Abstract
Lipids are the most abundant organic constituents in many humans. The rise in obesity prevalence has prompted a need for a more refined understanding of the effects of lipid molecules on cell physiology. In skeletal muscle, deposition of lipids can be associated with insulin resistance that contributes to the development of diabetes. Here, we review the evidence that muscle cells are equipped with the molecular machinery to convert and sequester lipid molecules, thus rendering them harmless. Induction of mitochondrial and lipogenic flux in the setting of elevated lipid deposition can protect muscle from lipid-induced “poisoning” of the cellular machinery. Lipid flux may also be directed toward the synthesis of ligands for nuclear receptors, further enhancing the capacity of muscle for lipid metabolism to promote favorable physiology. Exploiting these mechanisms may have implications for the treatment of obesity-related diseases.
Keywords: insulin resistance, lipotoxicity, lipoexpediency, peroxisome proliferator-activated receptor
the obesity pandemic and its associated comorbidities have been attributed mostly to chronic excess calorie consumption. In healthy subjects, excess calories are directed to adipose tissues where they are stored as triglycerides (TGs). However, when some poorly defined threshold for fat storage is exceeded, as occurs in metabolic syndrome, adipose tissue becomes insulin resistant, suppression of lipolysis is diminished, and the increased lipolysis of adipose TGs elevates circulating free fatty acids (FFAs) (19, 90). This excess circulating fat can be deposited in nonadipose tissues, which can result in cellular dysfunction commonly referred to as lipotoxicity (9, 108). In skeletal muscle, lipid-mediated signaling appears to be involved in insulin resistance (58, 80) through the effects of several molecular species (58, 96). Skeletal muscle insulin resistance is important in the development of type 2 diabetes (19, 21, 86).
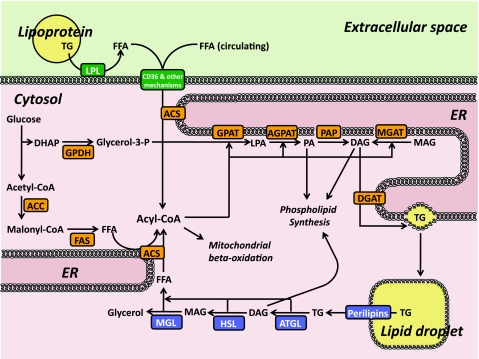
Figure 1 summarizes lipid metabolism in skeletal muscle. Lipoprotein-associated lipids are broken down into FFAs by lipoprotein lipase (LPL) on capillary endothelial cells (103). These FFAs, along with circulating FFAs that are derived from adipose lipolysis, are imported into skeletal muscle through multiple pathways involving the multifunctional receptor CD36 (16, 28), fatty acid transport proteins (FATPs), and noncarrier mechanisms (102). Intracellular FFAs are converted into acyl-CoAs by the membrane-associated enzyme acyl-CoA synthases (ACSs) (24, 63) or FATPs (102). ACSs also mediate synthesis of acyl-CoAs from FFAs that are derived from two additional pathways: de novo lipogenesis by acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) (104) and intramuscular lipolysis by adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (MGL) (111, 118). FAS is not as abundantly expressed in skeletal muscle as in classically lipogenic tissues such as liver or adipose tissues (K. Funai and C. F. Semenkovich, unpublished data); thus, it is assumed that the contribution of de novo lipogenesis to intramuscular lipid composition is low (85). Acyl-CoAs derived from these distinct pathways may become compartmentalized in separate intramuscular pools. At rest, FFAs that enter muscle cells appear initially to be directed to the intramuscular TG pool, whereas acyl-CoAs destined for mitochondrial β-oxidation appear to originate from TG lipolysis (18, 55). It is unclear whether utilization of these putative acyl-CoA pools is interchangeable when muscle FFA oxidation is accelerated such as during muscle contraction (22, 94, 111), when muscles have defective intramuscular lipolysis (51), or in the setting of diabetes, starvation, or catabolic disease. In the absence of demand for fatty acid oxidation, acyl-CoAs undergo a series of esterification reactions with glycerol initiated by glycerophosphate acyltransferase (GPAT) that ultimately yield TGs or phospholipids (17, 25, 105).
Fig. 1.
Skeletal muscle lipid flux. ACC, acetyl-CoA carboxylase; ACS, acyl-CoA synthase; AGPAT, acylglycerophosphate acyltransferase; ATGL, adipose triglyceride lipase; CD36, cluster of differentiation 36; CoA, coenzyme A; DAG, diacylglycerol; DGAT, diacylglycerol acyltransferase; DHAP, dihydroxyacetone phosphate; ER, endoplasmic reticulum; FAS, fatty acid synthase; FFA, free fatty acids; GDPH, glycerol-3-phosphate dehydrogenase; glycerol-3-P, glycerol-3-phosphate; GPAT, glycerophosphate acyltransferase; HSL, hormone-sensitive lipase; LPA, lysophosphatidic acid; LPL, lipoprotein lipase; MAG, monoacylglycerol; MGAT, monoacylglycerol acyltransferase; MGL, monoacylglycerol lipase; PA, phosphatidic acid; PAP, phosphatidate phosphatase (aka lipin); TG, triglycerides. Both PA and DAG can be used for phospholipid synthesis, details of which are not depicted here.
A Case for Lipoexpediency in Skeletal Muscle
Although excess fat deposition in nonadipose tissues may induce pathology, moderate fat deposition in those same tissues is often important for maintaining normal physiology. In skeletal muscle, lipids provide fuel for contractile activity. During exercise, skeletal muscle endurance is highly dependent on its capacity to utilize lipids and spare muscle glycogen (45, 95). Exercise increases circulating FFAs by promoting adipose tissue lipolysis through several mechanisms, including a decrease in insulin and increases in catecholamines (50, 61). In addition to mass action driven by increased FFA availability, lipid uptake by skeletal muscle is also enhanced through increased cell surface translocation of CD36 (47). Exercise also stimulates intramuscular TG lipolysis (50, 109, 111). In trained athletes, intramuscular acyl-CoA concentrations are elevated, and ATP synthesis may increase more than 100-fold compared with these variables in the resting state (2). Each exercise bout also appears to be associated with the diversion of a portion of the acyl-CoA pool toward intramuscular TG synthesis (49). Therefore, exercise-induced increases in skeletal muscle lipid flux are an essential component of increasing endurance.
With endurance training, skeletal muscle metabolism shifts so that substrate abundance and enzyme activities promote lipid utilization and storage. Exercise training is known to increase intramuscular TG content, an observation sometimes described as the “athlete's paradox” (29, 48, 78) but one that might also be considered an “obesity paradox”. TG content alone cannot predict muscle physiology, since elevated muscle TG content in endurance-trained athletes is an adaptive response to increased demand for fuel utilization (45, 93), whereas elevated muscle TG content in obese individuals is a maladaptive response to energy excess (30, 65). In trained muscles, exercise enhances intramuscular TG breakdown, which promotes ATP resynthesis to spare glycogen (the depletion of which probably leads to muscle exhaustion) (8, 32). Exercise training also induces an increase in protein abundance for enzymes that are associated with synthesis, breakdown, and utilization of intramuscular TG (1, 42, 52, 60, 87, 98). Thus, skeletal muscle lipid flux caused by each bout of exercise is further enhanced in endurance-trained athletes compared with untrained individuals. Notably, endurance-trained muscles are exquisitely insulin sensitive (20, 31, 40, 112).
One emerging notion is that it is not the increased content of FFA in skeletal muscle per se but rather the accumulation of lipid intermediates in muscle that may be deleterious. The specific identities of potentially harmful lipid intermediates are controversial, but ceramide, diacylglycerol (DAG), and certain acyl-CoAs are implicated in skeletal muscle insulin resistance, endoplasmic reticulum (ER) stress, mitochondrial stress, and apoptosis (9, 58, 80, 96, 108). Since a single bout of exercise in humans can protect against skeletal muscle insulin resistance induced by an overnight lipid infusion (98), it is likely that in the setting of obesity exercise activates pathways to shunt potentially harmful lipid molecules toward mitochondrial oxidation, TG storage, or phospholipid synthesis. Thus, even with the lipid overload that occurs in obesity, cells have the capacity to alter proportions of different classes of lipid molecules so that lipotoxicity is minimized. We recently coined the term “lipoexpediency” to describe the ability of cells to modify lipid metabolism to promote favorable physiology (72).
Data from transgenic and knockout models support the idea that muscle can protect against lipotoxic agents by producing neutral lipid storage. Increasing net TG synthesis by diacylglycerol acyltransferase-1 (DGAT1) overexpression (70, 106) or ATGL inactivation (34, 59) promoted skeletal muscle insulin sensitivity. Conversely, isolated skeletal muscle from whole body DGAT1 knockout mice exhibited insulin resistance (70). However, insulin sensitivity alone may not reflect improved muscle function, since DGAT1 overexpression and ATGL knockout were accompanied by muscle abnormalities not observed in trained muscles (51, 70, 101). Mechanisms underlying the apparently beneficial increases in intramuscular TG levels observed in trained muscles likely involve a complex set of coordinated physiological responses. A comprehensive understanding of exercise-induced lipid flux will be required if it will be possible to develop a strategy to mimic or enhance exercise and its benefits.
The idea that muscle with higher oxidative demands for ATP synthesis can protect against lipid-induced insulin resistance is also supported by data derived from transgenic mice expressing uncoupling protein-1 (UCP1) (35, 64) or UCP3 (15) in muscle. Fuel utilization ultimately depends on ATP turnover, so increasing mitochondrial oxidative capacity alone would not promote lipid flux toward oxidation in the resting condition (44, 80, 95). At rest, the mitochondrial electron transport system operates at a small fraction of its maximal capacity. Only in the context of increased demand for ATP resynthesis, such as occurs with exercise, do muscles with increased mitochondrial content exhibit greater capacity for lipid oxidation. Therefore, it is not likely that elevated mitochondrial mass alone (73, 79) in the absence of increased energy demand can protect muscle from lipotoxicity.
Roles of Specific Lipid Molecules as Ligands for PPARs
A series of novel observations over the past decade have helped clarify some of the mechanisms by which endurance training enhances the capacity of skeletal muscle to metabolize lipids. Several lines of evidence suggest that peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-1α) coordinates a broad spectrum of adaptive responses in muscle (3, 37–39, 66, 67, 89, 117). Skeletal muscle PGC-1α coactivates transcription factors such as PPARα and PPARβ/δ to promote expression of proteins critical for lipid metabolism (62, 81, 88). PGC-1α-dependent transcription is induced by multiple signaling events that are known to be activated by exercise. These events include activation of enzymes that serve as energy sensors, including 5′-adenosine monophosphate-activated protein kinase (AMPK) and the sirtuins, as well as calcium sensors, including the Ca2+/calmodulin-dependent kinases (CaMKs) (54, 92, 114, 115). These enzymes are known to activate the skeletal muscle transcriptional machinery involved in lipid metabolism (53, 83, 113, 116). Therefore, it seems plausible that PGC-1α serves as a signaling node for muscle adaptations occurring in response to exercise.
PPARα and PPARβ/δ are members of a nuclear receptor family important for regulating both metabolism and inflammation (23, 33, 74, 110). PPARs form heterodimers with retinoid X receptors (RXRs) and bind to specific DNA sites known as PPAR response elements (PPREs). Transcription is activated by ligand binding to PPARs, and a wide variety of lipid molecules have been implicated as PPAR ligands. Ligand binding induces conformational changes resulting in dissociation of corepressors and recruitment of coactivators such as PGC-1α. It is likely, although not proved, that exercise-induced activation of PPARs requires the binding of a lipid ligand to the receptor. One plausible scenario for skeletal muscle lipid metabolism in response to exercise would thus include the synthesis and binding of lipid ligands to PPARα or PPARβ/δ concurrently with the activation of PGC-1α.
Additional clues as to how synthesis of lipid ligands may participate in exercise-induced activation of the skeletal muscle transcriptional machinery come from studies of altering the lipid milieu without exercise. Raising circulating FFA in the absence of an exercise stimulus is sufficient to increase mitochondrial biogenesis and endurance in skeletal muscle (27, 36, 75, 107), outcomes that might involve PPARα, PPARβ/δ, and/or PGC-1α-dependent transcription (27, 36). These findings suggest that at least a portion of the exercise adaptations that enhance the skeletal muscle's capacity for lipid metabolism can be induced by simply replicating the increased exposure of that tissue to an appropriate lipid source. However, this lipid intervention also caused skeletal muscle insulin resistance (27, 36), the opposite of the insulin-sensitizing response to an exercise intervention (10, 26, 31, 43, 91). These studies reinforce the idea that enhancing mitochondria mass alone does not increase resting fat oxidation and that increased mitochondrial mass alone is not sufficient to afford skeletal muscle protection from fat-induced insulin resistance (44, 80). These studies did not address effects of chronically increased circulating FFAs on TG stores or lipid enzymes in muscle, so it is possible that the processes of lipid synthesis and breakdown were unaffected (65). Perhaps muscle contractile activity is required to promote synthesis of specific lipid mediators that promote transcriptional programs resulting in protection of skeletal muscle from fat-induced insulin resistance.
Because the identities of physiologically relevant ligands for PPARs in skeletal muscle are unknown, the origin(s) of these putative ligands is also obscure. Multiple lipogenic or lipolytic sites might generate potential ligands. Data from several transgenic and knockout mouse models suggest that such ligands are likely to be derived from branches of the futile cycle of lipid synthesis and breakdown. Upregulation of DGAT1 increased skeletal muscle expression of genes involved in mitochondrial and fatty acid metabolism (68, 106). Conversely, inactivation of DGAT1 decreased the expression of PPAR-dependent genes in skeletal muscle (69). ATGL overexpression increased skeletal muscle oxidative capacity and PPARβ/δ target genes (111). However, skeletal muscle from the whole body ATGL knockout mouse does not have decreased expression of genes relevant to fatty acid oxidation (51), an effect that may be different in cardiac muscle (34). Skeletal muscle CD36 deficiency decreased PPARβ/δ gene expression and mitochondrial fat oxidation (46, 82). In nonmuscle tissues, including liver, brain, and macrophages, the lipogenic enzyme FAS regulates PPARα-dependent gene expression (12, 13, 100). FAS in adipose tissue is probably involved in regulation of PPARγ activity (71, 72, 99). Lipolytic pathways also impact PPAR activation, since ATGL (34, 84, 97) and LPL (6, 7, 14, 119) regulate PPARα at several sites.
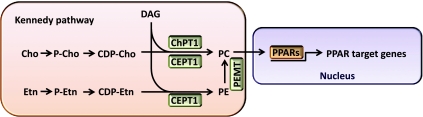
What are the physiologically relevant skeletal muscle ligands that activate PPARα and/or PPARβ/δ to coordinate exercise-induced adaptive responses? A distinct phosphatidylcholine species, 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC), was identified as an endogenous PPARα ligand in the liver (11). Phosphatidylcholine synthesis in eukaryotic cells occurs primarily through the Kennedy pathway (56, 57). In this pathway (Fig. 2), CDP-choline is synthesized from the sequential reaction of choline with ATP and CTP; then the phosphocholine group on CDP-choline is transferred to DAG (directly sequestering a potentially toxic lipid intermediate) by choline phosphotransferase-1 (ChPT1) found in the Golgi apparatus or choline/ethanolamine phosphotransferase-1 (CEPT1) found in the nucleus and ER (41). In liver, knockdown of CEPT1, but not ChPT1, resulted in downregulation of PPARα-dependent gene expression, whereas overexpression of liver CEPT1 increased PPARα-dependent gene expression (11), suggesting that generation of GPC in the ER or nucleus is a relevant physiological event in liver.
Fig. 2.
A working model for PPAR ligand synthesis in skeletal muscle. CDP, cytidine-diphosphate; CEPT1, choline/ethanolamine phosphotransferase-1; Cho, choline; ChPT1, choline-phosphotransferase-1; DAG, diacylglycerol; Etn, ethanolamine; P, phosphate; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PEMT, phosphatidylethanolamine N-methyltransferase; PPAR, peroxisome proliferator-activated receptor.
Do similar phosphatidylcholine species serve as endogenous ligands for PPARs in skeletal muscle? It is unknown whether the ligand for PPARα is the same in liver as in other tissues. CEPT1 protein is abundantly expressed in liver, skeletal muscle and heart (K. Funai and C. F. Semenkovich unpublished results). Abundance of CEPT1 protein varies considerably in skeletal muscle, with slow-twitch muscles such as diaphragm and soleus exhibiting approximately two- to fivefold more CEPT1 than fast-twitch muscles such as vastus lateralis and extensor digitorum longus (K. Funai and C. F. Semenkovich unpublished results). Slow-twitch muscles have a higher intramuscular TG content as well as more robust expression of enzymes involved in lipid metabolism (4), so it is possible that CEPT1 contributes to the oxidative phenotype by generating PPAR ligands in skeletal muscle. The role of exercise in modulating expression of CEPT1 or related Kennedy pathways enzymes is unknown, but AMPK, an important mediator of the energy depletion associated with exercise, is known to induce the expression of genes encoding enzymes involved in phospholipid biosynthesis (5).
Exercise training increases phosphatidylcholine in muscle (78), and the effects of exercise on numerous phospholipid species in skeletal muscle have been examined in rats fed a standard chow diet (77) or a high-fat diet (76). With chow feeding, exercise training increased the abundance of two phosphatidylcholine species (77): 16:0/18:1-GPC, the species also identified as an endogenous PPARα ligand in liver, and 16:0/18:2-GPC (1-palmitoyl-2-linoleoyl-sn-glycerol-3-phosphocholine). The latter phosphatidylcholine species was also found to be associated with PPARα in liver, but it was not displaced with the potent PPARα agonist Wy14,643 (11). It is possible that multiple endogenous ligands exist for PPARs. The affinity for phosphatidylcholine may be similar between PPARα and PPARβ/δ (11), so 16:0/18:1-GPC and 16:0/18:2-GPC may be candidates for PPARα and/or PPARβ/δ ligands in skeletal muscle. In addition, exercise training enhanced the abundance of 16:0/18:2-GPA (phosphatidic acid), 18:1/18:2-GPA, 16:0/18:2-plasmenyl-GPE (phosphatidylethanolamine), and 18:0/22:5-GPI (phosphatidylinositol) with the normal chow diet (77). With high-fat feeding (76), exercise training also increased 16:0/18:2-GPC, 18:1/18:2-GPA, and 16:0/18:2-plasmenyl-GPE but additionally resulted in increases in isobaric 18:0/18:2-GPE or 18:1/18:1-GPE, emphasizing the importance of diet in modulating skeletal muscle phospholipid responses to exercise that may be relevant to PPAR signaling.
Conclusion
Exercise is the ideal treatment for chronic conditions associated with individuals in positive energy balance, and this may be due, in part, to its ability to partition skeletal muscle lipid molecules to compartments where they are unlikely to “poison” muscle by disrupting insulin signaling or energy generation. Upregulation of intramuscular TG synthesis or lipid oxidation appears to protect skeletal muscles from fat-induced insulin resistance. Exercise also stimulates phospholipid biosynthesis, a process that might be involved in the production of endogenous ligands for PPARs. Identifying and characterizing physiologically relevant endogenous ligands for PPARs in skeletal muscle as well as the pathways involved in their generation could lead to novel approaches for promoting lipid flux and possibly preventing the poisoning of muscle physiology by fats.
GRANTS
This work was supported by Grants DK-076729, DK-088083, and T32 DK-007210 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
C. F. Semenkovich is a member of the Merck Speakers Bureau.
REFERENCES
- 1.Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296: E445–E453, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat 171: 259–272, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Athea Y, Viollet B, Mateo P, Rousseau D, Novotova M, Garnier A, Vaulont S, Wilding JR, Grynberg A, Veksler V, Hoerter J, Ventura-Clapier R. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes 56: 786–794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem 279: 25050–25057, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D'Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem 281: 8716–8723, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Barnes M, Gibson LM, Stephenson DG. Increased muscle glycogen content is associated with increased capacity to respond to T-system depolarisation in mechanically skinned skeletal muscle fibres from the rat. Pflügers Arch 442: 101–106, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Brookheart RT, Michel CI, Schaffer JE. As a matter of fat. Cell Metab 10: 9–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartee GD, Funai K. Exercise and insulin: convergence or divergence at AS160 and TBC1D1? Exerc Sport Sci Rev 37: 188–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravarthy MV, Lodhi IJ, Yin L, Malapaka RR, Xu HE, Turk J, Semenkovich CF. Identification of a physiologically relevant endogenous ligand for PPARalpha in liver. Cell 138: 476–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309–322, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest 117: 2539–2552, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla A, Lee CH, Barak Y, He W, Rosenfeld J, Liao D, Han J, Kang H, Evans RM. PPARdelta is a very low-density lipoprotein sensor in macrophages. Proc Natl Acad Sci USA 100: 1268–1273, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi CS, Fillmore JJ, Kim JK, Liu ZX, Kim S, Collier EF, Kulkarni A, Distefano A, Hwang YJ, Kahn M, Chen Y, Yu C, Moore IK, Reznick RM, Higashimori T, Shulman GI. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. J Clin Invest 117: 1995–2003, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 275: 32523–32529, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Coleman RA, Lewin TM, Muoio DM. Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr 20: 77–103, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest 58: 421–431, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Defronzo RA, Banting Lecture From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 773–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Sherwin RS, Kraemer N. Effect of physical training on insulin action in obesity. Diabetes 36: 1379–1385, 1987 [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32, Suppl 2: S157–S163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyck DJ, Miskovic D, Code L, Luiken JJ, Bonen A. Endurance training increases FFA oxidation and reduces triacylglycerol utilization in contracting rat soleus. Am J Physiol Endocrinol Metab 278: E778–E785, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol Rev 61: 373–393, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol 21: 212–217, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139: 855–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frosig C, Richter EA. Improved insulin sensitivity after exercise: focus on insulin signaling. Obesity (Silver Spring) 17, Suppl 3: S15–S20, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA 104: 10709–10713, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res 50, Suppl: S86–90, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism 49: 467–472, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49: 235–261, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Green HJ. How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol 69: 290–297, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Grimaldi PA. Metabolic and nonmetabolic regulatory functions of peroxisome proliferator-activated receptor beta. Curr Opin Lipidol 21: 186–191, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312: 734–737, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Han DH, Nolte LA, Ju JS, Coleman T, Holloszy JO, Semenkovich CF. UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am J Physiol Endocrinol Metab 286: E347–E353, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem 282: 30014–30021, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, Kulkarni RN, Shulman GI, Spiegelman BM. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–3474, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev 21: 770–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxford, England) 192: 127–135, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of Fatty Acid and lipid head group specificity. Mol Biol Cell 13: 3148–3161, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem 242: 2278–2282, 1967 [PubMed] [Google Scholar]
- 43.Holloszy JO. A forty-year memoir of research on the regulation of glucose transport into muscle. Am J Physiol Endocrinol Metab 284: E453–E467, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89: 463S–466S, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984 [DOI] [PubMed] [Google Scholar]
- 46.Holloway GP, Jain SS, Bezaire V, Han XX, Glatz JF, Luiken JJ, Harper ME, Bonen A. FAT/CD36-null mice reveal that mitochondrial FAT/CD36 is required to upregulate mitochondrial fatty acid oxidation in contracting muscle. Am J Physiol Regul Integr Comp Physiol 297: R960–R967, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Holloway GP, Luiken JJ, Glatz JF, Spriet LL, Bonen A. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview. Acta Physiol (Oxford, England) 194: 293–309, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Hoppeler H, Lindstedt SL. Malleability of skeletal muscle in overcoming limitations: structural elements. J Exp Biol 115: 355–364, 1985 [DOI] [PubMed] [Google Scholar]
- 49.Horowitz JF. Exercise-induced alterations in muscle lipid metabolism improve insulin sensitivity. Exerc Sport Sci Rev 35: 192–196, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr 72: 558S–563S, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Huijsman E, van de Par C, Economou C, van der Poel C, Lynch GS, Schoiswohl G, Haemmerle G, Zechner R, Watt MJ. Adipose triacylglycerol lipase deletion alters whole body energy metabolism and impairs exercise performance in mice. Am J Physiol Endocrinol Metab 297: E505–E513, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun 296: 395–400, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31: 212–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanaley JA, Shadid S, Sheehan MT, Guo Z, Jensen MD. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J Physiol 587: 5939–5950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem 222: 193–214, 1956 [PubMed] [Google Scholar]
- 57.Kent C. Regulatory enzymes of phosphatidylcholine biosynthesis: a personal perspective. Biochim Biophys Acta 1733: 53–66, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Kewalramani G, Bilan PJ, Klip A. Muscle insulin resistance: assault by lipids, cytokines and local macrophages. Curr Opin Clin Nutr Metab Care 13: 382–390, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G, Flier JS, Zechner R, Kim YB, Kershaw EE. Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem 284: 30218–30229, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kiens B. Effect of endurance training on fatty acid metabolism: local adaptations. Med Sci Sports Exerc 29: 640–645, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Kiens B. Skeletal muscle lipid metabolism in exercise and insulin resistance. Physiol Rev 86: 205–243, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Kleiner S, Nguyen-Tran V, Bare O, Huang X, Spiegelman B, Wu Z. PPAR(delta) agonism activates fatty acid oxidation via PGC-1(alpha) but does not increase mitochondrial gene expression and function. J Biol Chem 284: 18624–18633, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewin TM, Kim JH, Granger DA, Vance JE, Coleman RA. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J Biol Chem 276: 24674–24679, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med 6: 1115–1120, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Li M, Paran C, Wolins NE, Horowitz JF. High muscle lipid content in obesity is not due to enhanced activation of key triglyceride esterification enzymes or to the suppression of lipolytic proteins. Am J Physiol Endocrinol Metab 300: E699–E707, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 67.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Liu L, Shi X, Choi CS, Shulman GI, Klaus K, Nair KS, Schwartz GJ, Zhang Y, Goldberg IJ, Yu YH. Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes 58: 2516–2524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y, Huggins LA, Eriksson JW, Buckett LK, Turnbull AV, Ginsberg HN, Blaner WS, Huang LS, Goldberg IJ. DGAT1 deficiency decreases PPAR expression and does not lead lipotoxicity in cardiac and skeletal muscle. J Lipid Res 52: 732–744, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu YH. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J Clin Invest 117: 1679–1689, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu LH, Wang XK, Hu YD, Kang JL, Wang LL, Li S. Effects of a fatty acid synthase inhibitor on adipocyte differentiation of mouse 3T3-L1 cells. Acta Pharmacol Sin 25: 1052–1057, 2004 [PubMed] [Google Scholar]
- 72.Lodhi IJ, Wei X, Semenkovich CF. Lipoexpediency: de novo lipogenesis as a metabolic signal transmitter. Trends Endocrinol Metab 22: 1–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 74.Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol 44: 968–975, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol 56: 78–83, 1984 [DOI] [PubMed] [Google Scholar]
- 76.Mitchell TW, Turner N, Else PL, Hulbert AJ, Hawley JA, Lee JS, Bruce CR, Blanksby SJ. The effect of exercise on the skeletal muscle phospholipidome of rats fed a high-fat diet. Int J Mol Sci 11: 3954–3964, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell TW, Turner N, Hulbert AJ, Else PL, Hawley JA, Lee JS, Bruce CR, Blanksby SJ. Exercise alters the profile of phospholipid molecular species in rat skeletal muscle. J Appl Physiol 97: 1823–1829, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Morgan TE, Short FA, Cobb LA. Effect of long-term exercise on skeletal muscle lipid composition. Am J Physiol 216: 82–86, 1969 [DOI] [PubMed] [Google Scholar]
- 79.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55, Suppl 2: S9–S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim Biophys Acta 1801: 281–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Appl Physiol Nutr Metab 32: 874–883, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Nahle Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPAR delta/beta-mediated adaptation to metabolic stress. J Biol Chem 283: 14317–14326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc 63: 275–278, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53: 116–126, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pender C, Trentadue AR, Pories WJ, Dohm GL, Houmard JA, Youngren JF. Expression of genes regulating malonyl-CoA in human skeletal muscle. J Cell Biochem 99: 860–867, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Perseghin G, Ghosh S, Gerow K, Shulman GI. Metabolic defects in lean nondiabetic offspring of NIDDM parents: a cross-sectional study. Diabetes 46: 1001–1009, 1997 [DOI] [PubMed] [Google Scholar]
- 87.Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54: 1048–1055, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-alpha. Int J Obes (Lond) 29, Suppl 1: S5–9, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1(alpha) regulates a HIF2(alpha)-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA 107: 21866–21871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reaven GM. Banting lecture. Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988 [DOI] [PubMed] [Google Scholar]
- 91.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest 69: 785–793, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265: E380–E391, 1993 [DOI] [PubMed] [Google Scholar]
- 94.Sacchetti M, Saltin B, Osada T, van Hall G. Intramuscular fatty acid metabolism in contracting and non-contracting human skeletal muscle. J Physiol 540: 387–395, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahlin K, Harris RC. Control of lipid oxidation during exercise: role of energy state and mitochondrial factors. Acta Physiol (Oxford, England) 194: 283–291, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375: 2267–2277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res 50: 1621–1629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmid B, Rippmann JF, Tadayyon M, Hamilton BS. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem Biophys Res Commun 328: 1073–1082, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Schneider JG, Yang Z, Chakravarthy MV, Lodhi IJ, Wei X, Turk J, Semenkovich CF. Macrophage fatty-acid synthase deficiency decreases diet-induced atherosclerosis. J Biol Chem 285: 23398–23409, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schoiswohl G, Schweiger M, Schreiber R, Gorkiewicz G, Preiss-Landl K, Taschler U, Zierler KA, Radner FP, Eichmann TO, Kienesberger PC, Eder S, Lass A, Haemmerle G, Alsted TJ, Kiens B, Hoefler G, Zechner R, Zimmermann R. Adipose triglyceride lipase plays a key role in the supply of the working muscle with fatty acids. J Lipid Res 51: 490–499, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schwenk RW, Holloway GP, Luiken JJ, Bonen A, Glatz JF. Fatty acid transport across the cell membrane: regulation by fatty acid transporters. Prostaglandins Leukot Essent Fatty Acids 82: 149–154, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Seip RL, Semenkovich CF. Skeletal muscle lipoprotein lipase: molecular regulation and physiological effects in relation to exercise. Exerc Sport Sci Rev 26: 191–218, 1998 [PubMed] [Google Scholar]
- 104.Semenkovich CF. Regulation of fatty acid synthase (FAS). Prog Lipid Res 36: 43–53, 1997 [DOI] [PubMed] [Google Scholar]
- 105.Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Timmers S, de Vogel-van den Bosch J, Hesselink MK, van Beurden D, Schaart G, Ferraz MJ, Losen M, Martinez-Martinez P, De Baets MH, Aerts JM, Schrauwen P. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLoS ONE 6: e14503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, Cooney GJ. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 56: 2085–2092, 2007 [DOI] [PubMed] [Google Scholar]
- 108.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801: 209–214, 2010 [DOI] [PubMed] [Google Scholar]
- 109.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, Saris WH, Wagenmakers AJ. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 536: 295–304, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang YX. PPARs: diverse regulators in energy metabolism and metabolic diseases. Cell Res 20: 124–137, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Watt MJ, Spriet LL. Triacylglycerol lipases and metabolic control: implications for health and disease. Am J Physiol Endocrinol Metab 299: E162–E168, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem 42: 31–46, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Wright DC. Mechanisms of calcium-induced mitochondrial biogenesis and GLUT4 synthesis. Appl Physiol Nutr Metab 32: 840–845, 2007 [DOI] [PubMed] [Google Scholar]
- 114.Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem 282: 18793–18799, 2007 [DOI] [PubMed] [Google Scholar]
- 115.Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282: 194–199, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296: 349–352, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Bostrom P, Tyra HM, Crawford RW, Campbell KP, Rutkowski DT, Kaufman RJ, Spiegelman BM. The Unfolded Protein Response Mediates Adaptation to Exercise in Skeletal Muscle through a PGC-1alpha/ATF6alpha Complex. Cell Metab 13: 160–169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50: 3–21, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Lipolysis of triglyceride-rich lipoproteins generates PPAR ligands: evidence for an antiinflammatory role for lipoprotein lipase. Proc Natl Acad Sci USA 100: 2730–2735, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]