Specificity in Arf1 GEF recruitment to the trans-Golgi, and thus in localized Arf1 activation, is provided by an Arf-like G protein.
Abstract
The small G protein Arf1 regulates Golgi traffic and is activated by two related types of guanine nucleotide exchange factor (GEF). GBF1 acts at the cis-Golgi, whereas BIG1 and its close paralog BIG2 act at the trans-Golgi. Peripheral membrane proteins such as these GEFs are often recruited to membranes by small G proteins, but the basis for specific recruitment of Arf GEFs, and hence Arfs, to Golgi membranes is not understood. In this paper, we report a liposome-based affinity purification method to identify effectors for small G proteins of the Arf family. We validate this with the Drosophila melanogaster Arf1 orthologue (Arf79F) and the related class II Arf (Arf102F), which showed a similar pattern of effector binding. Applying the method to the Arf-like G protein Arl1, we found that it binds directly to Sec71, the Drosophila ortholog of BIG1 and BIG2, via an N-terminal region. We show that in mammalian cells, Arl1 is necessary for Golgi recruitment of BIG1 and BIG2 but not GBF1. Thus, Arl1 acts to direct a trans-Golgi–specific Arf1 GEF, and hence active Arf1, to the trans side of the Golgi.
Introduction
The members of the ADP ribosylation factor (Arf) family of small G proteins are essential regulators of membrane traffic and cytoskeletal systems (D’Souza-Schorey and Chavrier, 2006; Gillingham and Munro, 2007; Donaldson and Jackson, 2011). Distinct from the other members of the Ras superfamily of small G proteins, they are attached to membranes by an amphipathic N-terminal helix, which is often N myristoylated (Antonny et al., 1997; Pasqualato et al., 2002). The founding member of the family, Arf1, was initially shown to be required for the recruitment of COPI vesicle coats to Golgi membranes (Serafini et al., 1991; Donaldson et al., 1992). Arf1 is one of four close paralogs in humans, which are divided into class I (Arf1 and Arf3) and class II (Arf4 and Arf5), with a single member of each class being present in invertebrates (Tsuchiya et al., 1991; Lee et al., 1994). Most work has been performed on Arf1, although the other Arfs are thought to have similar roles in Golgi function but be less abundant. GTP-bound Arf1 has been shown to bind directly to vesicle coat proteins on both the cis-Golgi (COPI) and on the trans-Golgi (AP-1, AP-3, and GGAs; Stamnes and Rothman, 1993; Traub et al., 1993; Boman et al., 2000; Dell’Angelica et al., 2000; Drake et al., 2000). In addition, Arf1 has been shown to be involved in the Golgi recruitment of a coiled-coil protein as well as proteins involved in lipid transport and metabolism (Brown et al., 1993; Cockcroft et al., 1994; Gillingham et al., 2004).
The fact that Arfs1–5 function throughout the Golgi requires that they are activated in multiple parts of the Golgi stack. Two distinct Arf guanine nucleotide exchange factors (GEFs) have been found: the Gea1/GBF1 family and the Sec7/BIG family (Morinaga et al., 1997; Claude et al., 1999). These large proteins are related over much of their length and share a conserved Sec7 domain, which mediates nucleotide exchange (Chardin et al., 1996; Morinaga et al., 1999). However, the proteins are clearly distinct, with members of both families being found in all eukaryotic kingdoms, implying that they diverged before the last common eukaryotic ancestor and, hence, that the two types of GEF have fundamentally different roles (Cox et al., 2004; Mouratou et al., 2005; Bui et al., 2009). GBF1 acts on the early parts of the Golgi stack, whereas on the trans-Golgi are BIG1 and its close paralog BIG2 (whose human orthologs are encoded by the genes ARFGEF1/2; Zhao et al., 2002; Ishizaki et al., 2008; Manolea et al., 2008). This raises the question of how the two proteins are recruited to different ends of the Golgi stack.
Arfs1–5 are members of a larger Arf family that includes Sar1 and several Arf-like proteins (Arls; Pasqualato et al., 2002; Gillingham and Munro, 2007; Donaldson and Jackson, 2011). Some Arls have roles in membrane traffic, signaling, and cilia formation, although less is known about their regulation, and several lack known effectors. Two of the Arls, ARFRP1 and Arl1, are known to be localized on the trans-Golgi and to have been proposed to function in both exocytosis and in retrograde traffic from endosomes (Lowe et al., 1996; Behnia et al., 2004; Nishimoto-Morita et al., 2009; Cheryl Chia and Gleeson, 2011). Arl1 recruits several long coiled-coil golgins to the Golgi by binding to a conserved golgin-97, RanBP2a, Imh1p, and p230/golgin-245 (GRIP) domain at their C termini and also binds to the Bin–Amphiphysin–Rvs (BAR) domain protein arfaptin (Lu and Hong, 2003; Panic et al., 2003a; Setty et al., 2003; Derby et al., 2004; Man et al., 2011). ARFRP1 is required for the localization of Arl1 to Golgi membranes but has no known effectors (Panic et al., 2003b; Setty et al., 2003; Shin et al., 2005; Zahn et al., 2006; Nishimoto-Morita et al., 2009). Effectors for Arfs and Arls have been typically found by affinity chromatography using GST fusion proteins or yeast two-hybrid screens. However, it has been observed that at least one Arf1 effector, the coiled-coil protein GMAP-210, only shows detectable binding in vitro when Arf1-GTP is present on liposomes rather than being attached to beads as a GST fusion (Gillingham et al., 2004; Drin et al., 2008). This is a result of an amphipathic helix next to the GRIP-related Arf-binding (GRAB) domain, which stabilizes the GRAB–Arf1–GTP interaction by binding to the adjacent lipid bilayer (Drin et al., 2008). Given that all members of the Arf family are likely to sit close to the membrane and that several Arf family effectors have been found to also bind or modify lipids, this requirement for additional lipid interactions for stable binding may be a feature of at least some of their effectors (Levine and Munro, 2002; Shin and Nakayama, 2004; Cohen et al., 2007; Hofmann et al., 2007; Ménétrey et al., 2007). Thus, we developed a method for using G protein–coated liposomes for affinity purification of Arf effectors. Applying this to Drosophila melanogaster cell extracts revealed a direct interaction between Arl1 and Sec71, the Drosophila ortholog of the trans-Golgi GEFs BIG1/2. In mammalian cells, knockdown of Arl1 affects the Golgi recruitment of BIG1 and BIG2 but not of GBF1. Thus, Arl1 acts upstream of the trans-Golgi population of Arf1 and may therefore coordinate the tethering of arriving vesicles with the recruitment of coats for vesicle departure.
Results and discussion
Liposome-based isolation of Arf family effectors
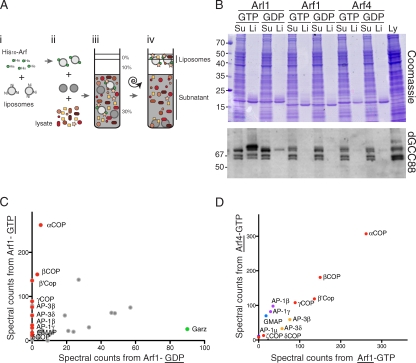
Arf family members bind to membranes only in the GTP-bound state because GTP binding drives the displacement of the amphipathic helix and N-terminal myristoyl group from a hydrophobic pocket (Goldberg, 1998; Pasqualato et al., 2002). This presents challenges for attaching native Arfs to liposomes, as they require an exchange factor to activate the protein before liposome binding, and mutant forms that are locked in the GTP-bound state are poorly soluble as a result of the exposed lipid-binding surface (Antonny et al., 1997; Béraud-Dufour et al., 1999). Thus, to uncouple nucleotide state from membrane attachment, we replaced the amphipathic N-terminal helices of Drosophila Arl1 and ARFRP1 with an N-terminal His10 tag. Such His-tagged forms of Sar1 and Arf1 have been previously shown to bind to liposomes containing low levels of a lipid with an Ni–nitrilotriacetic acid (NTA) head group and to then recruit effectors (Lee et al., 2005; Drin et al., 2008). For controls, we also expressed Drosophila orthologs of the class I and class II Arfs. The Drosophila genes for these four proteins are named CG7039 (ARFRP1), Arf72A (Arl1), Arf79F (class I Arf), and Arf102F (class II Arf), but to aid clarity, we will refer to the proteins by the names of their mammalian orthologs (ARFRP1, Arl1, Arf1, and Arf4).
All four G proteins were expressed with mutations previously shown to lock them in the GDP-bound (T to N) or GTP-bound (Q to L) states (Dascher and Balch, 1994; Lu et al., 2001) and then loaded with nucleotide and bound to Ni-NTA lipid-containing liposomes. The coated liposomes were suspended in extracts prepared from Drosophila S2 cells using a detergent-free protocol. The cell lysates were then adjusted to be of a higher density than the interior of the liposomes, and at the end of the binding reaction, the liposomes were floated out of the lysate by centrifugation (Fig. 1 A). A second flotation from washing buffer was used to reduce the background, and proteins were then extracted from the coated liposomes. Examination of the liposome-associated proteins showed relatively little background, and antibody staining revealed that the GRIP domain coiled-coil protein GCC88 was selectively bound to the Arl1-GTP–coated liposomes (Fig. 1 B). This demonstrates that Arf-coated liposomes can be used to enrich effectors from cell lysates.
Figure 1.
Liposome-based method for isolating Arf family effectors. (A) A schematic of the liposome-based purification method. His10-tagged Arf is bound to light liposomes (i), mixed with cell lysate and heavy liposomes (intended to reduce nonspecific binding to the light liposomes; ii), overlayed with less dense layers (iii), and, after centrifugation, liposomes and bound proteins are separated from the rest of the lysate (iv). Liposomes were then washed by diluting in buffer and repeating step iv. (B) Proteins from lysates of Drosophila S2 cells that bound to liposomes coated with nucleotide-locked forms of Drosophila Arl1, Arf1, and Arf4 prepared as in A. Lanes contain material from liposomes (Li) or 1/200 of subnatant (Su) or input lysate (Ly). Gels were either stained with Coomassie blue or immunoblotted for Drosophila GCC88 (dGCC88) as indicated. Molecular mass is indicated in kilodaltons. (C) Comparison of the peptide spectral counts for proteins on liposomes coated with GTP- or GDP-locked Arf1. Coloring indicates known Arf1 effectors (red) and the known Arf1 GEF Garz (green). COP, coatomer protein. (D) Comparison of the peptide spectral counts for known Arf effectors on liposomes coated with GTP-locked Arf1 or GTP-locked Arf4. Proteins of the same complex are shown in the same color (coat proteins COPI in red, AP-1 in purple, AP-3 in yellow, and the coiled-coil Golgi microtubule–associated protein [GMAP] in blue). (C and D) The graphs are representative of two independent experiments.
Isolation of effectors for class I Arf
As Arf1 is likely to be the most abundant of the four small G proteins, we initially investigated whether the material bound to the Arf1-coated liposomes was in sufficient yield to allow mass spectrometric identification of individual effectors. Protein gels of the liposome-bound material were cut into three sections, and tryptic peptides from in-gel digestion were analyzed by liquid chromatography tandem mass spectrometry (MS/MS). This revealed that there was sufficient material for peptide sequences to be obtained from many different proteins. Comparison of the number of spectra from a particular protein that were obtained from the Arf1-GTP and the Arf1-GDP samples provides an approximate measure of the relative amount in each sample (Neilson et al., 2011). Several known Arf effectors were readily detectable on the Arf1-GTP liposomes, whereas they were represented by few, or no, peptides in the material from the Arf1-GDP liposomes (Fig. 1 C and Table S1). Several proteins were present at approximately similar levels on both liposomes, but many of these were also readily detectable on uncoated liposomes, indicating that they are nonspecific background (Table S1). Interestingly, the Drosophila ortholog of GBF1, Gartenzwerg, was the only protein showing a clear enrichment on the Arf1-GDP liposomes, consistent with Arf1-GDP being its substrate (Fig. 1 C).
Liposomes coated with the class II Arf, Arf4, showed similar results, and comparison of peptide spectral counts for known Arf1 effectors did not reveal any that were exclusive to Arf1 or to Arf4, although there may be some slight preferences in binding (Fig. 1 D and Table S1). This is consistent with a study in mammalian cells, which have indicated considerable redundancy between class I and class II Arfs (Volpicelli-Daley et al., 2005). Collectively, these results show that Arf-1–coated liposomes can be used to enrich effectors with sufficient yield and purity to allow the effectors to be identified by mass spectrometry.
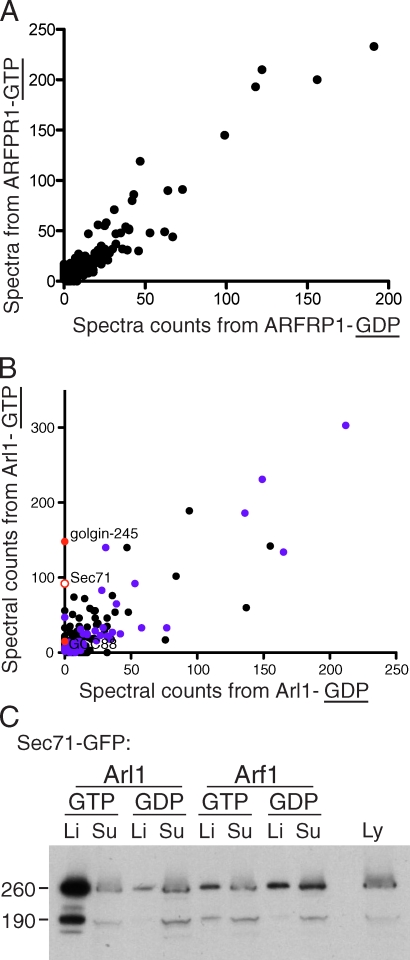
Affinity chromatography with ARFRP1 and Arl1
Next, we applied the same large-scale purification approach to liposomes coated with Drosophila ARFRP1 and Arl1. As with Arf1, mass spectrometry of bound material identified many proteins (Table S1). In the case of ARFRP1, the population of proteins bound to the GTP form appeared similar to that bound to the GDP form, and, thus, no candidate effectors could be identified (Fig. 2 A). However, several proteins showed preferential binding to Arl1-GTP over Arl1-GDP (Fig. 2 B). These included two GRIP domain proteins, as expected (golgin-245 and GCC88), as well as several proteins not previously associated with Arl1. Some of the less abundant examples were also found associated with free liposomes, suggesting that they actually represent nonspecific binding (e.g., Sbf; Table S1). However, the GTP-specific protein with the second highest number of peptide spectra after golgin-245 was Sec71. This corresponded to 25 unique peptides, whereas no spectra were found for this protein in the Arl1-GDP sample or from the ARFRP1 samples or free liposomes. Sec71 is the Drosophila ortholog of human BIG1/BIG2, although it has not been extensively characterized.
Figure 2.
Sec71 binds to Arl1-coated liposomes in a GTP-dependent manner. (A) Comparison of the peptide spectral counts for proteins bound to liposomes coated with GTP- or GDP-locked forms of ARFRP1. (B) As in A, except for Arl1. Known effectors dGolgin-245 and dGCC88 are shown (closed red circles) along with Sec71 (open red circles), and proteins among the top 100 found on liposomes are indicated in purple. (A and B) The graphs are representative of two independent experiments. (C) Anti-GFP immunoblot of a small-scale liposome-binding assay using lysates (Ly) of S2 cells expressing Sec71-GFP as input and the indicated forms of Arl1 or Arf1. Li, liposomes; Su, subnatant. Molecular mass is indicated in kilodaltons.
Arl1 binds directly to Sec71 to recruit it to the trans-Golgi
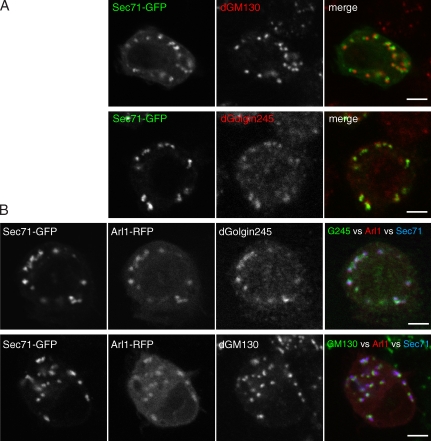
To verify the interaction between Arl1 and Sec71, the latter protein was expressed in S2 cells with a C-terminal GFP tag. When extracts from the transfected cells were used for liposome-binding assays, the protein bound to liposomes coated with GTP-locked, but not GDP-locked, Arl1, confirming the mass spectrometric data (Fig. 2 C). Examination of S2 cells expressing GFP-tagged Sec71 revealed that it was associated with the Golgi and overlapped with a trans-Golgi marker (dGolgin245) while being clearly distinct from a cis-Golgi marker (dGM130), indicating a trans-Golgi localization (Fig. 3 A). When Arl1-RFP was coexpressed with Sec71-GFP, the two proteins showed a very similar distribution, and, again, this overlapped the trans-Golgi protein while being clearly distinct from the cis-Golgi (Fig. 3 B). Thus, Sec71 binds to Arl1-coated liposomes in vitro and colocalizes with Arl1 on the trans-Golgi in vivo.
Figure 3.
Sec71 and Arl1 colocalize on the trans-Golgi. (A) Confocal micrographs of S2 cells expressing GFP-tagged Sec71 and stained for dGM130 (cis-Golgi) or dGolgin245 (trans-Golgi). (B) Confocal micrographs of S2 cells coexpressing Sec71-GFP and Arl1-RFP and stained as in A. (A and B) Sec71 is closer to the dGolgin245 than to dGM130 but does not colocalize with the former. However, the significance of this is uncertain, as the antibody to dGolgin245 was raised to the N-terminal 200 residues of this 1,489-residue coiled-coil protein (Sinka et al., 2008) and so may be binding up to 200 nm from the membrane to which dGolgin245 is anchored. Bars, 5 µm.
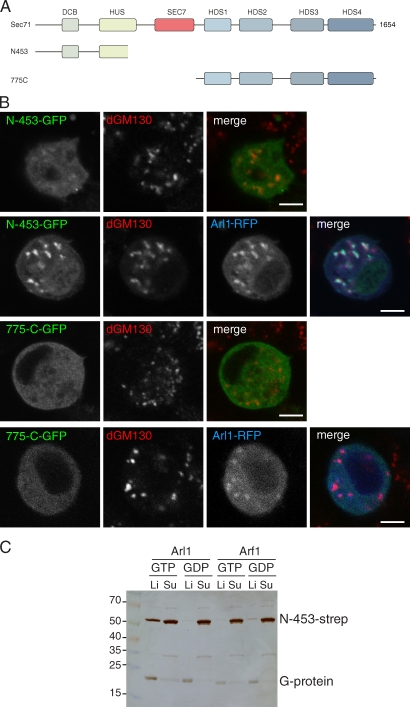
Sec71 binds directly to Arl1 via the region N terminal to the Sec7 domain
Sec71, like its mammalian counterparts, has large conserved regions flanking the central Sec7 domain (Fig. 4 A). It has been reported that the N-terminal 561 residues of human BIG1 are sufficient for Golgi targeting in mammalian cells (Mansour et al., 1999). When the equivalent region of Sec71 was expressed in S2 cells, it showed only weak Golgi staining (Fig. 4 B). However, when it was coexpressed with Drosophila Arl1, this N-terminal region was efficiently recruited to the Golgi (Fig. 4 C). In contrast, the region of Sec71 C terminal to the Sec7 domain was diffusely distributed in the cytosol, irrespective of Arl1 overexpression. This indicates that Arl1 recruits Sec71 to Golgi membranes, but it appears that Arl1’s effects are augmented by additional interactions elsewhere in the protein. These could be via the Sec7 domain itself, perhaps via direct interaction with its substrate Arf1.
Figure 4.
The N terminus of Sec71 binds to the Golgi and Arl1-GTP. (A) A schematic of Sec71 showing the catalytic Sec7 domain and other regions conserved in orthologs. The DCB (dimerization and Cyp5 binding), HUS (homology upstream of Sec7), and HDS (homology downstream of Sec7) domains are as previously described (Mouratou et al., 2005; Bui et al., 2009). (B) Confocal micrographs of S2 cells expressing GFP-tagged truncations of Sec71-GFP shown in A, in some cases along with Arl1-RFP. Cells were stained with an antibody against dGM130. Bars, 5 µm. (C) Silver-stained protein gel showing the binding of a purified recombinant form of the N terminus of Sec71 to Ni-NTA liposomes coated with His-tagged Arl1 or Arf1. The indicated forms of the G protein were bound to Ni-NTA liposomes, after which purified Strep-tagged Sec71(N-453) was added. After a 1-h incubation, liposomes were floated through an OptiPrep gradient, and protein samples were prepared from the liposome (Li) and subnatant (Su; 16% of input). Molecular mass is indicated in kilodaltons.
To determine whether Arl1 was interacting directly with Sec71, the N-terminal region of Sec71 was expressed in Escherichia coli and purified. This recombinant fragment of the protein bound specifically to the recombinant GTP-bound form of Arl1 on liposomes, confirming that the interaction is direct (Fig. 4 C).
Mammalian Arl1 is required for the Golgi recruitment of BIG1 and BIG2
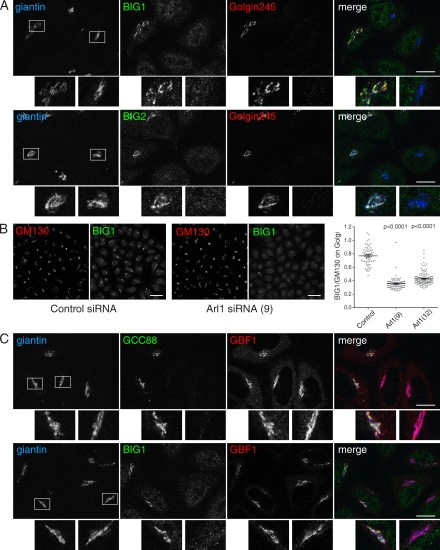
To test the relevance of these results for mammalian cells, we determined whether BIG1 and BIG2 require Arl1 for their Golgi localization. Arl1 could be efficiently knocked down in HeLa cells using two independent siRNAs, and in such cells, the GRIP domain golgins were displaced from the Golgi as expected (Fig. S1, A and B). Strikingly, knockdown of Arl1 also resulted in both BIG1 and BIG2 being displaced from the Golgi, whereas the levels of the proteins were not affected (Figs. 5 A and S1 A). Similar results were obtained with the two independent siRNAs and three independent antibodies to BIG1. Quantitation of the level of BIG1 staining relative to another Golgi marker demonstrated that the effect was seen throughout the cell population and was highly statistically significant (Fig. 5 B). Moreover, the displacement of BIG1 after Arl1 knockdown could be rescued by expression of an siRNA-resistant form of Arl1 (Fig. S1 C). Finally, overexpression of two known Arl1 effectors (golgin-245 and arfaptin; Lu and Hong, 2003; Man et al., 2011) displaced BIG1 from the Golgi (Fig. S1 D), consistent with Arl1 binding to BIG1 in a manner mutually exclusive with binding to the GRIP domain or arfaptin.
Figure 5.
Arl1 is required for recruitment of BIG1 and BIG2 to the Golgi. (A) Confocal micrographs of HeLaM cells treated with siRNA against Arl1 and mixed 50:50 with untreated cells before plating on slides for staining with antibodies to the indicated endogenous proteins. Representative Golgi regions in the boxed areas are shown magnified in the insets. In cells lacking Arl1, the GRIP domain protein golgin-245 or GCC88 is displaced from the Golgi as expected. In such cells, BIG1 and BIG2 are also displaced from the Golgi, whereas other Golgi proteins are apparently unaffected. Bars, 15 µm. (B) Confocal micrographs of z stack projections of fields of HeLaM cells treated with a nontargeting siRNA (control) or an Arl1 siRNA, stained for the indicated proteins, and imaged with identical settings. The ratio of BIG1 staining to GM130 staining was quantified for all the cells in the field (n > 60), and the difference between the control and two different Arl1 siRNAs is highly statistically significant (two-tailed unpaired t test). Error bars show the SEM. Bars, 50 µm. (C) As in A, except that the cells were stained for the indicated endogenous proteins to show that the cis-Golgi Arf GEF, GBF1, retains its Golgi localization when Arl1 is knocked down. Bars, 15 µm.
In contrast to these results with BIG1 and BIG2, other Golgi markers were relatively unaffected by Arl1 removal, including GBF1, the Arf GEF that is localized to the cis-Golgi (Fig. 5 C). Thus, it seems that, as in Drosophila, Arl1 is required for the recruitment of the Arf exchange factors of the trans-Golgi. This finding is consistent with several previously reported observations on mammalian Arl1 and BIG1/2. First, when cells are treated with the Arf GEF inhibitor Brefeldin A, BIG1 and BIG2, like Arl1, are displaced from the Golgi more slowly than Arf1 effectors, suggesting that all are upstream of Arf1 activity on the trans-Golgi (Lowe et al., 1996; Yamaji et al., 2000). Second, overexpression of either BIG1 or the GTP-locked form of Arl1 increases the Golgi recruitment of Arf effectors AP-1 and GGAs and blocks their Brefeldin A–induced redistribution, indicating that Arl1 and BIG1/2 both act upstream of Arf1-GTP (Lu et al., 2001; Shinotsuka et al., 2002). These previous observations fit well with the notion of a Golgi Arf cascade from ARFRP1 to Arl1 and then to the TGN Arfs, with at least the latter step being mediated by a direct interaction between a G protein and a downstream GEF. Such cascades of a small G protein recruiting the GEF for a second G protein have been found to occur with some members of the Rab family of G proteins (Ortiz et al., 2002; Kinchen and Ravichandran, 2010; Nordmann et al., 2010). In addition, the Arf GEFs of the ARF nucleotide–binding site opener/cytohesin family have been shown to be recruited to the plasma membrane by the Arf family members Arl4 and Arf6 (Cohen et al., 2007; Hofmann et al., 2007; Stalder et al., 2011). Thus, such G protein cascades may be a dominant theme in the organization of internal membranes.
Our data show that Arf GEFs require Arl1 for recruitment to liposomes and to the trans-Golgi. Our results also show that liposome-based binding can be used to identify novel effectors from total cell extracts, and so it may be of use for analyzing at least some of the other Arf family members. However, it does have the limitation that detergent washing is impossible, and so it may only be applicable to more abundant or higher affinity effectors, and it did not produce an effector for ARFRP1. Although Arl1 is sufficient for Sec71 recruitment when present at high levels on liposomes, it is likely that further interactions are required to stabilize binding to the trans-Golgi. This is perhaps not surprising, given the large size of these GEFs and the importance of ensuring that they are only active in the correct compartment. Several other trans-Golgi proteins bind PtdIns(4)P (Levine and Munro, 2002; Santiago-Tirado and Bretscher, 2011), but overexpression of a pleckstrin homology (PH) domain that binds the Golgi pool of PtdIns(4)P did not displace BIG1, suggesting that the latter may not rely on PI(4)P for recruitment in vivo (Fig. S1 D). Alternatively, Arf1 itself could contribute to Sec71/BIG1 recruitment either via Arf1-GDP binding to the Sec7 domain or by Arf1-GTP recognizing Sec71 as an effector and so enhancing recruitment in a positive feedback loop. This might also explain that fact that the yeast ortholog Sec7 appears to remain at least partially Golgi localized when yeast Arl1 is deleted (Panic et al., 2003b; Behnia et al., 2004). We did detect Drosophila Sec71 bound to Arf1-GTP liposomes (Table S1), but we did not detect binding of the overexpressed Sec71-GFP (Fig. 2 C), suggesting that the association may be indirect. Nonetheless, if there are any such additional interactions, they are clearly not sufficient for Golgi targeting of BIG1 and BIG2 in the absence of Arl1. The notion that Arl1 is the critical determinant of BIG1/2 localization is appealing, as this G protein also recruits golgins that are believed to tether carriers arriving from endosomes (Yoshino et al., 2005; Cheryl Chia and Gleeson, 2011; Munro, 2011). Thus, Arl1 would be well placed to ensure that as maturing Golgi cisternae acquire the ability to receive traffic from endosomes, they are also able to generate the vesicles that will return recycling receptors to endosomes.
Materials and methods
Antibodies and plasmids
Human or Drosophila versions of Arf family proteins without the residues comprising the N-terminal amphipathic helix (typically 14 amino acids) were inserted into pET-based vectors for expression with an N-terminal His10 tag. Full-length and truncated forms of Drosophila Sec71 (CG7578) were amplified and cloned into the pDONR221 vector and transferred to appropriate destination vectors (Gateway; Invitrogen). The N-terminal 453 residues of Drosophila Sec71 were cloned into pASK-IBA3C (IBA GmbH) for expression with a C-terminal Strep tag.
GFP–human BIG1 and HA–human BIG1 constructs were gifts from M. Vaughan (National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD) and D. Stephens (University of Bristol School of Medical Sciences, Bristol, England, UK; Yamaji et al., 2000; Boal and Stephens, 2010), and GFP-arfaptin was a gift from Y. Vallis (Medical Research Council Laboratory of Molecular Biology, Cambridge, England, UK). GFP-GRIP (golgin-245 GRIP domain) and GFP–oxysterol-binding protein (OSBP) PH was as previously described (Levine and Munro, 1998; Munro and Nichols, 1999).
Mouse antibodies were against GFP, human AP-1 (100/3; Sigma-Aldrich), human GM130 (558712; BD), human golgin-245 (611280; BD), and human GBF1 (612116; BD). Rabbit anti-Ar11 was provided by W. Hong (Institute of Molecular and Cell Biology, Singapore, Singapore), and other rabbit antibodies were against Drosophila GM130 (ab30637; Abcam), Drosophila GRIP domain–containing proteins (Sinka et al., 2008), BIG1 (HPA023399 [Sigma-Aldrich] and H-200 [Santa Cruz Biotechnology, Inc.]), and BIG2 (HPA026078; Sigma-Aldrich). Goat anti-giantin (N-18; Santa Cruz Biotechnology, Inc.), goat anti-BIG1 (K-19; Santa Cruz Biotechnology, Inc.), and sheep anti-TGN46 (AHP500; AbD Serotec) were used.
Protein expression and purification
Expression constructs were transformed into E. coli BL21-GOLD (DE3; Agilent Technologies). Cells grown to OD600 = 0.8–1.0 at 37°C were induced with 0.1 mM IPTG at 17°C overnight. Cell pellets were frozen in liquid nitrogen and stored at −80°C until use. To purify the His-tagged proteins, cell pellets from 1 L of culture were resuspended in 40 ml of lysis buffer (50 mM Hepes, 120 mM KOAc, 1 mM MgCl2, and 1 mM DTT) further containing 10 mM imidazole and protease inhibitors and lysed by Dounce homogenization and sonication. The lysate was precleared by centrifugation at 15,000 rpm (JA25.50 rotor; Beckman Coulter) for 15 min and added to 1 ml of equilibrated Ni-NTA agarose beads (QIAGEN). After 1 h of incubation at 4°C, the beads were washed three times in batch (two times with 25 ml of lysis buffer containing 20 mM imidazole and once in 25 ml of lysis buffer containing 40 mM imidazole), poured into columns, and washed once more with 10 ml of the same buffer. Protein was eluted using lysis buffer containing 250 mM imidazole. Fractions containing His-tagged protein (typically 8× 0.5-ml fractions were collected, with protein eluted in fraction 2, 3, and 4) were combined and dialyzed overnight against lysis buffer without imidazole, containing either GDP or nonhydrolyzable guanosine 5′-[β,γ-imido]triphosphate (Sigma-Aldrich). To purify the Strep-tagged proteins, the same procedure was followed to lyze and preclear the lysate, with the exception that the lysis buffer did not contain imidazole. Protein was bound to equilibrated Strep-Tactin Sepharose beads (IBA GmbH) by gravity flow over a column. The column was washed five times with one column volume of lysis buffer, and protein was eluted with lysis buffer containing 2.5 mM desthiobiotin (Sigma-Aldrich). Desthiobiotin was removed from the purified protein using spin-desalting columns (Zeba; Thermo Fisher Scientific).
Liposome flotation assay
All lipids were obtained from Avanti Polar Lipids, Inc. Lipids and cholesterol were combined in chloroform in the following ratio: 50% egg phosphatidylcholine, 19% liver phosphatidylethanolamine, 5% brain phosphatidylserine, 10% liver phosphatidylinositol, and 16% cholesterol. To allow binding of His-tagged protein, 5% Ni-NTA–labeled lipid (18:1 DGS-NTA(Ni)/1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl]) was added as well as 0.2% NBD-labeled phosphatidylethanolamine to allow distinction of the Ni-NTA liposomes from cellular vesicles and unlabeled liposomes. Solvent was evaporated using argon flow, and the mixture was further dried for 1 h under vacuum. The mixture was rehydrated using lysis buffer lacking imidazole added to a lipid concentration of 1 mg/ml for at least 1 h. Liposomes were prepared with the Mini-Extruder (Avanti Polar Lipids, Inc.) using Nuclepore track-etched membranes with 400-nm pores (GE Healthcare).
0.05 mg of protein was added to 0.4 ml of liposome suspension and incubated for 30 min in the presence of the appropriate nucleotide. Precleared cell lysate was prepared by lysing cells using Dounce homogenization and sonication in an equal-volume lysis buffer containing protease inhibitors and subsequent centrifugation for 2 h at 100k g. Either 1.5 ml of precleared cell lysate and 0.3 ml of heavy liposomes (liposomes without Ni-NTA lipids, prepared in lysis buffer containing 30% OptiPrep [Axis-Shield]) or purified protein was added, and the mixture was incubated for 1 h at room temperature. OptiPrep was added to a final concentration of 30%, and the mixture was overlaid with 0.55 ml of a 10% OptiPrep solution in lysis buffer and a 0.3-ml layer of lysis buffer. Liposomes were floated in an SW60 rotor at 45k rpm for 30 min. The Ni-NTA liposome layer that floated to the 10–0% OptiPrep interface was collected and resuspended in lysis buffer. OptiPrep was added again to 30%, and the mixture was overlaid as described before. Liposomes were floated again and collected. Bound protein was precipitated using methanol and chloroform and dissolved in SDS-PAGE sample buffer.
Mass spectrometry and data analysis
Samples obtained from the liposome flotation assay were loaded onto 6% Tris-glycine SDS-PAGE gels and run for a few centimeters. Proteins were stained with Coomassie brilliant blue. The entire gel lane between the stacking gel and the His fusion protein was excised in three parts (40–60, 60–90, and >90 kD), and each was submitted to in-gel tryptic digestion, and peptides were eluted.
Data-dependent liquid chromatography MS/MS was performed by nanoflow reverse-phase liquid chromatography (Dionex U3000; Thermo Fisher Scientific) coupled online to a mass spectrometer (LTQ Orbitrap XL; Thermo Fisher Scientific). In brief, the liquid chromatography separation was performed using a Dionex C18 PepMap column (75 µm ID × 150 mm; Thermo Fisher Scientific), and the peptides were eluted using a linear gradient from 5 to 50% B over 2 h at a flow rate of 200 nL/min (solvent A: 98% H2O and 2% acetonitrile in 0.1% formic acid; solvent B: 90% acetonitrile in 0.1% formic acid). A cycle of one full Fourier transform scan mass spectrum (m/z 350–2,000, with a resolution of 60,000 at m/z 400) was followed by 10 data-dependent MS/MS acquired in the linear ion trap. All the measurements in the Orbitrap were performed with the lock mass option (lock mass: m/z 445.120025) for internal calibration. Mascot generic files were generated using Proteome Discoverer software (version 1.0; Thermo Fisher Scientific). All spectra were searched using Mascot (version 2.2; Matrix Science). Mascot was set up to search the Drosophila all-translation database (version 4.3; 38,778 entries) assuming the digestion enzyme trypsin. Mascot was searched with a fragment ion mass tolerance of 0.80 D and a parent ion tolerance of 10.0 ppm. Iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification. Conversion to pyroglutamate of the N-terminal glutamine, oxidation of methionine, and acetylation of the protein N terminus were specified in Mascot as variable modifications.
Scaffold software (Proteome Software Inc.) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at >90.0% probability, as specified by the Peptide Prophet algorithm (Keller et al., 2002). Protein identifications were accepted if they could be established at >99.0% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (Nesvizhskii et al., 2003). Proteins that contained similar peptides and that could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Cell culture and microscopy
Drosophila S2 cells were grown at 25°C in serum-free medium (Express Five; Invitrogen) containing penicillin, streptomycin, and l-glutamine. COS and HeLaM cells were grown at 37°C in 10% CO2 in DME (Invitrogen) in the presence of penicillin, streptomycin, n-glutamine, and 10% FCS. Cells were fixed (4% formaldehyde in PBS for 15 min) and blocked for 1 h (PBS, 0.1% Triton X-100, and 20% FCS). Primary and secondary (Alexa Fluor; Invitrogen) antibodies in blocking buffer were applied for 1 h, and cells were washed five times with PBS, mounted in VECTASHIELD (Vector Laboratories), and imaged on a confocal microscope with a Plan-Apochromat 63× 1.4 NA objective (LSM 510 controlled with Zen software; Carl Zeiss). Images were further processed with Photoshop (CS5; Adobe) to increase brightness without altering contrast. BIG1 levels on the Golgi were quantified by using Imaris (Bitplane) to analyze image stacks taken at low magnification. GM130 staining was used to segment the Golgi region of each cell, with a 250-µm3 minimum size to remove mitotic fragments, etc. BIG1 and GM130 staining levels within each Golgi segment were determined and then expressed as a ratio.
siRNA
HeLaM cells were transfected with ON-TARGETplus siRNA oligonucleotides (Thermo Fisher Scientific) using Oligofectamine (Invitrogen). HeLaM cells were seeded at 2 × 105 cells per 9.4 cm2 and transfected with 100 µM siRNA after 4 h. Cells were fixed in 4% formaldehyde in PBS for 15 min or lysed in SDS-PAGE sample buffer 4 d after the initial transfection. 24 h before fixing, cells were split onto coverslips. siRNAs were nontargeting siRNA1 (D-001810-01-20) or human ARL1 (J-019265-12 and J-019265-09).
Online supplemental material
Fig. S1 shows experiments in mammalian cells to validate the Arl1 siRNA, demonstrates the rescue of BIG1 displacement by siRNA-resistant Arl1, and shows the displacement of BIG1 by other Arl1 effectors. Table S1 lists the mass spectrometric peptides from proteins bound to liposomes coated with GDP- and GTP-bound forms of Drosophila Arf1, Arf4, Arl1, and ARFRP1. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201107115/DC1.
Acknowledgments
We thank Wanjin Hong, Andrew Peden, David Stephens, Yvonne Vallis, and Martha Vaughan for reagents and advice; Farida Begum and Elaine Stephens for mass spectrometry advice and analysis; Nick Barry for help with image quantitation; and Alison Gillingham, Ben Nichols, and Katja Röper for comments on the manuscript.
Footnotes
Abbreviations used in this paper:
- GEF
- guanine nucleotide exchange factor
- MS/MS
- tandem mass spectrometry
- NTA
- nitrilotriacetic acid
- OSBP
- oxysterol-binding protein
- PH
- pleckstrin homology
References
- Antonny B., Beraud-Dufour S., Chardin P., Chabre M. 1997. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 36:4675–4684 10.1021/bi962252b [DOI] [PubMed] [Google Scholar]
- Behnia R., Panic B., Whyte J.R., Munro S. 2004. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat. Cell Biol. 6:405–413 10.1038/ncb1120 [DOI] [PubMed] [Google Scholar]
- Béraud-Dufour S., Paris S., Chabre M., Antonny B. 1999. Dual interaction of ADP ribosylation factor 1 with Sec7 domain and with lipid membranes during catalysis of guanine nucleotide exchange. J. Biol. Chem. 274:37629–37636 10.1074/jbc.274.53.37629 [DOI] [PubMed] [Google Scholar]
- Boal F., Stephens D.J. 2010. Specific functions of BIG1 and BIG2 in endomembrane organization. PLoS ONE. 5:e9898 10.1371/journal.pone.0009898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A.L., Zhang C., Zhu X., Kahn R.A. 2000. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 11:1241–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H.A., Gutowski S., Moomaw C.R., Slaughter C., Sternweis P.C. 1993. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 75:1137–1144 10.1016/0092-8674(93)90323-I [DOI] [PubMed] [Google Scholar]
- Bui Q.T., Golinelli-Cohen M.-P., Jackson C.L. 2009. Large Arf1 guanine nucleotide exchange factors: Evolution, domain structure, and roles in membrane trafficking and human disease. Mol. Genet. Genomics. 282:329–350 10.1007/s00438-009-0473-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chardin P., Paris S., Antonny B., Robineau S., Béraud-Dufour S., Jackson C.L., Chabre M. 1996. A human exchange factor for ARF contains Sec7- and pleckstrin-homology domains. Nature. 384:481–484 10.1038/384481a0 [DOI] [PubMed] [Google Scholar]
- Cheryl Chia P.Z., Gleeson P.A. 2011. The regulation of endosome-to-Golgi retrograde transport by tethers and scaffolds. Traffic. 12:939–947 10.1111/j.1600-0854.2011.01185.x [DOI] [PubMed] [Google Scholar]
- Claude A., Zhao B.P., Kuziemsky C.E., Dahan S., Berger S.J., Yan J.P., Armold A.D., Sullivan E.M., Melançon P. 1999. GBF1: A novel Golgi-associated BFA-resistant guanine nucleotide exchange factor that displays specificity for ADP-ribosylation factor 5. J. Cell Biol. 146:71–84 [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Thomas G.M., Fensome A., Geny B., Cunningham E., Gout I., Hiles I., Totty N.F., Truong O., Hsuan J.J. 1994. Phospholipase D: A downstream effector of ARF in granulocytes. Science. 263:523–526 10.1126/science.8290961 [DOI] [PubMed] [Google Scholar]
- Cohen L.A., Honda A., Varnai P., Brown F.D., Balla T., Donaldson J.G. 2007. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domains. Mol. Biol. Cell. 18:2244–2253 10.1091/mbc.E06-11-0998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Mason-Gamer R.J., Jackson C.L., Segev N. 2004. Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol. Biol. Cell. 15:1487–1505 10.1091/mbc.E03-06-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Balch W.E. 1994. Dominant inhibitory mutants of ARF1 block endoplasmic reticulum to Golgi transport and trigger disassembly of the Golgi apparatus. J. Biol. Chem. 269:1437–1448 [PubMed] [Google Scholar]
- Dell’Angelica E.C., Puertollano R., Mullins C., Aguilar R.C., Vargas J.D., Hartnell L.M., Bonifacino J.S. 2000. GGAs: A family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 149:81–94 10.1083/jcb.149.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby M.C., van Vliet C., Brown D., Luke M.R., Lu L., Hong W., Stow J.L., Gleeson P.A. 2004. Mammalian GRIP domain proteins differ in their membrane binding properties and are recruited to distinct domains of the TGN. J. Cell Sci. 117:5865–5874 10.1242/jcs.01497 [DOI] [PubMed] [Google Scholar]
- Donaldson J.G., Jackson C.L. 2011. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 12:362–375 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J.G., Cassel D., Kahn R.A., Klausner R.D. 1992. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc. Natl. Acad. Sci. USA. 89:6408–6412 10.1073/pnas.89.14.6408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.T., Zhu Y., Kornfeld S. 2000. The assembly of AP-3 adaptor complex-containing clathrin-coated vesicles on synthetic liposomes. Mol. Biol. Cell. 11:3723–3736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G., Morello V., Casella J.F., Gounon P., Antonny B. 2008. Asymmetric tethering of flat and curved lipid membranes by a golgin. Science. 320:670–673 10.1126/science.1155821 [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C., Chavrier P. 2006. ARF proteins: Roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7:347–358 10.1038/nrm1910 [DOI] [PubMed] [Google Scholar]
- Gillingham A.K., Munro S. 2007. The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 23:579–611 10.1146/annurev.cellbio.23.090506.123209 [DOI] [PubMed] [Google Scholar]
- Gillingham A.K., Tong A.H., Boone C., Munro S. 2004. The GTPase Arf1p and the ER to Golgi cargo receptor Erv14p cooperate to recruit the golgin Rud3p to the cis-Golgi. J. Cell Biol. 167:281–292 10.1083/jcb.200407088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. 1998. Structural basis for activation of ARF GTPase: Mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell. 95:237–248 10.1016/S0092-8674(00)81754-7 [DOI] [PubMed] [Google Scholar]
- Hofmann I., Thompson A., Sanderson C.M., Munro S. 2007. The Arl4 family of small G proteins can recruit the cytohesin Arf6 exchange factors to the plasma membrane. Curr. Biol. 17:711–716 10.1016/j.cub.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Ishizaki R., Shin H.-W., Mitsuhashi H., Nakayama K. 2008. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-Golgi network and endosomes. Mol. Biol. Cell. 19:2650–2660 10.1091/mbc.E07-10-1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A., Nesvizhskii A.I., Kolker E., Aebersold R. 2002. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74:5383–5392 10.1021/ac025747h [DOI] [PubMed] [Google Scholar]
- Kinchen J.M., Ravichandran K.S. 2010. Identification of two evolutionarily conserved genes regulating processing of engulfed apoptotic cells. Nature. 464:778–782 10.1038/nature08853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.J., Stevens L.A., Hall L.M., Murtagh J.J., Jr, Kao Y.L., Moss J., Vaughan M. 1994. Characterization of class II and class III ADP-ribosylation factor genes and proteins in Drosophila melanogaster. J. Biol. Chem. 269:21555–21560 [PubMed] [Google Scholar]
- Lee M.C., Orci L., Hamamoto S., Futai E., Ravazzola M., Schekman R. 2005. Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell. 122:605–617 10.1016/j.cell.2005.07.025 [DOI] [PubMed] [Google Scholar]
- Levine T.P., Munro S. 1998. The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr. Biol. 8:729–739 10.1016/S0960-9822(98)70296-9 [DOI] [PubMed] [Google Scholar]
- Levine T.P., Munro S. 2002. Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12:695–704 10.1016/S0960-9822(02)00779-0 [DOI] [PubMed] [Google Scholar]
- Lowe S.L., Wong S.H., Hong W. 1996. The mammalian ARF-like protein 1 (Arl1) is associated with the Golgi complex. J. Cell Sci. 109:209–220 [DOI] [PubMed] [Google Scholar]
- Lu L., Hong W. 2003. Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/p230 onto the Golgi. Mol. Biol. Cell. 14:3767–3781 10.1091/mbc.E03-01-0864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Horstmann H., Ng C., Hong W. 2001. Regulation of Golgi structure and function by ARF-like protein 1 (Arl1). J. Cell Sci. 114:4543–4555 [DOI] [PubMed] [Google Scholar]
- Man Z., Kondo Y., Koga H., Umino H., Nakayama K., Shin H.-W. 2011. Arfaptins are localized to the trans-Golgi by interaction with Arl1, but not Arfs. J. Biol. Chem. 286:11569–11578 10.1074/jbc.M110.201442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolea F., Claude A., Chun J., Rosas J., Melançon P. 2008. Distinct functions for Arf guanine nucleotide exchange factors at the Golgi complex: GBF1 and BIGs are required for assembly and maintenance of the Golgi stack and trans-Golgi network, respectively. Mol. Biol. Cell. 19:523–535 10.1091/mbc.E07-04-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S.J., Skaug J., Zhao X.H., Giordano J., Scherer S.W., Melançon P. 1999. p200 ARF-GEP1: A Golgi-localized guanine nucleotide exchange protein whose Sec7 domain is targeted by the drug brefeldin A. Proc. Natl. Acad. Sci. USA. 96:7968–7973 10.1073/pnas.96.14.7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménétrey J., Perderiset M., Cicolari J., Dubois T., Elkhatib N., El Khadali F., Franco M., Chavrier P., Houdusse A. 2007. Structural basis for ARF1-mediated recruitment of ARHGAP21 to Golgi membranes. EMBO J. 26:1953–1962 10.1038/sj.emboj.7601634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga N., Moss J., Vaughan M. 1997. Cloning and expression of a cDNA encoding a bovine brain brefeldin A-sensitive guanine nucleotide-exchange protein for ADP-ribosylation factor. Proc. Natl. Acad. Sci. USA. 94:12926–12931 10.1073/pnas.94.24.12926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga N., Adamik R., Moss J., Vaughan M. 1999. Brefeldin A inhibited activity of the sec7 domain of p200, a mammalian guanine nucleotide-exchange protein for ADP-ribosylation factors. J. Biol. Chem. 274:17417–17423 10.1074/jbc.274.25.17417 [DOI] [PubMed] [Google Scholar]
- Mouratou B., Biou V., Joubert A., Cohen J., Shields D.J., Geldner N., Jürgens G., Melançon P., Cherfils J. 2005. The domain architecture of large guanine nucleotide exchange factors for the small GTP-binding protein Arf. BMC Genomics. 6:20 10.1186/1471-2164-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. 2011. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb. Perspect. Biol. 3 10.1101/cshperspect.a005256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Nichols B.J. 1999. The GRIP domain - a novel Golgi-targeting domain found in several coiled-coil proteins. Curr. Biol. 9:377–380 10.1016/S0960-9822(99)80166-3 [DOI] [PubMed] [Google Scholar]
- Neilson K.A., Ali N.A., Muralidharan S., Mirzaei M., Mariani M., Assadourian G., Lee A., van Sluyter S.C., Haynes P.A. 2011. Less label, more free: Approaches in label-free quantitative mass spectrometry. Proteomics. 11:535–553 10.1002/pmic.201000553 [DOI] [PubMed] [Google Scholar]
- Nesvizhskii A.I., Keller A., Kolker E., Aebersold R. 2003. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75:4646–4658 10.1021/ac0341261 [DOI] [PubMed] [Google Scholar]
- Nishimoto-Morita K., Shin H.W., Mitsuhashi H., Kitamura M., Zhang Q., Johannes L., Nakayama K. 2009. Differential effects of depletion of ARL1 and ARFRP1 on membrane trafficking between the trans-Golgi network and endosomes. J. Biol. Chem. 284:10583–10592 10.1074/jbc.M900847200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann M., Cabrera M., Perz A., Bröcker C., Ostrowicz C., Engelbrecht-Vandré S., Ungermann C. 2010. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr. Biol. 20:1654–1659 10.1016/j.cub.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Ortiz D., Medkova M., Walch-Solimena C., Novick P. 2002. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J. Cell Biol. 157:1005–1015 10.1083/jcb.200201003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic B., Perisic O., Veprintsev D.B., Williams R.L., Munro S. 2003a. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol. Cell. 12:863–874 10.1016/S1097-2765(03)00356-3 [DOI] [PubMed] [Google Scholar]
- Panic B., Whyte J.R., Munro S. 2003b. The ARF-like GTPases Arl1p and Arl3p act in a pathway that interacts with vesicle-tethering factors at the Golgi apparatus. Curr. Biol. 13:405–410 10.1016/S0960-9822(03)00091-5 [DOI] [PubMed] [Google Scholar]
- Pasqualato S., Renault L., Cherfils J. 2002. Arf, Arl, Arp and Sar proteins: A family of GTP-binding proteins with a structural device for ‘front-back’ communication. EMBO Rep. 3:1035–1041 10.1093/embo-reports/kvf221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Tirado F.H., Bretscher A. 2011. Membrane-trafficking sorting hubs: Cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 21:515–525 10.1016/j.tcb.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Orci L., Amherdt M., Brunner M., Kahn R.A., Rothman J.E. 1991. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: A novel role for a GTP-binding protein. Cell. 67:239–253 10.1016/0092-8674(91)90176-Y [DOI] [PubMed] [Google Scholar]
- Setty S.R., Shin M.E., Yoshino A., Marks M.S., Burd C.G. 2003. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr. Biol. 13:401–404 10.1016/S0960-9822(03)00089-7 [DOI] [PubMed] [Google Scholar]
- Shin H.-W., Nakayama K. 2004. Dual control of membrane targeting by PtdIns(4)P and ARF. Trends Biochem. Sci. 29:513–515 10.1016/j.tibs.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Shin H.W., Kobayashi H., Kitamura M., Waguri S., Suganuma T., Uchiyama Y., Nakayama K. 2005. Roles of ARFRP1 (ADP-ribosylation factor-related protein 1) in post-Golgi membrane trafficking. J. Cell Sci. 118:4039–4048 10.1242/jcs.02524 [DOI] [PubMed] [Google Scholar]
- Shinotsuka C., Yoshida Y., Kawamoto K., Takatsu H., Nakayama K. 2002. Overexpression of an ADP-ribosylation factor-guanine nucleotide exchange factor, BIG2, uncouples brefeldin A-induced adaptor protein-1 coat dissociation and membrane tubulation. J. Biol. Chem. 277:9468–9473 10.1074/jbc.M112427200 [DOI] [PubMed] [Google Scholar]
- Sinka R., Gillingham A.K., Kondylis V., Munro S. 2008. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J. Cell Biol. 183:607–615 10.1083/jcb.200808018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder D., Barelli H., Gautier R., Macia E., Jackson C.L., Antonny B. 2011. Kinetic studies of the Arf activator Arno on model membranes in the presence of Arf effectors suggest control by a positive feedback loop. J. Biol. Chem. 286:3873–3883 10.1074/jbc.M110.145532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes M.A., Rothman J.E. 1993. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 73:999–1005 10.1016/0092-8674(93)90277-W [DOI] [PubMed] [Google Scholar]
- Traub L.M., Ostrom J.A., Kornfeld S. 1993. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 123:561–573 10.1083/jcb.123.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M., Price S.R., Tsai S.C., Moss J., Vaughan M. 1991. Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells. J. Biol. Chem. 266:2772–2777 [PubMed] [Google Scholar]
- Volpicelli-Daley L.A., Li Y., Zhang C.J., Kahn R.A. 2005. Isoform-selective effects of the depletion of ADP-ribosylation factors 1-5 on membrane traffic. Mol. Biol. Cell. 16:4495–4508 10.1091/mbc.E04-12-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji R., Adamik R., Takeda K., Togawa A., Pacheco-Rodriguez G., Ferrans V.J., Moss J., Vaughan M. 2000. Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc. Natl. Acad. Sci. USA. 97:2567–2572 10.1073/pnas.97.6.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino A., Setty S.R., Poynton C., Whiteman E.L., Saint-Pol A., Burd C.G., Johannes L., Holzbaur E.L., Koval M., McCaffery J.M., Marks M.S. 2005. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J. Cell Sci. 118:2279–2293 10.1242/jcs.02358 [DOI] [PubMed] [Google Scholar]
- Zahn C., Hommel A., Lu L., Hong W., Walther D.J., Florian S., Joost H.G., Schürmann A. 2006. Knockout of Arfrp1 leads to disruption of ARF-like1 (ARL1) targeting to the trans-Golgi in mouse embryos and HeLa cells. Mol. Membr. Biol. 23:475–485 10.1080/09687860600840100 [DOI] [PubMed] [Google Scholar]
- Zhao X., Lasell T.K., Melançon P. 2002. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: Evidence for distinct functions in protein traffic. Mol. Biol. Cell. 13:119–133 10.1091/mbc.01-08-0420 [DOI] [PMC free article] [PubMed] [Google Scholar]