39H40O11PS+, 747.2023); anal. C 62.71, H 5.31%, calcd for C39H39O11PS, C 62.73, H 5.26%.
(E)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[(para-toluenesulfonyl)oxy]-3″-[(disodium)phosphate]-4″-methoxyphenyl] ethene (3b)
To a solution of dibenzylphosphate 2b (0.16 g, 0.21 mmol) in acetonitrile (12 mL cooled to −10 °C under nitrogen) was added freshly distilled TMSBr (0.15 mL, 1.14 mmol), and the mixture was stirred for 3 h at −10 °C. The solution was added dropwise to a suspension of NaOMe (0.15 g, 2.78 mmol) in MeOH (10 mL) cooled to −10 °C. The mixture was stirred for 3 h, allowed to slowly return to ambient temperature, and the solvent was evaporated in vacuo. The crude product was separated by flash chromatography using a prepacked 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.19 min (1 CV), 100% A/0% B →45% A/55% B over 18.28 min (14 CV), 0% A/100% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] to afford 3b (0.042 g, 32%): 1H NMR (D2O, 500 MHz) δ 7.52 (2H, d, J = 8.0 Hz, H-2″, H-6″), 7.08 (1H, d, J = 9.0 Hz, H-6′), 6.91 (2H, d, J = 8.0 Hz, H-3″, H-5″), 6.84 (1H, d, J = 9.0 Hz, H-5′), 6.42 (1H, d, J = 16.5 Hz, H-1a), 6.36 (1H, d, J = 16.5 Hz, H-1′a), 6.34 (2H, s, H-2, H-6), 3.82 (3H, s, OCH3-4′), 3.73 (6H, s, OCH3-3, -5), 3.71 (3H, s, OCH3-4), 1.90 (3H, s, CH3-4″); 13C NMR (D2O, 125 MHz) δ 152.3 (C, C-4′), 152.1 (C, C-3, C-5), 146.5 (C, C-4″), 140.2 (C, C-2′), 136.1 (C, C-4), 135.7 (C, C-3′), 133.5 (C, C-1), 131.8 (C, C-1″), 129.8 (CH, C-2″, C-6″), 128.3 (CH, C-1a), 127.9 (CH, C-3″, C-5″), 124.2 (C, C-1′), 121.4 (CH, C-6′), 121.2 (CH, C-1′a), 111.8 (CH, C-5′), 103.7 (CH, C-2, C-6), 60.7 (CH3, OCH3-4), 56.1 (CH3, OCH3-4′), 55.8 (CH3, OCH3-3, -5), 20.5 (CH3, CH3-4″); 31P NMR (D2O, 202 MHz) δ −3.99; HRESIMS m/z 611.0713 [M + H]+ (calcd for C25H26Na2O11PS+, 611.0723).
(E)-1-[3′,4′,5′-Trimethoxyphenyl]-2-[2″-[hydroxy]-3″-[(disodium)phosphate]-4″-methoxyphenyl] ethene 4b
A solution of NaOH (3 mL, 2 M) was added to sulfonate ester 3b (0.050 g, 0.082 mmol) in MeOH (17 mL, in a 20 mL microwave safe vial with a stir bar). The vial was capped and placed in the microwave. The solution was pre-stirred for 2 min and then heated at 50 °C in a microwave reactor for 30 min. (Caution: temperatures higher than 50 °C may lead to isomerization.) Reverse phase TLC (30:70 acetonitrile-water) was used to monitor the reaction course. After the reaction was complete, the solvent was evaporated in vacuo. The crude product was subjected to flash chromatographic separation using a prepacked 30 g RP-18 silica column [eluents, solvent A, water, solvent B, acetonitrile; gradient, 100% A/0% B over 1.19 min (1 CV), 100% A/0% B →60% A/40% B over 13.12 min (10 CV), 0% A/100% B over 3.57 min (3 CV); flow rate 25.0 mL/min; monitored at 254 and 280 nm] to yield 4b (0.035 g, 0.077 mmol, 94%): 1H NMR (D2O, 500 MHz) δ 7.36 (1H, d, J = 16.35 Hz, H-1′a), 7.29 (1H, d, J = 8.75 Hz, H-6′), 6.98 (1H, d, J = 16.35 Hz, H-1a), 6.86 (2H, s, H-2, H-6), 6.67 (1H, d, J = 8.65 Hz, H-5′), 3.88 (3H, s, OCH3-4′), 3.86 (6H, s, OCH3-3, -5), 3.77 (3H, s, OCH3-4); 13C NMR (D2O, 125 MHz): δ 152.4 (C, C-3, C-5), 151.9 (C, C-4′), 147.6 (C, C-2′), 135.8 (C, C-4), 134.7 (C, C-1), 131.3 (C, C-3′), 126.5 (C, C-1a), 123.3 (CH, C-1′a), 120.6 (CH, C-6′), 119.6 (CH, C-1′), 104.7 (CH, C-5′), 103.4 (CH, C-2, C-6), 60.8 (CH3, OCH3-4), 55.9 (CH3, OCH3-4′), 55.8 (CH3, OCH3-3, -5).; 31P NMR (D2O, 202 MHz) δ −3.74; HRESIMS m/z 457.0631 [M + 1]+ (calcd for C18H20Na2O9P+, 457.0635).
Supplementary Material
Acknowledgments
The authors are grateful to OXiGENE, Inc. (grants to K.G.P. and M.L.T.), and the Welch Foundation (grant no. AA-1278 to K.G.P.), and one of us (G.R.P.) for grants R01 CA90441-01 and SR01 CA090441-07-08 from The Division of Cancer Treatment Diagnosis, NCI, DHHS for their financial support of this project, and to the NSF for funding the Varian 500 MHz NMR spectrometer (grant no. CHE-0420802). In addition, portions of this project were partially supported by Award Number 5R01CA140674 (to KGP and MLT) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors also thank Dr. Alejandro Ramirez (Mass Spectrometry Core Facility, Baylor University) for mass spectroscopic analysis, Dr. Craig Moehnke for assistance with NMR studies, and Dr. James Karban and Dr. Michelle Nemec (Director) for use of the shared Molecular Biosciences Center at Baylor University.
Footnotes
Supporting Information Available. General experimental details regarding tubulin and cytotoxicity assays, 1H NMR, 13C NMR, gHSQC, gHMBC, HRESIMS, and HPLC. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pettit GR, Singh SB, Niven ML, Hamel E, Schmidt JM. J Nat Prod. 1987;50:119–131. doi: 10.1021/np50049a016. [DOI] [PubMed] [Google Scholar]
- 2.Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D. Experientia. 1989;45:209–211. doi: 10.1007/BF01954881. [DOI] [PubMed] [Google Scholar]
- 3.Lin CM, Singh SB, Chu PS, Dempcy RO, Schmidt JM, Pettit GR, Hamel E. Mol Pharmacol. 1988;34:200–208. [PubMed] [Google Scholar]
- 4.Lin CM, Ho HH, Pettit GR, Hamel E. Biochemistry. 1989;28:6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- 5.NCI. Cancer Screening Data. DTP; 2010. [Google Scholar]
- 6.Pettit GR, Toki B, Herald DL, Verdier-Pinard P, Boyd MR, Hamel E, Pettit RK. J Med Chem. 1998;41:1688–1695. doi: 10.1021/jm970644q. [DOI] [PubMed] [Google Scholar]
- 7.Pettit GR, Grealish MP, Herald DL, Boyd MR, Hamel E, Pettit RK. J Med Chem. 2000;43:2731–2737. doi: 10.1021/jm000045a. [DOI] [PubMed] [Google Scholar]
- 8.Pettit GR, Lippert JW., III Anticancer Drug Des. 2000;15:203–216. [PubMed] [Google Scholar]
- 9.Pettit GR, Rhodes MR. Anticancer Drug Des. 1998;13:183–191. [PubMed] [Google Scholar]
- 10.Pettit GR, Temple C, Jr, Narayanan VL, Varma R, Simpson MJ, Boyd MR, Rener GA, Bansal N. Anticancer Drug Des. 1995;10:299–309. [PubMed] [Google Scholar]
- 11.Lippert JW., III Bioorg Med Chem. 2007;15:605–615. doi: 10.1016/j.bmc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Patterson DM, Rustin GJ. Clin Oncol (R Coll Radiol) 2007;19:443–456. doi: 10.1016/j.clon.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Patterson DM, Ross P, Koetz B, Saleem A, Stratford M, Stirling J, Padhani A, Asselin M, Price P, Rustin GJ. J Clin Oncol (Meeting Abstracts) 2007;25:14146. [Google Scholar]
- 14.Siemann DW, Chaplin DJ, Walicke PA. Expert Opin Investig Drugs. 2009;18:189–197. doi: 10.1517/13543780802691068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaplin DJ, Pettit GR, Hill SA. Anticancer Res. 1999;19:189–195. [PubMed] [Google Scholar]
- 16.Mooney CJ, Nagaiah G, Fu P, Wasman JK, Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS, Flick SM, Hartman PH, Ortiz JD, Lavertu PN, Remick SC. Thyroid. 2009;19:233–240. doi: 10.1089/thy.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akerley WL, Schabel M, Morrell G, Horvath E, Yu M, Johnsson B, Arbogast K. J Clin Oncol (Meeting Abstracts) 2007;25:14060. [Google Scholar]
- 18.Nathan PD, Judson I, Padhani A, Harris A, Carden CP, Smythe J, Collins D, Leach M, Walicke P, Rustin GJ. J Clin Oncol (Meeting Abstracts) 2008;26:3550. [Google Scholar]
- 19.www.Oxigene.com (accessed Aug 04, 2010).
- 20.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Cancer Res. 1997;57:1829–1834. [PubMed] [Google Scholar]
- 21.Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC. Br J Cancer. 1999;81:1318–1327. doi: 10.1038/sj.bjc.6692174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinney KG. Vascular-Targeted Therapies in Oncology. John Wiley & Sons, Ltd; 2006. Molecular Recognition of the Colchicine Binding Site as a Design Paradigm for the Discovery and Development of Vascular Disrupting Agents; pp. 95–121. [Google Scholar]
- 23.Pinney KG, Jelinek C, Edvardsen K, Chaplin DJ, Pettit GR. The Discovery and Development of the Combretastatins. In: Cragg GR, Kingston DGI, Newman DJ, editors. Anticancer Agents from Natural Products. CRC Press/Taylor & Francis; Boca Raton, FL: 2005. pp. 23–46. [Google Scholar]
- 24.Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, Marme D, Lorusso PM. Clin Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 25.Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafii S. J Clin Invest. 2005;115:2992–3006. doi: 10.1172/JCI24586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan MA, Wilson L. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 27.Tozer GM, Kanthou C, Baguley BC. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 28.Monk KA, Siles R, Hadimani MB, Mugabe BE, Ackley JF, Studerus SW, Edvardsen K, Trawick ML, Garner CM, Rhodes MR, Pettit GR, Pinney KG. Bioorg Med Chem. 2006;14:3231–3244. doi: 10.1016/j.bmc.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 29.Kanthou C, Tozer GM. Expert Opin Thera Targets. 2007;11:1443–1457. doi: 10.1517/14728222.11.11.1443. [DOI] [PubMed] [Google Scholar]
- 30.Tozer GM, Akerman S, Cross NA, Barber PR, Björndahl MA, Greco O, Harris S, Hill SA, Honess DJ, Ireson CR, Pettyjohn KL, Prise VE, ReyesAldasoro CC, Ruhrberg C, Shima DT, Kanthou C. Cancer Res. 2008;68:2301–2311. doi: 10.1158/0008-5472.CAN-07-2011. [DOI] [PubMed] [Google Scholar]
- 31.Hill SA, Lonergan SJ, Denekamp J, Chaplin DJ. Eur J Cancer. 1993;29:1320–1324. doi: 10.1016/0959-8049(93)90082-q. [DOI] [PubMed] [Google Scholar]
- 32.Hua J, Sheng Y, Pinney KG, Garner CM, Kane RR, Prezioso JA, Pettit GR, Chaplin DJ, Edvardsen K. Anticancer Res. 2003;23:1433–1440. [PubMed] [Google Scholar]
- 33.Folkes LK, Christlieb M, Madej E, Stratford MRL, Wardman P. Chem Res Toxicol. 2007;20:1885–1894. doi: 10.1021/tx7002195. [DOI] [PubMed] [Google Scholar]
- 34.Pettit GR, Thornhill AJ, Moser BR, Hogan F. J Nat Prod. 2008;71:1561–1563. doi: 10.1021/np800179g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirwan IG, Loadman PM, Swaine DJ, Anthoney DA, Pettit GR, Lippert JW, Shnyder SD, Cooper PA, Bibby MC. Clin Cancer Res. 2004;10:1446–1453. doi: 10.1158/1078-0432.ccr-0518-03. [DOI] [PubMed] [Google Scholar]
- 36.Stratford MRL. J Chromatogr A. 2008;1181:162–165. doi: 10.1016/j.chroma.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 37.Tanpure RP, Strecker TE, Chaplin DJ, Siim BG, Trawick ML, Pinney KG. J Nat Prod. 2010;73:1093–1101. doi: 10.1021/np100108e. [DOI] [PubMed] [Google Scholar]
- 38.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 39.Vichai V, Kirtikara K. Nat Protocols. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 40.Monk KA, Siles R, Hadimani MB, Mugabe BE, Ackley JF, Studerus SW, Edvardsen K, Trawick ML, Garner CM, Rhodes MR, Pettit GR, Pinney KG. Bioorg Med Chem. 2006;14:3231–3244. doi: 10.1016/j.bmc.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 41.Batch prepared by Dr. Ming Zhou (Baylor University).
- 42.Gift from Oxigene Inc.
- 43.Chaplin DJ, Garner CM, Kane RR, Pinney KG, Prezioso JA, Edvardsen K. 7384925. United States Patent. 2005
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.