Abstract
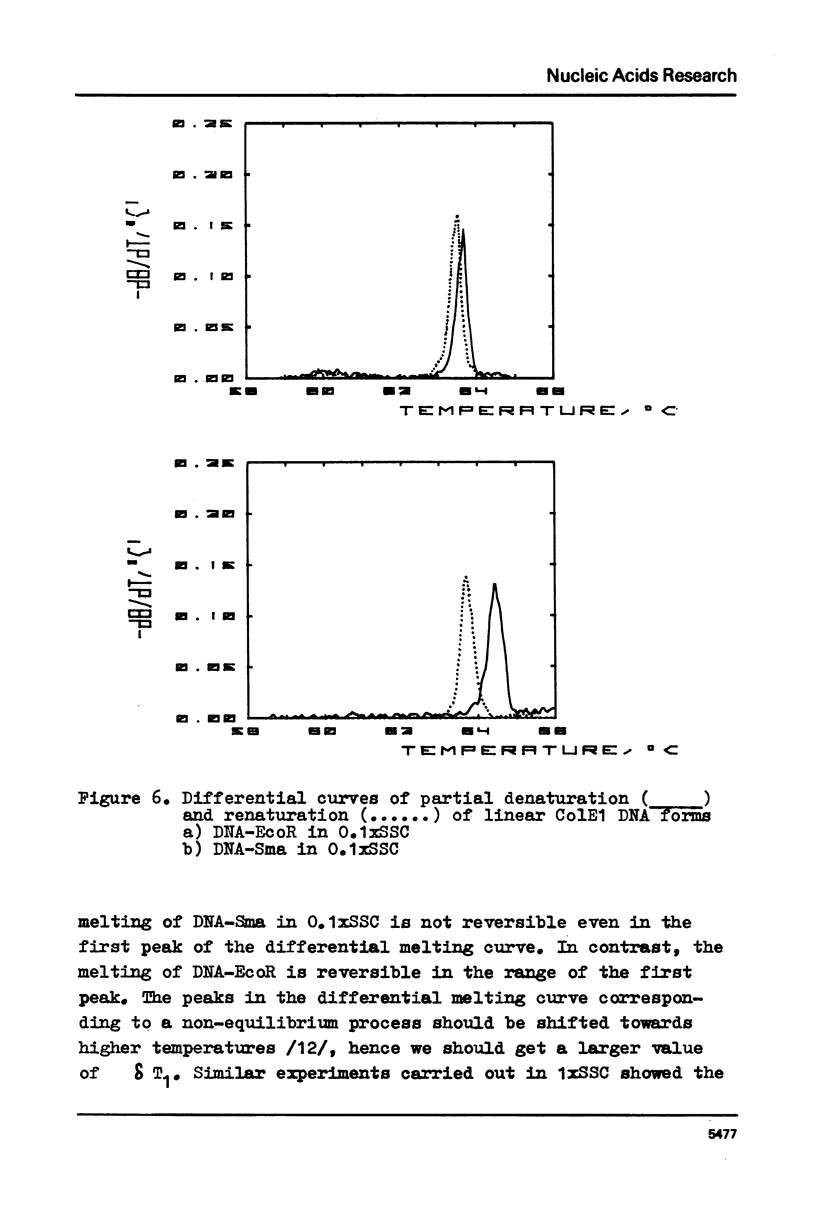
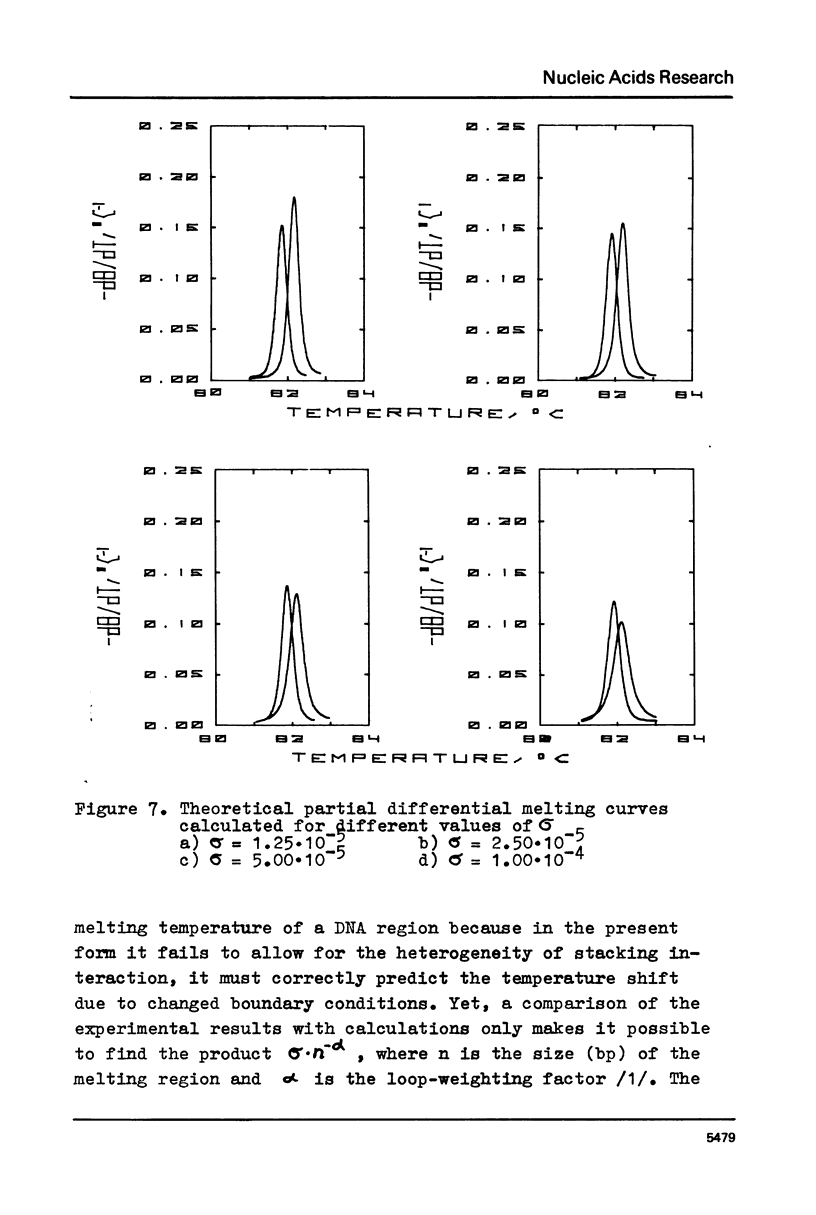
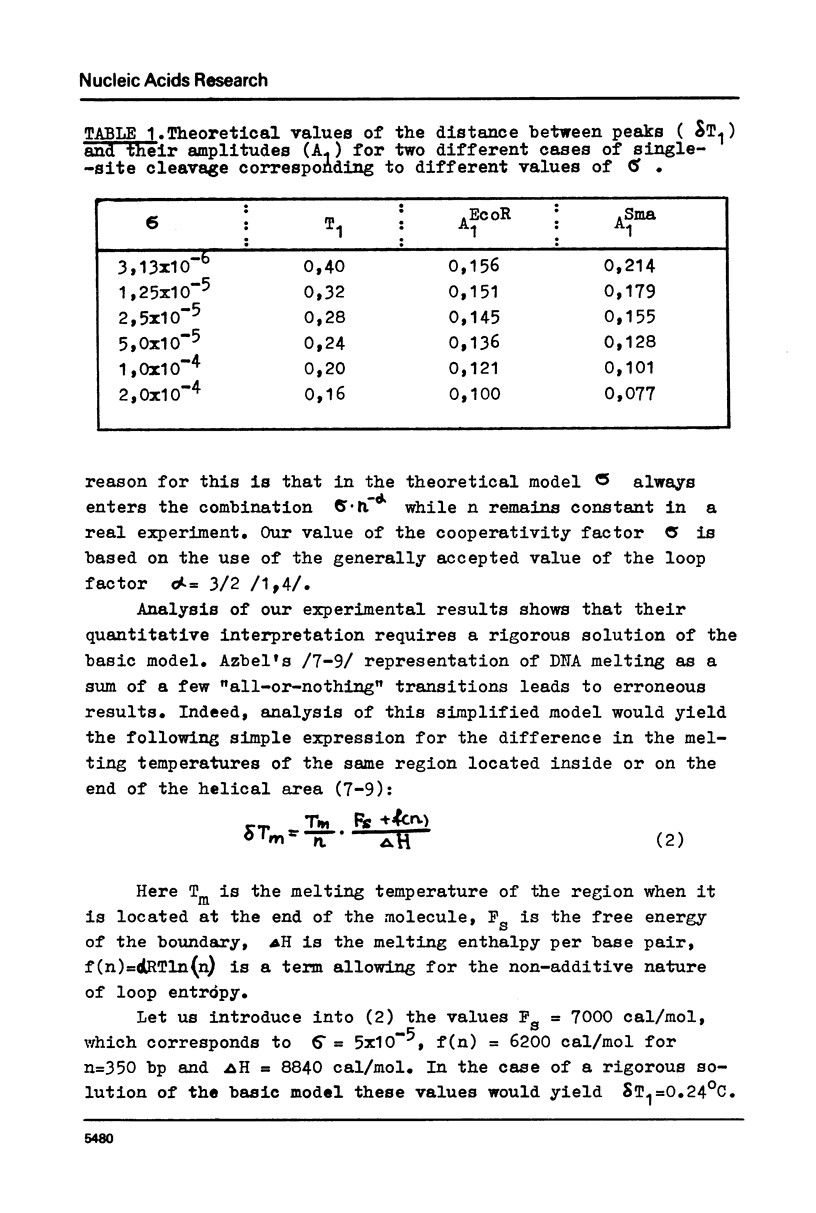
The paper presents measurements of the difference in the melting temperature of a colE1 DNA region when it is located inside the DNA helix and at its end. A direct comparison of calculations based on the rigorous theory of helix-coil transition with experimental data for .2 M Na+ (the conditions for fully reversible melting) yielded the value of 2.5-5x10(-5) for the cooperatively factor sigma. We discuss the reversibility of DNA melting and the possibility of applying the "all-or-nothing" concept to the melting of DNA regions.
Full text
PDF



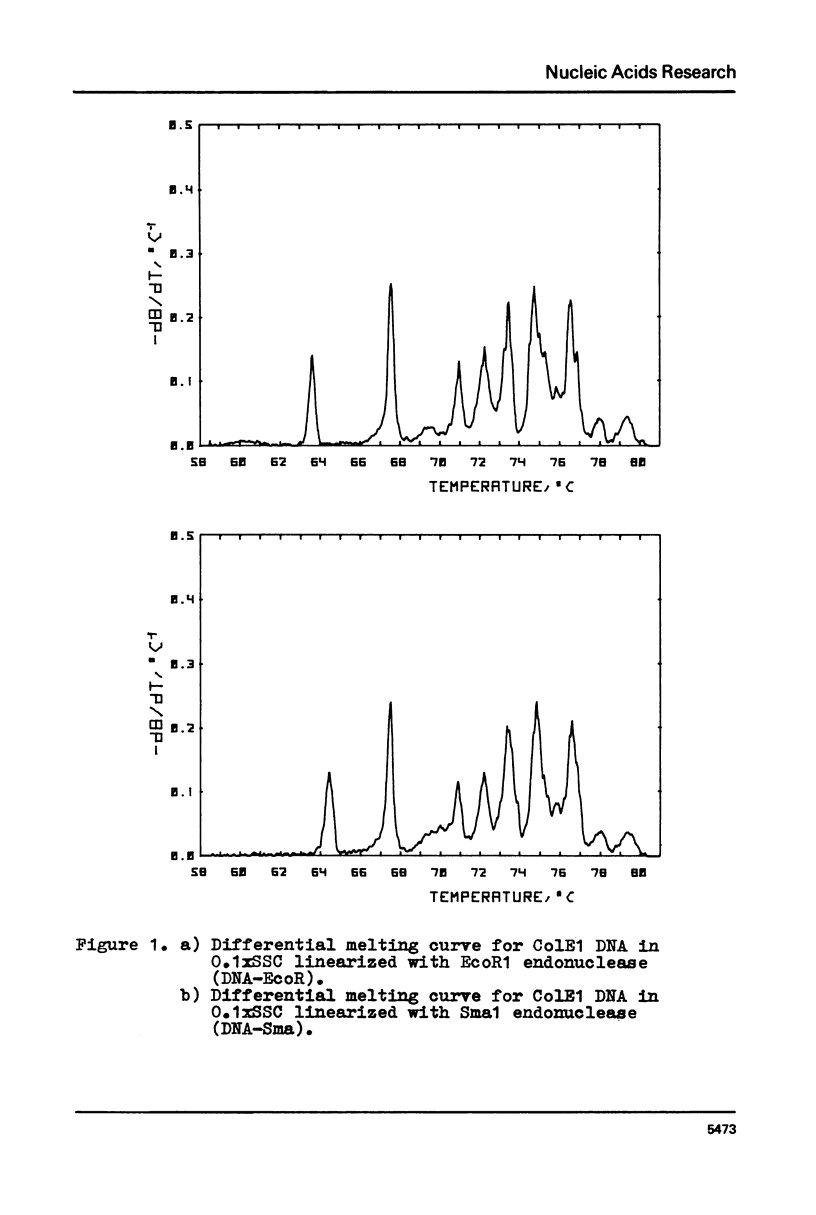
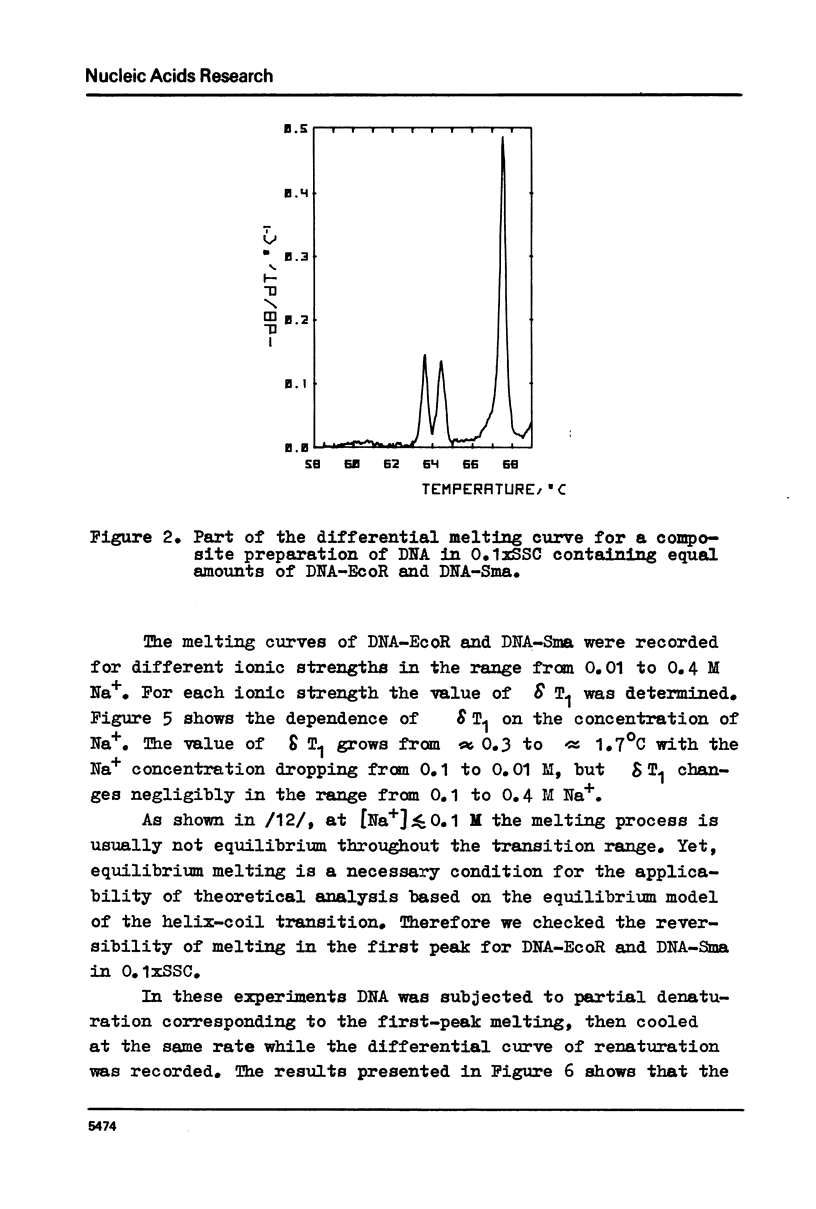
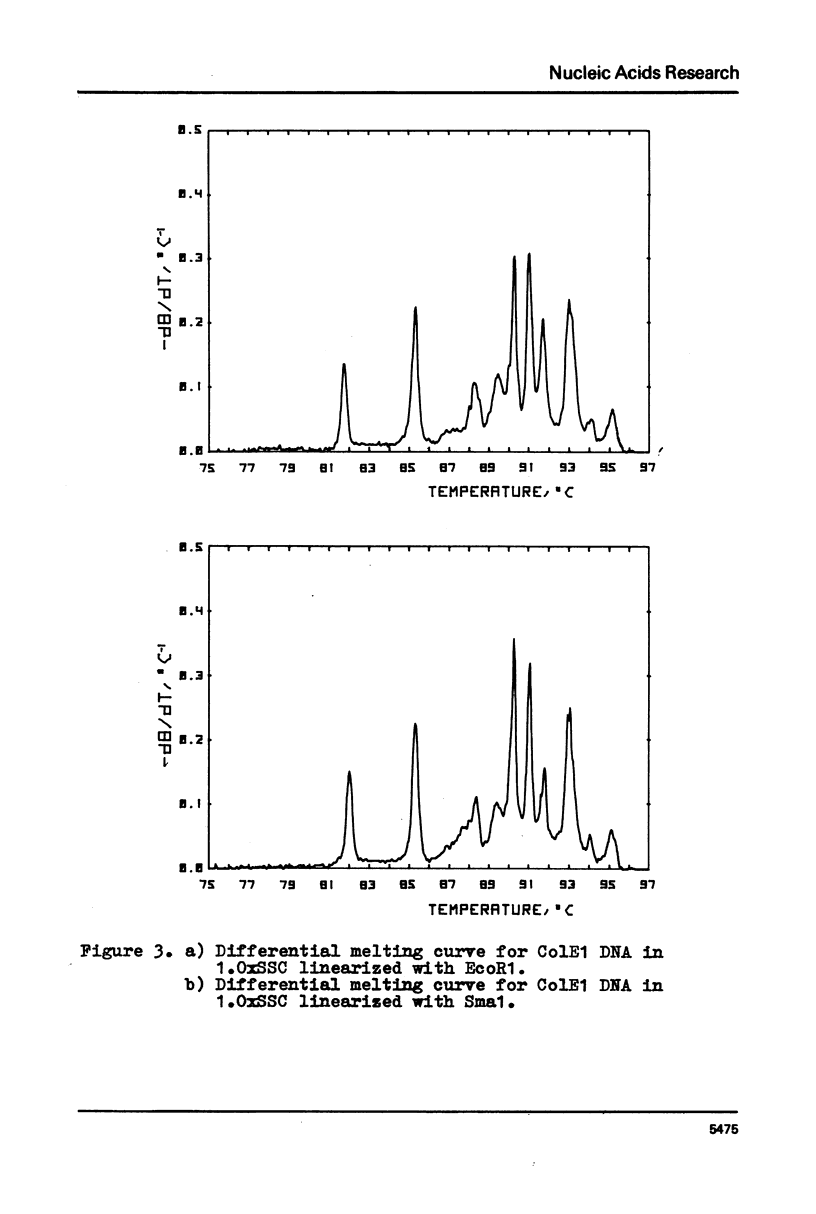
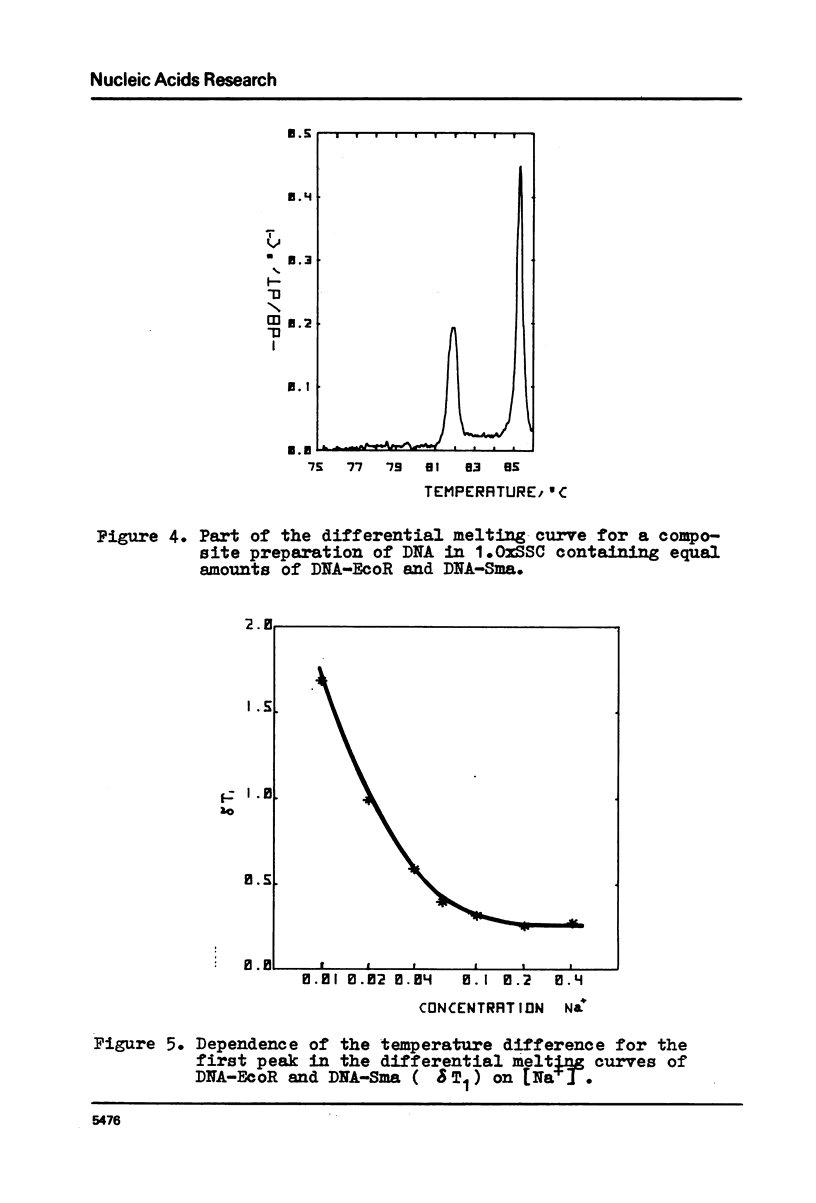



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azbel M. Y. DNA sequencing and helix-coil transition. III. DNA sequencing. Biopolymers. 1980 Jan;19(1):95–109. doi: 10.1002/bip.1980.360190107. [DOI] [PubMed] [Google Scholar]
- Azbel M. Y. DNA sequencing and melting curve. Proc Natl Acad Sci U S A. 1979 Jan;76(1):101–105. doi: 10.1073/pnas.76.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovik A. S., Kalambet Y. A., Lyubchenko Y. L., Shitov V. T., Golovanov E. I. Equilibrium melting of plasmid ColE1 DNA: electron-microscopic visualization. Nucleic Acids Res. 1980 Sep 25;8(18):4165–4184. doi: 10.1093/nar/8.18.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman M., Freire J. J. Theory of DNA melting curves. Biopolymers. 1977 Dec;16(12):2693–2704. doi: 10.1002/bip.1977.360161209. [DOI] [PubMed] [Google Scholar]
- Frank-Kamenetskii F. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers. 1971;10(12):2623–2624. doi: 10.1002/bip.360101223. [DOI] [PubMed] [Google Scholar]
- Gruenwedel D. W. Salt effects on the denaturation of DNA. 3. A calorimetric investigation of the transition enthalpy of calf thymus DNA in Na2SO4 solutions of varying ionic strength. Biochim Biophys Acta. 1974 Feb 27;340(1):16–30. doi: 10.1016/0005-2787(74)90170-1. [DOI] [PubMed] [Google Scholar]
- Gruenwedel D. W. Salt effects on the denaturation of DNA. IV. A calorimetric study of the helix-coil conversion of the alternating copolymer poly[d(A-T)]. Biochim Biophys Acta. 1975 Jul 7;395(3):246–257. doi: 10.1016/0005-2787(75)90195-1. [DOI] [PubMed] [Google Scholar]
- Oka A., Nomura N., Morita M., Sugisaki H., Sugimoto K., Takanami M. Nucleotide sequence of small ColE1 derivatives: structure of the regions essential for autonomous replication and colicin E1 immunity. Mol Gen Genet. 1979 May 4;172(2):151–159. doi: 10.1007/BF00268276. [DOI] [PubMed] [Google Scholar]
- Oliver A. L., Wartell R. M., Ratliff R. L. Helix coil transitions of d(A)n-d(T)n, d(A-T)n-d(A-T)n, and d(A-A-T)n-d(A-T-T)n; evaluation of parameters governing DNA stability. Biopolymers. 1977 May;16(5):1115–1137. doi: 10.1002/bip.1977.360160512. [DOI] [PubMed] [Google Scholar]
- Perelroyzen M. P., Lyamichev V. I., Kalambet YuA, Lyubchenko YuL, Vologodskii A. V. A study of the reversibility of helix-coil transition in DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4043–4059. doi: 10.1093/nar/9.16.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada A., Yabuki S., Husimi Y. Fine structure in the thermal denaturation of DNA: high temperature-resolution spectrophotometric studies. CRC Crit Rev Biochem. 1980;9(2):87–144. doi: 10.3109/10409238009105432. [DOI] [PubMed] [Google Scholar]


