Several organizations worldwide have developed procedure-based guidelines and/or position statements regarding various aspects of quality and safety indicators, and credentialing for endoscopy. Although important, they do not specifically address patient needs or provide a framework for their adoption in the context of endoscopy services. The consensus guidelines reported in this article, however, aimed to identify processes and indicators relevant to the provision of high-quality endoscopy services that will support ongoing quality improvement across many jurisdictions, specifically in the areas of ethics, facility standards and policies, quality assurance, training and education, reporting standards and patient perceptions.
Keywords: Digestive system, Endoscopy, Guideline, Health care, Quality assurance
Abstract
BACKGROUND:
Increasing use of gastrointestinal endoscopy, particularly for colorectal cancer screening, and increasing emphasis on health care quality, highlight the need for clearly defined, evidence-based processes to support quality improvement in endoscopy.
OBJECTIVE:
To identify processes and indicators of quality and safety relevant to high-quality endoscopy service delivery.
METHODS:
A multidisciplinary group of 35 voting participants developed recommendation statements and performance indicators. Systematic literature searches generated 50 initial statements that were revised iteratively following a modified Delphi approach using a web-based evaluation and voting tool. Statement development and evidence evaluation followed the AGREE (Appraisal of Guidelines, REsearch and Evaluation) and GRADE (Grading of Recommendations, Assessment, Development and Evaluation) guidelines. At the consensus conference, participants voted anonymously on all statements using a 6-point scale. Subsequent web-based voting evaluated recommendations for specific, individual quality indicators, safety indicators and mandatory endoscopy reporting fields. Consensus was defined a priori as agreement by 80% of participants.
RESULTS:
Consensus was reached on 23 recommendation statements addressing the following: ethics (statement 1: agreement 100%), facility standards and policies (statements 2 to 9: 90% to 100%), quality assurance (statements 10 to 13: 94% to 100%), training, education, competency and privileges (statements 14 to 19: 97% to 100%), endoscopy reporting standards (statements 20 and 21: 97% to 100%) and patient perceptions (statements 22 and 23: 100%). Additionally, 18 quality indicators (agreement 83% to 100%), 20 safety indicators (agreement 77% to 100%) and 23 recommended endoscopy-reporting elements (agreement 91% to 100%) were identified.
DISCUSSION:
The consensus process identified a clear need for high-quality clinical and outcomes research to support quality improvement in the delivery of endoscopy services.
CONCLUSIONS:
The guidelines support quality improvement in endoscopy by providing explicit recommendations on systematic monitoring, assessment and modification of endoscopy service delivery to yield benefits for all patients affected by the practice of gastrointestinal endoscopy.
Abstract
HISTORIQUE :
L’utilisation croissante de l’endoscopie gastrointestinale, notamment dans le cadre du dépistage du cancer colorectal, et l’accent grandissant mis sur la qualité des soins mettent en lumière la nécessité d’établir des processus probants clairement définis pour étayer l’amélioration de la qualité en endoscopie.
OBJECTIF :
Déterminer les processus et les indicateurs de qualité et de sécurité pertinents pour la prestation de services d’endoscopie de qualité.
MÉTHODOLOGIE :
Un groupe multidisciplinaire de 35 participants ayant droit de vote a élaboré des énoncés de recommandations et des indicateurs de rendement. Des analyses bibliographiques systématiques ont permis d’obtenir 50 énoncés initiaux qui ont été révisés de manière itérative conformément à la méthode Delphi modifiée au moyen d’une évaluation virtuelle et d’un outil de vote. L’élaboration des énoncés et l’évaluation des données probantes respectaient les lignes directrices AGREE (acronyme anglais d’appréciation de lignes directrices, de recherches et d’évaluations) et GRADE (notation de recommandations, d’appréciation, d’élaboration et d’évaluation). À la conférence consensuelle, les participants ont exprimé leur vote de manière anonyme à l’égard de tous les énoncés, au moyen d’une échelle de six points. Les votes virtuels subséquents ont permis d’évaluer les recommandations relatives à des indicateurs de qualité spécifiques et individuels, à des indicateurs de sécurité et à des champs de déclaration d’endoscopie. Le consensus a été défini a priori-par une entente entre 80 % des participants.
RÉSULTATS :
Les chercheurs sont arrivés à un consensus à l’égard de 23 énoncés de recommandation portant sur les points suivants : l’éthique (énoncé 1 : entente 100 %), normes et politiques de facilité (énoncés 2 à 9 : 90 % à 100 %), assurance qualité (énoncés 10 à 13 : 94 % à 100 %), formation, éducation, compétence et privilèges (énoncés 14 à 19 : 97 % à 100 %), normes de déclaration d’endoscopie (énoncés 20 et 21 : 97 % à 100 %) et perceptions des patients (énoncés 22 et 23 : 100 %). De plus, ils ont repéré 18 indicateurs de qualité (entente de 83 % à 100 %), 20 indicateurs de sécurité (entente de 77 % à 100 %) et 23 éléments de déclaration d’endoscopie recommandés (entente de 91 % à 100 %).
EXPOSÉ :
Le processus consensuel a permis de déterminer un besoin clair de recherches cliniques et d’issues de qualité pour étayer l’amélioration de la qualité dans la prestation des services d’endoscopie.
CONCLUSIONS :
Les lignes directrices appuient l’amélioration de la qualité en endoscopie en fournissant des recommandations explicites sur la surveillance, l’évaluation et la modification systématiques de la prestation des services d’endoscopie qui seront bénéfiques à tous les patients touchés par la pratique de l’endoscopie gastrointestinale.
Quality in digestive endoscopy has been central to the emphasis on quality care in gastroenterology worldwide. Publications, both in the gastroenterological and surgical literature, have primarily addressed issues of procedural and technical quality and of procedural and process safety (1–8). However, recognition of the need for greater patient focus in health care has led to greater interest in patient access to procedures, in the appropriateness and timeliness of procedures, and in patient comfort and satisfaction.
Guidelines or position statements on various aspects of quality indicators, safety indicators and credentialing for endoscopy have been developed by the British Society of Gastroenterology (BSG) (1), the American Society for Gastrointestinal Endoscopy and the American College of Gastroenterology (2,4), the Organisation Mondiale d’Endoscopie Digestive/World Organisation of Digestive Endoscopy (OMGE/OMED) (3), the International Agency for Research in Cancer (9) and the Canadian Association of Gastroenterology (CAG) (5–8). However, these guidelines are all procedure-based and, while they are very important, they either do not address patient needs or do not provide a framework for adoption in the overall context of endoscopy services.
The Global Rating Scale (GRS) was developed from the patients’ perspective as a quality improvement tool relevant to the entirety of endoscopy service delivery (10); it was based on quality and safety indicators developed by the BSG (1) and was designed for the English National Health Service in the context of a nascent colorectal cancer screening program. The GRS has been credited with a marked improvement in the quality and availability of endoscopy services in England, and has been adopted in other parts of the United Kingdom. However, although the general principles of the GRS are broadly applicable, specific elements of the GRS and the associated BSG quality and safety indicators are not, necessarily, directly applicable to other health care systems. The development and implementation of endoscopy quality guidelines relevant to different jurisdictions should be based on the needs of the patients and the realities of clinical practice evaluated by providers and users familiar with the relevant health care environment.
AIMS
The primary aim in developing the present consensus guidelines was to identify processes and indicators relevant to the provision of high-quality endoscopy services and to achieve consensus on broadly applicable standards and key indicators that will support continuing quality improvement for endoscopy services across many jurisdictions (1,10). The literature review and guideline development addressed only the most commonly performed endoscopic procedures: esophagogastroduodenoscopy and colonoscopy. However, the principal issues addressed in this process are also applicable to more specialized endoscopic procedures, although specific indicators relevant to these procedures fall beyond of the scope of the current project.
METHODS
Initial stages and identification of the consensus group
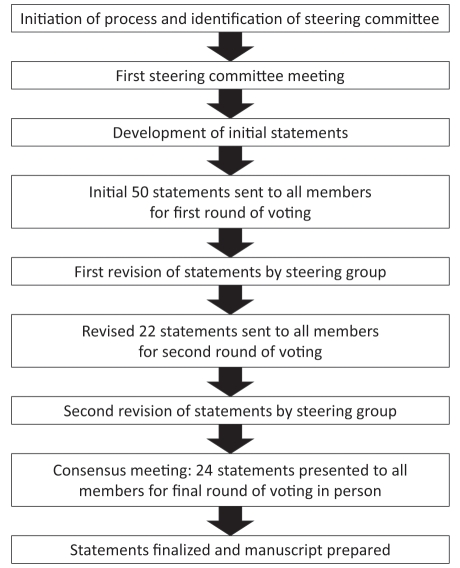
The guideline development process was performed in accordance with the Appraisal of Guidelines, REsearch and Evaluation (AGREE) guidelines (Figure 1) (11).
Figure 1).
Guideline development process
The goals of the consensus process were defined by the steering committee (DA, AB, RB, RC, CD, RE, CG, RH and DM) and endorsed by the CAG. The voting participants, invited by the steering committee, were selected to ensure multidisciplinary expert input from gastroenterologists, surgeons, nurses and administrators, as well as individuals with expertise in endoscopy, colorectal cancer screening, quality and patient safety (Appendix); two participants (MB and SF) joined the steering group as leads to two of the working groups. Four international experts (BP, PC, EK and RV) and a nonvoting moderator (JM) were also invited. No patients participated; however, consensus participants were provided with a summary of focus-group sessions conducted in a multicentre, qualitative research study that identified patient-derived quality indicators for endoscopy services.
Systematic literature searches
Systematic literature searches of PubMed were performed using the search terms: “colonoscopy”, “quality of healthcare”, “quality control”, “colorectal neoplasms”, “rectal neoplasms”, “adenomatous polyps”, “colonic polyps”, “intestinal polyps”, “digestive system neoplasms”, “diagnosis”, “diagnostic errors/adverse effects”, “diagnostic errors/standards”, “safety”, “mortality”, “complications” and “adverse events”. Searches were limited to articles published in English since 1990. Case reports were excluded. Additional searches (using the same search terms) were used to identify relevant abstracts from Digestive Disease Week 2007, 2008 and 2009, and United European Gastroenterology Week 2007 and 2008.
These searches identified 2475 articles. The citations and abstracts were assigned in batches to pairs of assessors from the steering committee; a dedicated, purpose-designed, online voting system was used to identify relevant articles on the basis of the article title and abstract. All conflicts were resolved by consensus. After two rounds of reviewing, 817 references were retained and the complete articles were uploaded to an online literature review site, used by the steering committee to develop draft statements. Additional literature searches were conducted during the statement development process, as needed.
Development of statements and iterative voting process
Fifty initial statements were developed, based on the literature review, analysis of the GRS and input from the steering committee; these were intended to be broad in nature rather than highly specific and they were used as a framework for continued development. An online system enabled the 35 voting participants (Table 1) to engage in three rounds of votes during the development process. At each voting round, participants voted on the importance and content of all statements using a 6-point scale (‘disagree strongly’, ‘disagree with major reservation’, ‘disagree with minor reservation’, ‘agree with major reservation’, ‘agree with minor reservation’ and ‘agree strongly’) and commented on the wording and validity of the statements. Results were compiled by the CAG to ensure voter anonymity.
TABLE 1.
Details of participants participating in the final round of voting (n=35)
| Specialty | |
| Gastroenterology | 28 (80) |
| Surgery | 3 (8.6) |
| Endoscopy nurse | 1 (2.9) |
| Other | 3 (8.6) |
| Country of employment | |
| Canada | 32 (91.4) |
| Other | 3 (8.6) |
| Endoscopic practice | |
| Perform upper endoscopy | 31 (88.6) |
| Perform colonoscopy | 30 (85.7) |
| Perform ERCP or other advanced procedures | 17 (48.6) |
| Location of endoscopic practice | |
| Academic health centre | 23 (65.7) |
| Hospital setting | 31 (88.6) |
| Out-of-hospital facility | 12 (34.3) |
Data presented as n (%). ERCP Endoscopic retrograde cholangiopancreatography
Round 1 vote: Using the 6-point scale, participants rated the extent to which they agreed that each of the 50 initial statements was important and relevant to the quality or safety of endoscopy. After reviewing the results of this round of voting, the steering committee reduced the number of statements to 22 and made iterative changes to the remaining statements to incorporate participants’ comments.
Round 2 vote: Participants voted to indicate the extent of their agreement with each of the 22 statements using the 6-point scale. After this round of voting, the 22 statements were assigned to nine working groups for further refinement and development of the evidence base before a third round of voting. The nine working groups were convened to address the following topics: quality indicators, safety indicators, endoscopy reporting standards, ethics, credentialing, quality assurance legislation, GRS development, patient perceptions of endoscopy quality and guideline dissemination. The conclusions of the working groups were then reported back to the steering committee.
Round 3 vote: Participants again voted to indicate the extent of their agreement with each of the statements, using the 6-point scale. After the third round of voting, the statements were revised and two additional statements were added. The resulting 24 statements were reviewed by the working groups, who allocated relevant references to each statement to enable evidence grading.
Grading of evidence
Evidence grading was performed by two independent evaluators (GL and KK) using a modified Grading of Recommendation Assessment, Development and Evaluation (GRADE) process (Table 2) (12). This process involved two steps: assessment of the methodological quality of individual studies and assessment of the overall level of evidence behind each statement. Each step was performed independently by the two evaluators and disagreements were resolved by consensus.
TABLE 2.
Categorization of evidence, classification of recommendations and voting options
|
Grade of evidence |
| High: Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| Low: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low: Any estimate of effect is very uncertain |
|
Category of recommendation |
| “Do it” or “don’t do it”: Indicates a judgment that most well-informed people would make |
| “Probably do it” or “probably don’t do it”: Indicates a judgment that the majority of well-informed people would make but a substantial minority would not |
|
First vote – should this statement be implemented? |
| Strong recommendation in favour – do it |
| Weak recommendation in favour – possibly do it |
| Weak recommendation against – possibly don’t do it |
| Strong recommendation against – don’t do it |
|
Second vote – how much do you agree with the overall recommendation for implementation? |
| Agree strongly |
| Agree moderately |
| Agree slightly |
| Disagree slightly |
| Disagree moderately |
| Disagree strongly |
The overall level of evidence across studies for each statement was assessed using a Summary of Findings table (13). The GRADE system classifies the quality of evidence into one of four levels – high, moderate, low or very low. Evidence based on randomized controlled trials begins as high-quality evidence, but may be decreased due to study limitations (identified through the risk of bias evaluation), inconsistency (heterogeneity), indirectness, imprecision or other considerations such as reporting bias. Evidence based on case-control or cohort studies starts as low-quality evidence, but may be further decreased (for the same reasons as for randomized controlled trials) or increased if the magnitude of the treatment effect is very large, if there is evidence of a dose-response relationship or if all plausible biases would decrease the magnitude of an apparent treatment effect (12). The grading was subsequently reviewed and approved by all consensus members.
Consensus meeting and final voting
At the three-day consensus meeting, held in June 2010, each section was introduced by a member of the relevant working group who summarized the relevant literature and key issues. Each statement was subsequently discussed by the consensus group under the direction of the nonvoting moderator. A statement about withdrawal of consent was incorporated into a more general statement on consent (statement 1). Thus, the final vote included 23 statements.
Statement 1. For a patient to give a physician informed consent to perform an elective endoscopic procedure, the patient must be advised, in a timely fashion, of all relevant information about the procedure, its risks, benefits and alternatives, if any, and be given an opportunity to ask questions that the physician must answer.
Evidence grade: Low/very low
Strength of recommendation: Do it, 91%; possibly do it, 6%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 65%; agree moderately, 29%; agree slightly, 6%)
Following discussion, all voting participants used electronic keypads to record two separate anonymous votes on each statement. The first vote asked whether participants believed the statement should be implemented, and the second asked the extent to which they agreed with the overall recommendation for implementation (Table 2). In voting on the strength of recommendation, participants were asked to consider desirable and undesirable effects, patient values and preferences, the quality of the evidence and the judicious use of resources (12).
After the meeting, voting participants used the online system to identify specific, individual quality indicators, safety indicators and mandatory endoscopy reporting fields that they would recommend, again using a six-point scale for each item: ‘disagree strongly’, ‘disagree moderately’, ‘disagree slightly’, ‘agree slightly’, ‘agree moderately’ and ‘agree strongly’.
For each vote, a recommendation was considered to have been adopted by consensus if 80% or more of participants selected ‘agree slightly’, ‘agree moderately’ or ‘agree strongly’.
Ethics and financial support
The consensus process and meeting were administered by the CAG, supported by Canadian Partnership Against Cancer/Partenariat Canadien Contre le Cancer and Canadian Institutes of Health Research/ Instituts de Recherche en Santé du Canada: Institute of Nutrition, Metabolism, and Diabetes. All members provided conflict of interest statements before the meeting. Honoraria for participation were provided for international experts and participants who were not members of the CAG. Travel and accommodation were provided for all participants. After review of all disclosures, it was determined that there should be no limitations placed on any participant (14).
CONSENSUS STATEMENTS SECTION 1: ETHICS
Introduction
Informed consent must be obtained from a patient before any endoscopic procedure is performed. This statement provides the legal and ethical background to this position.
Discussion
This statement describes the minimum required to document acceptable provision of informed consent, consistent with Canadian law. It is necessary to obtain informed consent in a way that satisfies the patient’s expectation of full, informed consent based on individual needs for information to address personal concerns. The patient can be provided with information or instructions (eg, for bowel preparation) in several formats, including videotapes (15–17), brochures, information sessions led by allied health care professionals (nurses or assistants) or computer-assisted instructions/interactive software (18). However, these forms of communication do not obviate the need for a conversation with the endoscopist; it is the endoscopist who should obtain informed consent. Trainees may obtain consent if they are to perform the procedure and if disclosure is complete. However, in one study, up to one-third of trainees obtaining consent for colonoscopy had not disclosed the risks of perforation and hemorrhage (19). Ultimately, it is the supervisor who is responsible for ensuring that complete information is provided and that questions have been appropriately answered.
For an elective procedure, disclosure should allow the patient sufficient time to understand the information, reflect on it, ask questions and decide whether to proceed or to select an alternative. In an emergency situation, informed consent should be obtained as soon as possible. For direct-access endoscopy, the patient may be sent information before the procedure, although, in one study, 37% of patients would have preferred meeting a gastroenterologist before the colonoscopy and 20% said that they received the most useful information after taking the bowel preparation (20). In all cases, full verbal disclosure, documented in the patient record, is still required before the procedure (Box 1).
BOX 1. Full disclosure for informed consent: Required elements for review.
Indication for the procedure
Nature of the procedure
- Need for preparation
- ○ Risks and benefits of preparation
- ○ Alternative preparations
Patients’ concerns about discomfort and pain
- Alternatives to the procedure
- ○ Option of no investigations or treatment
- ○ Potential alternative therapies including risks and benefits
- Sedation options
- ○ Risks, benefits and adverse events of all options
- ▪ No sedation
- ▪ Conscious sedation and deep sedation/general anesthesia
- ▪ Alternatives including hypnotic relaxation, music, electro-acupuncture and nitrous oxide
The choice of sedation (or no sedation) should be a shared decision, made on a case-by-case basis, between the attending physician/ endoscopist and patient, based on the patient’s expectations of comfort, the patient’s medical condition, and complexity and duration of the procedure. Regardless of the type of sedation recommended, patients should understand that they may decline sedation.
The consent process should account for the patient’s competence and understanding. Language, cognitive ability, severity of illness, pain and analgesia may affect a patient’s capacity to understand and consent to a procedure. Provision of informed consent is not a guarantee that the patient has understood the information provided; recall of informed consent is often suboptimal. In one study, 50% of patients could not recall important information on their procedure’s indication or risks or when consent was obtained (26). Effort should be made to ensure that the patient has the capacity to consent and to maximize their understanding of the information provided, possibly by complementing oral with written information.
Consent must be given without coercion; this implies that consent can be withdrawn at any time. However, it is possible that a patient under sedation may not be fully cognizant of their request to halt a procedure and the associated risks; nonetheless, if a patient requests that the procedure be stopped, the endoscopist should review his or her technique, the patient’s comfort and the need to continue. Procedure cessation may put the patient at risk of increased complications and may not be appropriate.
SECTION 2: FACILITY STANDARDS AND POLICIES
Introduction
Endoscopy facilities must meet or exceed defined standards of quality for operating standards, appropriateness of procedure, and technical and personnel resources. Facilities also must have policies in place that ensure best practice before, during and after endoscopic procedures.
Statement 2. Endoscopy facilities should meet or exceed defined operating standards, in all domains, consistent with accreditation under the appropriate national or regional standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 91%; possibly do it, 6%; don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 85%; agree moderately, 12%; disagree slightly, 3%)
Discussion
Operating standards may be endorsed locally by many endoscopists and endoscopy facilities, but adoption of appropriate standards is not universal and approved; accepted national guidelines are unavailable in many jurisdictions. Although endoscopic facilities differ greatly with respect to structure, size and procedural case loads, operating standards should apply uniformly, regardless of the facility’s size, design or location. It is, however, recognized that standards may be achieved by different means in different facilities.
In many jurisdictions, accrediting bodies do not have specific directives for endoscopy facilities. If endoscopy facilities are to be included in hospital accreditation programs, comprehensive directives must be specified to ensure that high-quality care becomes the standard. Ideally, accreditation of hospital and out-of-hospital endoscopy facilities should be governed by comparable processes to support continuous quality improvement programs, with regular review in all cases.
The adoption of standards and procedures to ensure safety and quality should also support appropriate resource use; indicators of appropriate utilization include no-show rates, adequacy of preparation, nurse-to-patient ratios, safe working environment and appropriate flow of patients. Quality assurance activities should be defined from a patient perspective, and should target indicators of procedural completeness and accuracy, opportunities for patient feedback, and evidence of continued change and reassessment to demonstrate improvement (see section 3 below). Quality and safety standards should also address endoscope reprocessing, conscious sedation, monitoring protocols and resuscitation equipment. Although documented transmission of infection from endoscopic equipment is extremely rare (27), the United States Food and Drug Administration recommends that all institutions have a written program and appropriate administrative organization for endoscope processing, handling and storage (28). Every facility providing endoscopic services should ensure the ongoing availability of protocols, equipment, and trained, competent personnel necessary for the provision of safe and effective conscious sedation.
Statement 3. Endoscopic procedures are performed for an appropriate, clearly documented indication, consistent with current, evidence-based guidelines.
Evidence grade: Low/very low
Strength of recommendation: Do it, 97%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 85%; agree moderately, 6%; agree slightly, 6%; disagree slightly, 3%)
Discussion
The indication for every procedure should be documented in the procedure report, and the indication should be consistent with accepted guidelines. National or regional guidelines are preferable; however, if none are available or applicable, locally relevant guidelines should be developed.
Consensus guidelines provide explicit statements of appropriate indications for endoscopic procedures (29,30), and there is evidence that the diagnostic yield of endoscopy is significantly increased if procedures are performed for appropriate indications (31,32). Regrettably, reports indicate that 11% to 39% of endoscopic procedures are performed for inappropriate indications (31,33), and that surveillance endoscopies may be performed at inappropriate intervals (34) or unnecessarily (35–37). However, guidelines should not be the only determinants of procedural appropriateness; for example, esophagastroduodenoscopy may be an inappropriate initial step in dyspepsia management, but it may become appropriate if initial medical therapy fails (29). Similarly, a colonoscopic surveillance interval may, appropriately, be shorter than recommended if, for example, bowel preparation is poor. However, in all cases, the reason for deviation from guidelines or accepted indications should be clearly documented.
Statement 4. Endoscopy facilities should have the technical and personnel resources required by national and/or regional standards to complete all planned procedures safely and effectively.
Evidence grade: Low/very low
Strength of recommendation: Do it, 91%; possibly do it, 6%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 69%; agree moderately, 26%; agree slightly, 6%)
Discussion
Published, evidence-based guidelines do, on occasion, specify the technical and personnel resources needed for procedures, such as endoscopic reprocessing and urgent endoscopy, in the event of a major gastrointestinal bleed (28,38). The importance of experienced ancillary personnel for procedural quality and efficiency is highlighted, for example, by reports of higher polyp detections rates at screening colonoscopy in the presence of more experienced nurses (detection rate of 46% with more than six months of experience versus 40% with six months or less; OR 1.26 [95% CI 1.09 to 1.46]) (39) or of efficiency gains of up to 30% when additional personnel were available to gain intravenous access and administer medications (40).
A skills mix review (41) can identify the personnel resources necessary for a particular institution or facility, based on types of procedures performed, types of sedation offered and need for out-of-hours emergency services.
Statement 5. Endoscopy facilities should implement and monitor the effect of preprocedure policies that ensure best practice.
Evidence grade: Low/very low
Strength of recommendation: Do it, 97%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 86%; agree moderately, 9%; agree slightly, 3%; disagree slightly, 3%)
Discussion
Preprocedure assessment should encompass the risks of sedation, the preparation and the procedure, including documented assessments of patient age, comorbidity, American Society of Anesthesiologists classification, significant medical issues, medications (including type of bowel preparation) and potential complications of bowel preparation (such as dehydration). Relevant policies and guidelines should be implemented (Box 2).
BOX 2. Preprocedure guidelines that should be implemented.
Antibiotic prophylaxis guidelines for prevention of infective endocarditis; eg, American Heart Association (42) and BSG guidelines (43).
Antithrombotic agent guidelines on management of antithrombotic agents for endoscopic procedures; eg, American Society for Gastrointestinal Endoscopy guidelines (44) state that anticoagulant agents (eg, warfarin, unfractionated heparin, low-molecular-weight heparin) and antiplatelet agents (eg, acetylsalicylic acid, clopidogrel, ticlopidine, glycoprotein IIb/IIIa receptor inhibitors) increase the risks of procedure-related bleeding. Preprocedural assessment should encompass the benefits, risks and urgency of the procedure, the bleeding risks associated with antithrombotic therapy and the procedure, and the thromboembolic risk of stopping therapy.
Surveillance schedules (eg, Barrett’s esophagus, ulcerative colitis)
Anesthetic/sedation risk guidelines (47)
Allergy or drug sensitivity guidelines (48)
Procedural pause (48)
Statement 6. Endoscopy facilities should implement and monitor the effect of intraprocedural policies that ensure best practice.
Evidence grade: Low/very low
Strength of recommendation: Do it, 94%; possibly do it, 3%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 85%; agree moderately, 9%; agree slightly, 3%; disagree moderately, 3%)
Discussion
Intraprocedural policies are important to achieve and maintain maximal patient safety and comfort and optimal examination quality (Box 3). Completion of colonoscopy, with complete cecal visualization, is essential for detecting lesions in the proximal colon. Endoscopists with a higher cecal intubation rate have a higher rate of colonic neoplastic lesion detection (49). Documentation of cecal intubation using still image or, preferably, video recording (50) of the appendiceal orifice and ileocecal valve, should be confirmed by the endoscopy assistant/ nurse. Terminal ileum biopsy should be performed only if indicated clinically. Right iliac fossa transillumination and finger indentation are inadequate to confirm cecal intubation (51). Cecal intubation rates greater than 90%, documented in several case series (52–54), should be standard of practice for symptomatic patients and intubation rates should exceed 95% for screening colonoscopies (1,3).
BOX 3. Intraprocedural policies that should be implemented.
Verified, objective photo or video documentation of cecal intubation
Regular monitoring of sedation level (eg, implementation of an evidence-based sedation protocol)
Regular monitoring of relevant physiological parameters (blood pressure, pulse, oxygen saturation, etc)
Routine evaluation of bowel preparation (ie, a standardized tool such as the Ottawa Bowel Preparation scale [59] or the Boston Bowel Preparation scale [60])
Documentation of withdrawal time during colonoscopy – a minimum of 8 min is ideal (withdrawal time is correlated with a thorough examination)
Regular monitoring of patient comfort
Dual observation during examination (endoscopist and nurse)
Conscious sedation (intravenous benzodiazepines, with or without narcotics, or propofol) may improve endoscopy completion rates (55) but is resource intensive. An evidence-based sedation protocol can improve practice quality and reduce sedation-related adverse events (56,57).
Poor bowel preparation is associated with longer procedure time (58), greater patient discomfort (58), lower colonoscopy completion rates (55), and higher risks of adverse events and missed lesions. A standardized tool such as the Ottawa Bowel Preparation scale (59) or the Boston Bowel Preparation scale (60) should be used to assess bowel preparation quality and process changes should be implemented if inadequate bowel preparation is prevalent.
Endoscopy facilities should institute a policy to record the colonoscopy withdrawal time as a surrogate for a thorough examination. Longer withdrawal times are associated with greater detection rates for advanced neoplasia (adenomata of 10 mm or larger, lesions with villous change, high-grade dysplasia or cancer) (61) and advanced neoplasia detection is improved by a protocol specifying a minimum withdrawal time of 8 min (62).
Statement 7. The endoscopy facility should implement and monitor the effects of policies for the discharge of patients that ensure best practice.
Evidence grade: Very low
Strength of recommendation: Do it, 97%; possibly do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 89%; agree moderately, 9%; agree slightly, 3%)
Discussion
Clear specific discharge policies (Box 4), should be implemented and should accommodate differences in patients’ responses, particularly, to sedation. Elderly patients (70 years of age and older) and those with oxygen desaturation, hypotension, bradycardia and those needing reversal agents require longer recovery times (63). A list of criteria such as the Aldrete score (respiration, oxygen saturation, consciousness, circulation and activity levels) should be used to determine readiness for discharge (64,65). The half-life of reversal agents tends to be shorter than the half-life of sedatives; therefore, reversal agent effects may wane before those of the sedative. Discharge policies should specify patient transportation requirements and activity restrictions after the procedure, with respect to the type and dose of sedation.
BOX 4. Policies for discharge of patients that should be implemented.
Patient satisfaction with discharge processes should be assessed regularly, for example, using standardized postdischarge surveys.
Statement 8. Endoscopy facilities should ensure that there is a policy in place to notify patients of the need, and appropriate interval, for follow-up.
Evidence grade: Low/very low
Strength of recommendation: Do it, 76%; possibly do it, 18%; possibly don’t do it, 3%; don’t do it, 3%
Level of agreement with recommendation: Agree, 90% (agree strongly, 39%; agree moderately, 42%; agree slightly, 9%; disagree slightly, 6%; disagree moderately, 3%)
Statement 9. All patients, on discharge, are given written information regarding the procedural findings, plans for treatment and follow-up, worrisome symptoms to watch for, and steps to be taken.
Evidence grade: Very low
Strength of recommendation: Do it, 97%; possibly do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 86%; agree moderately, 6%; agree slightly, 9%)
Discussion
On discharge, written details of the procedure should be provided for the information of the patient and of any physician providing subsequent care (eg, for complications). Provision of a procedure report reduced patients’ postprocedure anxiety, improved their recall of findings and recommendations and improved adherence to recommendations (66).
The facility’s policies should specify the information to be provided in the discharge report (Box 5), the details of follow-up arrangements that have been or will be made and the person responsible for arranging the follow-up plan.
BOX 5. Discharge report – key elements.
Description of key findings, interventions, complications and sedation
Description of symptoms of potential complications
Instructions on actions to be taken if symptoms of complications arise
Contact details in the event that complications arise
Instructions on resumption of anticoagulants
Instructions for follow-up (why, when, where and with whom)
SECTION 3: QUALITY ASURANCE
Introduction
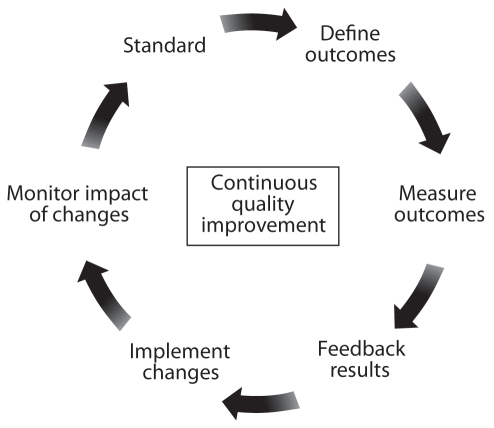
Maintaining and enhancing the quality and safety of endoscopy services should be a continuous process that measures aspects of endoscopic performance, implements changes based on these measurements, monitors the effect of these changes and evaluates these effects to achieve new standards (Figure 2).
Figure 2).
Continuous process of quality improvement
Discussion
In some jurisdictions, quality assurance activities are legislated and, in many jurisdictions, a quality assurance committee’s proceedings are protected from disclosure if it is constituted according to applicable regional, territorial, state or national legislation and regulations (66–77). Thus, endoscopy quality assurance committees must be developed within the appropriate legislative framework to oversee the performance of the endoscopy facility and all of its personnel. The potential conflicts arising from the disparate perspectives of different stakeholders can be resolved if quality in endoscopy is addressed from the perspective of the patient. In developing the GRS for evaluation of endoscopy, two patient-centred quality dimensions were devised (10): the “Clinical Quality and Safety” dimension addressed six items related to appropriateness of the procedure, information and consent, safety, patient comfort, quality and prompt communication of results, while the “Quality of Patient Experience” (customer care) dimension addressed six items related to equality, timeliness, patient choice, privacy and dignity, aftercare and the ability to provide feedback to the endoscopy service.
A quality improvement and assessment tool such as the GRS provides a framework to support evaluation of service delivery by identifying specific indicators, consistent with high-quality endoscopy that should be auditable (ie, relevant outcomes for which there are no set standards) or measurable (ie, relevant outcomes for which there is an established standard).
Statement 12. Endoscopy facilities should systematically and regularly review current indicators of quality for all endoscopic procedures and implement appropriate responses.
Evidence grade: Low/very low
Strength of recommendation: Do it, 88%; possibly do it, 9%; don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 88%; agree moderately, 9%; disagree strongly, 3%)
Statement 13. Endoscopy facilities should systematically and regularly review current indicators of safety for all endoscopic procedures and implement appropriate responses.
Evidence grade: Low/very low
Strength of recommendation: Do it, 91%; possibly do it, 9%
Level of agreement with recommendation: Agree, 100% (agree strongly, 82%; agree moderately, 15%; agree slightly, 3%)
Discussion
Quality indicators and safety indicators for endoscopy were considered sufficiently distinct that two working groups were charged with identifying measurable or auditable outcomes that would support quality improvement. After the consensus meeting, participants voted on their endorsement of each quality and safety indicator identified by the working groups (Boxes 6 and 7).
BOX 6. Indicators of quality in endoscopy.
| Indicator Comment | Consensus, % |
|---|---|
|
Indicator related to entire endoscopy facility | |
| 1. Participation in a recognized quality assurance program | 97.2 |
|
Indicators related to the technical performance of the procedure and appropriateness | |
| 2. Completion of procedure | 100.0 |
| Documented inspection of duodenum, cecum or terminal ileum | |
| 3. Appropriateness of procedure | 97.2 |
| Performed for an appropriate indication | |
| 4. Completeness of procedure | 97.2 |
| Inspection of all relevant areas, acquisition of appropriate biopsies and completion of all appropriate interventions | |
| 5. Withdrawal time | 85.6 |
| As a minimum, rapid withdrawal precludes complete inspection; especially colonoscopy | |
| 6. Adenoma detection rate | 100.0 |
| Requires reconciliation of pathology and endoscopy reports | |
| 7. Polyp detection rate | 91.4 |
| A surrogate marker for a careful examination | |
| 8. Appropriateness of endoscopic intervention | 97.2 |
| Interventions are performed, or eschewed, appropriately, according to the indication and findings | |
| 9. Completion of endoscopic intervention | 97.2 |
| Interventions are performed to completion (eg, polypectomy) | |
| 10. Appropriateness of biopsy | 94.3 |
| Biopsies are performed, or eschewed, appropriately, according to the indication and findings | |
|
Patient-centred indicators of quality | |
| 11. Quality of patient experience | 97.2 |
| There is a formal assessment of the patient’s experience, preferably using a standard tool | |
| 12. Sedation dosage | 82.8 |
| Systematic overuse or underuse of sedation is identified; usage is correlated with outcome | |
|
Indicators relevant to quality of preparation before procedure | |
| 13. Quality of bowel preparation | 97.2 |
| Assessed formally, using a validated tool or, at a minimum, a standard scale (eg, poor, fair, good) | |
| 14. Appropriateness of antithrombotic management | 91.4 |
| Consistent with accepted guidelines | |
| 15. Appropriateness of antibiotic prophylaxis management | 82.8 |
| Consistent with accepted guidelines | |
|
Indicator relevant to quality of communication regarding results | |
| 16. Completion of endoscopy reporting | 97.2 |
| Procedure results report should be complete, with all relevant information included, and should be available immediately | |
|
Indicator of procedural quality relevant, predominantly, to colonoscopy screening and surveillance programs | |
| 17. Interval cancer incidence | 97.2 |
| Requires reconciliation of endoscopy report and health records | |
|
Indicator of technical competence related, predominantly, to the endoscopist | |
| 18. Number of procedures performed annually | 97.2 |
| A marker for maintenance of competence | |
BOX 7. Indicators of safety compromise in endoscopy.
| Indicator Comment | Consensus, % |
|---|---|
|
Indicators of increased risk of complications | |
| 1. Use of reversal agents | 88.6 |
| An indication of inappropriate sedation practice | |
| 2. Sedation doses in patients older than 70 years | 82.8 |
| Evaluation of sedation use in susceptible patients who have greater risk of comorbidities | |
|
Indicators related to an increased risk of immediate complications | |
| 3. Need for cardiopulmonary resuscitation | 97.2 |
| For any cause – with assessment of causal relationship | |
| 4. Allergic reactions | 80.0 |
| For documented or undocumented allergens | |
| 5. Laryngospasm or bronchospasm | 80.0 |
| For any cause – with assessment of causal relationship | |
| 6. Hypoxia (oxygen saturation <85%) | 88.6 |
| For any cause – with assessment of causal relationship | |
| 7. Hypotension: <90/50 mmHg or fall of ≥20% from baseline | 88.6 |
| For any cause – with assessment of causal relationship | |
| 8. Hypertension: >190/130 mmHg or rise of ≥20% from baseline | 80.0 |
| For any cause – with assessment of causal relationship | |
| 9. Symptomatic metabolic complications | 80.0 |
| Symptomatic hypoglycemia or hyperglycemia; symptomatic disturbance of fluid and/or electrolyte status | |
| 10. Perforation | 100.0 |
| Occurring during or after procedure | |
| 11. Immediate postpolypectomy bleeding | 94.3 |
| This may have been treated successfully during the procedure or it may be persistent and/or requiring transfusion | |
| 12. Severe persistent abdominal pain | 91.4 |
| Requiring further evaluation but not proven as perforation | |
| 13. Impaction of instrument | 94.3 |
| Includes therapeutic accessories, eg, snare or basket | |
| 14. Instrument malfunction | 77.1 |
| Includes endoscope, accessories or ancillary equipment (eg, processor, monitor, lighting, computer, etc) | |
| 15. Admission or transfer to an emergency department | 94.3 |
| Includes transfer from endoscopy unit for any reason other than the underlying gastrointestinal condition | |
|
Indicators related to an increased risk of late complications | |
| 16. Infection | 88.6 |
| Including acute (eg, Clostridium difficile, abscess, endocarditis) and chronic (eg, hepatitis C) infections; presentation may be early or delayed | |
| 17. Gastrointestinal bleeding within 14 days of the procedure | 88.6 |
| Upper or lower gastrointestinal origin: eg, postpolypectomy or postbiopsy | |
| 18. Unplanned hospitalization within 14 days of the procedure | 94.3 |
| For any cause – with assessment of causal relationship | |
| 19. Unplanned contact with health care provider within 14 days of the procedure | 91.4 |
| For any reason – eg, for abdominal pain or infection – with assessment of causal relationship | |
| 20. Death within 30 days | 94.3 |
| For any reason – with assessment of causal relationship and evaluation of mortality attributable to the underlying gastrointestinal condition | |
Quality and safety indicators should be recorded systematically; the American College of Surgeons National Surgical Quality Improvement Program (78) is a potential model for a safety monitoring system, although it is labour intensive and would require modification for use in endoscopy.
Adverse events should be reviewed by the facility’s endoscopy quality review committee to determine the indication for the procedure and decision as to the level of attribution (eg, definitely, probably, possibly or unlikely to be due to the procedure), as recommended for good clinical practice in clinical research (79), the severity of the event and the appropriateness of the procedure. Changes should be implemented as appropriate and the impact of changes made should be reviewed within an acceptable time frame (such as three to six months).
Indicators of safety compromise may be monitored by phone call follow-up, questionnaire mail back or review of hospital admissions. At a minimum, relevant clinical events and near misses should be recorded, systematically, through a safety learning report system to identify risks, reduce the likelihood of complications, improve safety of the service and reassure patients and physicians.
SECTION 4: TRAINING, EDUCATION, COMPETENCY AND PRIVILEGES
Introduction
To provide high-quality endoscopy services, endoscopy staff’s skills must meet predefined standards. This requires that endoscopy facilities provide staff with the opportunities to evaluate, maintain and improve their skills on a regular basis while accommodating the disparate needs of patients, trainees, trainers and the facility.
Statement 14. Endoscopy facilities should provide high-quality education programs or opportunities for all staff.
Evidence grade: Very low
Strength of recommendation: Do it, 86%; possibly do it, 14%
Level of agreement with recommendation: Agree, 100% (agree strongly, 66%; agree moderately, 26%; agree slightly, 9%)
Discussion
Endoscopy facilities should provide continuing training and competency assessment for all staff in all their areas of activities, including clinical policies, patient monitoring, administration of intravenous medications, endoscope handling and maintenance, use of accessories, emergency procedures, decontamination, patient recovery, communication skills and new technologies. The benefits of nurse training, for example, include improved polyp detection rates (39) and nonpharmacological management of pain and anxiety.
This statement does not imply that the facility should pay for all education opportunities or support ‘growth opportunities’ that are not congruent with the facility’s needs. It does, however, imply that all staff members have the opportunity to evaluate and maintain skills relevant to their position.
Statement 15. All endoscopy facility personnel in training should be supervised and their performance monitored regularly until they have achieved competency to perform specified routine and/or emergency procedures according to appropriate current standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 97%; possibly do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 94%; agree moderately, 3%; agree slightly, 3%)
Statement 16. All endoscopy facility personnel engaged, directly or indirectly, in endoscopy service delivery should be trained and certified as having competency to perform specified routine and/ or emergency procedures according to appropriate current standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 97%; possibly do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 91%; agree moderately, 6%; disagree strongly, 3%)
Discussion
Competency is the minimal level of skill, knowledge and expertise derived through training and experience required to perform a task or procedure safely and proficiently, without assistance or supervision (80). Endoscopists must be competent to perform endoscopic procedures safely and to interpret and manage findings correctly. Competency for specific procedures should be assessed by objective measures with full documentation of the number and type of procedures performed. Methods for determining competency should include guidelines on the role of observers, number of cases observed and criteria assessed. Competency assessment should encompass proficiency in common therapeutic interventions, cognitive competency (ie, how to manage the patient overall) and technical competency.
Minimal levels of endoscopic competency should be defined, consistent with the OMGE/OMED guidelines on credentialing and quality assurance in digestive endoscopy (Box 8) (3). The minimum numbers of procedures recommended by many professional associations as thresholds for evaluation of competency, may be markedly lower than numbers required for documentation of proficiency (81,82).
BOX 8. Competencies required by end of training (OMGE/OMED) (3).
Successful completion of a recognized medical or surgical training program
Ability to integrate endoscopy into the clinical management plan (be this medical, surgical or referral for specialty services)
Understanding of indications, contraindications and risks related to procedures
Ability to clearly describe to the patient, in layman’s terms, details of the procedure including attendant risks and, thus, to obtain informed consent
Sound knowledge of endoscopic anatomy
Familiarity with the technical and safety features of the endoscope and accessories and an understanding of proper endoscope reprocessing and infection control
Ability to accurately identify and interpret endoscopic findings
Understanding of pharmacology, administration and risks of sedation/analgesia
Ability to perform procedures competently, including common methods for tissue sampling and therapy
Ability to diagnose and manage complications promptly and competently
Ability to recognize limitations of endoscopic technology and of their own skill in management or therapy of endoscopic findings
Ability to document findings and communicate them with patients and other health care providers
Ability to maintain a record of key performance indicators
Statement 17. Endoscopists should regularly review their endoscopic practice and outcome data with the aim of continuous professional development.
Evidence grade: Low, very low
Strength of recommendation: Do it, 94%; possibly do it, 6%
Level of agreement with recommendation: Agree, 97% (agree strongly, 66%; agree moderately, 29%; agree slightly, 3%; disagree slightly, 3%)
Discussion
Under the auspices of a properly constituted quality assurance committee (see statements 10 and 11) (66–77), endoscopists should review their endoscopy practice data regularly including the number and type of procedures, and standard quality and safety indicator outcomes (eg, completion rates, unplanned events, comfort scores, patient satisfaction). Practice audits (83–86) to facilitate maintenance of competence should be complemented by a formal annual evaluation of endoscopy performance. Any serious unplanned event or series of events should be reviewed by the endoscopist and quality assurance committee with documentation of deficiencies, discrepancies and any consequent actions. Audits can detect poor performance/inadequate numbers and can improve outcomes such as colonoscopy completion rate (52,87–89).
Statement 10. Endoscopy facilities should maintain a comprehensive quality improvement program incorporating formal, regular, scheduled review of performance reports.
Evidence grade: Low/very low
Strength of recommendation: Do it, 85%; possibly do it, 9%; possibly don’t do it, 3%; don’t do it, 3%
Level of agreement with recommendation: Agree, 94% (agree strongly, 76%; agree moderately, 12%; agree slightly, 6%; disagree slightly, 6%)
Statement 11. Endoscopy facilities should appoint a review committee to monitor and report back to management on adherence to and implementation of quality standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 79%; possibly do it, 21%
Level of agreement with recommendation: Agree, 97% (agree strongly, 71%; agree moderately, 24%; agree slightly, 3%; disagree strongly, 3%)
Unverified self-reporting is not encouraged as a formal performance indicator, although this does not preclude self-reflection based on structured practice audits directed at personal learning programs. Verifiable databases, generated from electronic reporting systems or web-based practice audits, have greater validity for documenting, but these do require that endoscopy facilities have tools available to monitor outcomes.
Statement 18. Endoscopists should be granted privileges to perform specified procedures based on a formal evaluation of their competence consistent with appropriate current standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 100%
Level of agreement with recommendation: Agree, 100% (agree strongly, 91%; agree moderately, 9%)
Statement 19. Endoscopists’ privileges should be subject to formal, regular, scheduled review to ensure that renewal is based on documented competence to perform specified procedures consistent with appropriate current standards.
Evidence grade: Low/very low
Strength of recommendation: Do it, 94%; possibly do it, 3%; possibly don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 89%; agree moderately, 9%; disagree slightly, 3%)
Discussion
Health care institutions are legally responsible for ensuring that individuals who perform procedures are competent (3). Endoscopy privileges are granted or renewed by health care institutions, including hospitals and out-of-hospital endoscopy facilities, to competent individuals based on regional and national guidelines and regulations.
Policies guiding the granting of endoscopic privileges should be applied uniformly for all endoscopists; privileges should be granted separately for each endoscopic procedure, based on competence determined according to current standards (5–8). Each institution should specify appropriate standards, monitor adherence to standards and update standards, as needed.
When applying for privileges, the applicant and training program director must provide details of training undertaken for each endoscopic procedure requested. Each application should be reviewed by a clinician who is knowledgeable about the relevant procedure; procedural competence should be documented from direct observation of the applicant’s performance at the privileging institution or by the training program director.
Endoscopic privileges should be granted for a finite period to permit regular re-evaluation of the applicant’s performance and competency; procedural volumes should also be evaluated because, for example, colonoscopic complications have been associated with procedures performed by lower-volume endoscopists (90). Renewal of privileges should be based on defined policies for addressing poor performance.
SECTION 5: ENDOSCOPY REPORTING STANDARDS
Introduction
A completed, comprehensive endoscopy report is an essential element of a quality endoscopy service. Traditional, narrative reporting is associated with marked variations in the documentation of positive findings, pertinent negative findings and other procedural details. Effective communication of procedural findings and successful practice audit and quality improvement processes are virtually impossible in the absence of a standardized, ideally electronic, endoscopy report format.
Statement 20. Endoscopic procedures should be reported in a standardized electronic format, including mandatory reporting fields, to provide full documentation of all necessary clinical and quality measures.
Evidence grade: Low/very low
Strength of recommendation: Do it, 82%; possibly do it, 15%; don’t do it, 3%
Level of agreement with recommendation: Agree, 97% (agree strongly, 76%; agree moderately, 15%; agree slightly, 6%; disagree strongly, 3%)
Discussion
Deficits in endoscopy reporting include marked variability in the definitions of inflammation in ulcerative colitis (91), as well as marked differences in the completion of different report elements such as lesion identification and removal (84% of reports), sedation procedure (75%), demographic data (69%), procedure interpretation (58%), patient history (57%) and procedure quality (40%) (92).
Electronic reporting offers a data collection method that can be more complete and cost-effective – but no more time-consuming – than handwritten reports or free-text dictated reports (93). Although electronic reporting is initially more expensive than handwritten or dictated reporting (94), the overall costs become comparable after five years, and cost-benefit outcomes are better after three years, in part, because automatic electronic transmission of reports reduces costs, administrative workload and communication delays.
Standardization of electronic reports, including mandatory reporting elements (Box 9) and accepted grading systems (Box 10), permits standardization of the data capture and long-term storage (at least 10 years) needed for audit and quality improvement processes. While electronic data (eg, digital transcripts of dictated reports) are preferable to handwritten reports, they are inferior to a full, structured electronic report.
BOX 9. Required endoscopy report elements.
| Report field Comment | Consensus, % |
|---|---|
| 1. Type of procedure | 100.0 |
| Esophagastoduodenoscopy, colonoscopy, etc | |
| 2. Date and time of procedure | 100.0 |
| 3. Name of endoscopist | 100.0 |
| Including trainee and supervisor | |
| 4. Name(s) of assistant(s) | 91.4 |
| Endoscopy nurse, respiratory technician, etc | |
| 5. Age and sex of patient | 100.0 |
| 6. Indication(s) for procedure | 100.0 |
| Consistent with guidelines for appropriate indications | |
| 7. Comorbidities | 91.4 |
| Assessed using American Society of Anesthesiologists physical status classification system (95), Mallampati score (96), etc | |
| 8. Type of bowel preparation | 91.4 |
| Including timing and adherence to prescribed regimen | |
| 9. Type and dose of sedation used | 100.0 |
| Including incremental dose adjustment | |
| 10. Other medication and related information | 97.2 |
| Administration route, reversal agents, antispasmodics, allergies, etc | |
| 11. Extent and completeness of examination | 100.0 |
| Confirmed by independent observer and/or photodocumentation; withdrawal time (colonoscopy) and retroflexion manoeuvres | |
| 12. Quality of bowel preparation | 97.2 |
| Assessed formally, using a validated tool or standard scale (59,60) | |
| 13. Relevant findings | 97.2 |
| Using relevant, standardized descriptions and validated scales | |
| 14. Pertinent negatives | 97.2 |
| Using relevant, standardized descriptions and validated scales | |
| 15. Adverse events and resulting interventions | 100.0 |
| Using relevant, standardized descriptions and validated scales | |
| 16. Patient comfort | 100.0 |
| Using formal descriptors and, if possible, a validated scale | |
| 17. Diagnoses | 100.0 |
| Using standard terminology and validated scales | |
| 18. Endoscopic interventions performed | 100.0 |
| Using standard terminology and descriptors | |
| 19. Details of pathology specimens | 100.0 |
| Number and location of biopsies; number, size and location of polyps | |
| 20. Details of follow-up arrangements | 97.2 |
| Identify person responsible for booking further tests and follow-up | |
| 21. Appended pathology report(s), when available | 94.3 |
| Requires reconciliation of endoscopy and pathology reports | |
| 22. Management recommendations | 100.0 |
| Including medication, tests and follow-up | |
| 23. Information provided to patient and/or family | 91.4 |
| Description of findings; contact details in the event of an emergency |
BOX 10. Grading systems appropriate for electronic reporting forms.
Patient status: American Society of Anesthesiologists physical status classification system (95), Mallampati score (96)
Bowel preparation: Boston Bowel Preparation scale (60) or Ottawa Bowel Preparation scale (59)
Reflux esophagitis severity: Los Angeles classification (97,98)
Barrett’s esophagus diagnosis and extent: Prague C & M criteria (99)
Crohn’s disease – SES-CD activity score (100)
Bleeding lesions – Forrest classification (103)
Statement 21. Endoscopy facilities should implement policies to monitor and ensure the timeliness and completeness of procedure reporting.
Evidence grade: Low/very low
Strength of recommendation: Do it, 100%
Level of agreement with recommendation: Agree, 100% (agree strongly, 91%; agree moderately, 6%; agree slightly, 3%)
Discussion
An important aspect of clinicians’ competency relates to the timely provision of a completed procedure report. Receipt of a procedure report improves patients’ adherence to follow-up appointments and therapies (66). Optimally, the endoscopy report should be available on the day of the endoscopy, either as a modified, patient-centred version or as the formal report, supplemented by a patient-centred summary. When relevant, pathology results should accompany the final endoscopy report but this can be a technical challenge because it requires linkage of separate databases. Patient concerns regarding their test results should be addressed in a timely manner although the nature and timeliness of the response may, legitimately, vary among institutions, depending on local needs and resources.
SECTION 6: PATIENT PERCEPTIONS
Introduction
Patient-centred care is predicated on a satisfactory patient experience in endoscopy, as in all other areas of health care delivery; thus, assessments of endoscopy quality must include domains that are important to patients.
Statement 22. Endoscopy facilities should ensure that the services they provide are patient-centred.
Evidence grade: Moderate to very low
Strength of recommendation: Do it, 85%; possibly do it, 12%; don’t do it, 3%
Level of agreement with recommendation: Agree, 100% (agree strongly, 71%; agree moderately, 26%; agree slightly, 3%)
Discussion
Acknowledgement of the patient’s perspective on all aspects of endoscopy service delivery and responsiveness to their concerns necessitates a collaborative relationship between the clinician and the patient to address the quality of the patient’s experience and the appropriateness, accuracy and safety of the procedure. Patients’ perspectives on quality endoscopy care were reviewed at the consensus conference based on the results of focus groups held in French and English Canada for adults who had undergone or were scheduled to undergo a colonoscopy. Focus group participants’ discussions of the total colonoscopy experience, defined as all factors and events occurring before, during and after the day of the procedure, yielded several major quality themes, including communication: quantity and quality; comfort: physical and psychological; and attitude and demeanor: physician and endoscopy unit staff. Endoscopist expertise, procedural safety and physical amenities were also important. Many patients expressed little concern about quality indicators (106–108), reporting that they trusted the accuracy and safety of the test on the presumption that tests were closely monitored and regulated to ensure adherence to standards.
Preprocedural educational interventions, such as provision of additional information, as an educational video (16,109), booklet (110), face-to-face discussion (111) or behavioural intervention (112) can support patients’ need to understand the procedure and improve their satisfaction and appointment keeping. Interventions may have a limited effect, due to high preprocedural satisfaction levels (113), but they can, nonetheless, improve patients’ knowledge about the procedure.
In theory, patients should be more likely to comply with follow-up examinations if they experienced high levels of satisfaction with their previous endoscopic procedure; to date however, this hypothesis remains unproven.
Statement 23. Endoscopy facilities should systematically and at least annually solicit patient feedback, report the results to the service and to the institution’s quality committee, and implement effective measures to address patients’ concerns.
Evidence grade: Very low
Strength of recommendation: Do it, 94%; possibly do it, 6%
Level of agreement with recommendation: Agree, 100% (agree strongly, 82%; agree moderately, 15%; agree slightly, 3%)
Discussion
The increasing and appropriate emphasis on patient-centred care requires that patients provide feedback on their experience to inform endoscopy facilities’ decisions on balancing cost and volumes, access and wait times, discomfort and completion rates, and resources and procedural times. Patient feedback should be sought, at least annually (10), using one or more methods including satisfaction surveys, focus groups and invited comments. Patient feedback is essential for assessing many aspects of endoscopy service delivery, such as the quality of the informed consent process (114), and offers measurable improvements in patient experience (115). Regular, structured measurement of patient satisfaction ensures that patients’ perspectives are evaluated alongside more traditional indicators such as appropriateness, accuracy and safety.
CONCLUSIONS
Gastrointestinal endoscopy is a complex diagnostic and therapeutic undertaking that demands a high level of skill and knowledge on the part of the operator (2,5). However, high-quality endoscopy requires more than a skilled operator – the delivery of high-quality endoscopy services, in a cost-effective manner consistent with the broader needs of a health care system, requires a formal quality improvement framework that addresses all aspects of endoscopy service delivery from the patient’s initial contact with a health care provider (eg, the identification of family history of colon cancer in an asymptomatic individual) through to documentation of long-term outcomes (eg, freedom from colon cancer over decades). Recognition of the patient as the focus of the endoscopy process provides a structure for integrating the efforts of the many diverse disciplines whose contribution is needed to ensure a high-quality service.
The fundamental principle underlying high-quality health care is the need for an iterative feedback loop centred on the patient’s needs. The feedback loop requires identification of the patient’s needs, measurement of the extent to which these needs are met, intervention to ensure that unmet needs are addressed and reassessment of the patient’s needs after intervention. The GRS (10) is a quality improvement tool that enables the adoption of iterative quality improvement processes in endoscopy, supported by the identification of quality and safety indicators relevant to specific aspects of service delivery.
Whenever possible, health care recommendations are based on the highest quality evidence available. Outcomes of this consensus conference confirm that there is a paucity of high-grade evidence relevant to endoscopy service delivery. However, among a large multidisciplinary group of health care professionals, there was a high level of agreement on key features that should be addressed to improve endoscopy quality. The fact that the supporting evidence was generally graded as ‘low’ or ‘very low’ does not indicate that the recommendations, themselves, are weak; it indicates, rather, the need for concerted, widespread efforts to document the short- and long-term effects of adopting new quality improvement processes in endoscopy.
The consensus process, presented in the current report, provides the framework for a quality improvement structure in endoscopy based on explicit recommendations that will support systematic monitoring, assessment and modifications in endoscopy service delivery; this framework is intended to yield benefits for all patients whose care may be affected by the practice of gastrointestinal endoscopy.
Acknowledgments
The authors thank Sandra Daniels, Palma Colacino and Paul Sinclair from the CAG for organizing the meeting, Dr Catherine Hill and Dr Anja Becher from Oxford PharmaGenesis Ltd™ for writing assistance, and Dr Khurram Khan for his work in grading the evidence.
APPENDIX.
List of attendees
Voting chair: David Armstrong, Hamilton, Ontario.
Nonvoting moderator: Jon Meddings, Calgary, Alberta.
Voting steering committee: Alan Barkun, Montreal, Quebec; Ron Bridges, Calgary; Rose Carter, Edmonton, Alberta; Chris de Gara, Edmonton; Catherine Dubé, Calgary; Robert Enns, Vancouver, British Columbia; Roger Hollingworth, Mississauga, Ontario; Don MacIntosh, Halifax, Nova Scotia.
Voting participants: Doug Bair, Oakville, Ontario; Simon Bergman, Montreal; Mark Borgaonkar, St John’s, Newfoundland and Labrador; Peter Cotton, Charleston, South Carolina, USA; Sylviane Forget, Montreal; Alan Forster, Ottawa, Ontario; George Ghattas, Montreal; Michael Gould, Etobicoke, Ontario; Pierre Hallé, Quebec City, Quebec; Robert Hilsden, Calgary; Lawrence Hookey, Kingston, Ontario; Betty Kennah, Hamilton; Ernst Kuipers, Rotterdam, The Netherlands; Eoin Lalor, Edmonton; Tammy MacDonald, Halifax; Gary May, Toronto, Ontario; Tony Nestel, Bridgewater, Nova Scotia; Neely Panton, Vancouver; Victor Plourde, Montreal; Craig Render, Kelowna, British Columbia; Dan Sadowski, Edmonton; Harminder Singh, Winnipeg, Manitoba; Jennifer Telford, Vancouver; Jill Tinmouth, Toronto; Frances Tse, Hamilton; Roland Valori, London, United Kingdom.
Advisors: Bret Peterson, Rochester, Minnesota, USA; Linda Rabeneck, Toronto, Ontario.
Nonvoting observers: Bernard Badley, Colorectal Cancer Prevention Program, Cancer Care Nova Scotia, Nova Scotia; Judy Budgell, Department of Health and Community Services, Government of Newfoundland and Labrador, Newfoundland and Labrador; Ford Bursey, Memorial University, St John’s, Newfoundland and Labrador; Tanya Chawla, Colonoscopy Program at the Joint Department of Medical Imaging, Mount Sinai Hospital, Toronto; Surinder Dhaliwal, McMaster University Medical Centre, Hamilton; Dan Faulkner, The College of Physicians and Surgeons of Ontario, Toronto; Susan Fekete, Canadian Partnership Against Cancer, Ontario; Wade Hillier, The College of Physicians and Surgeons of Ontario, Toronto; Nasir Jaffer, Department of Medical Imaging, Mount Sinai Hospital, Toronto; Margaret Keresteci, Canadian Partnership Against Cancer, Ontario; Jeff Kolbasnik, Ontario Association of General Surgeons, Ontario; Grigorios Leontiadis, McMaster University Medical Centre, Hamilton; Marnie MacKinnon, Cancer Care Ontario, Toronto; Diane Major, Institut National de Santé Publique du Québec, Quebec; Robin Reece, The College of Physicians and Surgeons of Ontario, Toronto; Janice Sanger, Department of Health and Community Services, Government of Newfoundland and Labrador, Newfoundland and Labrador; Giles Stevenson, McMaster University, Hamilton; Ross Stimpson, Manitoba CRC Screening Program Cancer Care, Manitoba.
Working groups
Quality indicators: Robert Enns (Lead), Craig Render, Harminder Singh, Jennifer Telford.
Safety indicators: Mark Borgaonkar (Lead), Roger Hollingworth, Lawrence Hookey, Ernst Kuipers.
Endoscopic reporting: Alan Barkun (Lead), Pierre Hallé, Jill Tinmouth.
Ethics: Sylviane Forget (Lead), David Armstrong, Rose Carter, Nanda Gopinath, Cliff Ottaway.
Training and credentials/privileges: Ron Bridges (Lead), Chris de Gara, Gary May, Frances Tse, Peter Cotton (expert resource).
Monitoring and quality assurance: Ron Bridges (Lead), Rose Carter, Neely Panton, Dan Sadowski, Roland Valori (expert resource).
GRS: Don MacIntosh (Lead), Catherine Dubé, Roger Hollingworth, Georges Ghattas, Sander van Zanten, Roland Valori (expert resource).
The patient’s perspective: Catherine Dubé (Lead), David Armstrong, Alan Barkun, Surinder Dhaliwal, Robert Hilsden, Maida Sewitch.
CONFLICT OF INTEREST/STUDY SUPPORT
Guarantor of the article: David Armstrong.
Specific author contributions: All authors played a role in providing the content for the manuscript (see ‘working groups’, above). All authors approved the final draft of the manuscript.
Financial support: The meeting was supported by Canadian Partnership Against Cancer/Partenariat Canadien Contre le Cancer and Canadian Institutes of Health Research/Instituts de Recherche en Santé du Canada: Institute of Nutrition, Metabolism, and Diabetes. Honoraria for participation were provided to the international experts and for participants who were not members of the CAG. Travel and accommodation was provided for all participants.
Footnotes
POTENTIAL COMPETING INTERESTS: No industry or government relationships to report: D Armstrong, M Borgaonkar, R Bridges, R Carter, P Cotton, C de Gara, A Forster, P Hallé, R Hilsden, R Hollingworth, B Kennah, E Kuipers, E Lalor, D MacIntosh, J Meddings, T MacDonald, T Nestel, B Petersen, V Ploude, L Rabeneck, C Render, D Sadowski, H Singh, J Telford, J Tinmouth, R Valori. Advisory board: Olympus (R Enns), Merck (S Forget). Consultation fees: Ethicon Endosurgical (N Panton), Johnson & Johnson (M Gould), Takeda (A Barkun), Oakville Endoscopy Centre – partner and active clinical practice/OHIP billings (D Bair).
RESEARCH GRANTS/CLINICAL TRIAL FUNDING: Boston Scientific (A Barkun), Olympus (C Dubé), UCB – Data Safety Monitoring Board (S Forget). Speakers’ bureau: Abbott (S Forget, M Gould), AstraZeneca (A Barkun), Nestlé (S Forget), Olympus (A Barkun).
REFERENCES
- 1.Valori R, Barton R. BSG quality and safety indicators for endoscopy. JAG: Joint Advisory Group on GI Endoscopy. 2007. pp. 1–13.
- 2.Faigel DO, Pike IM, Baron TH, et al. Quality indicators for gastrointestinal endoscopic procedures: An introduction. Gastrointest Endosc. 2006;63:S3–9. doi: 10.1016/j.gie.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Faigel DO, Cotton PB. The London OMED position statement for credentialing and quality assurance in digestive endoscopy. Endoscopy. 2009;41:1069–74. doi: 10.1055/s-0029-1215279. [DOI] [PubMed] [Google Scholar]
- 4.Eisen GM, Baron TH, Dominitz JA, et al. Methods of granting hospital privileges to perform gastrointestinal endoscopy. Gastrointest Endosc. 2002;55:780–3. doi: 10.1016/s0016-5107(02)70403-3. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D, Enns R, Ponich T, Romagnuolo J, Springer J, Barkun AN. Canadian credentialing guidelines for endoscopic privileges: An overview. Can J Gastroenterol. 2007;21:797–801. doi: 10.1155/2007/414967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer J, Enns R, Romagnuolo J, Ponich T, Barkun AN, Armstrong D. Canadian credentialing guidelines for endoscopic retrograde cholangiopancreatography. Can J Gastroenterol. 2008;22:547–51. doi: 10.1155/2008/582787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romagnuolo J, Enns R, Ponich T, Springer J, Armstrong D, Barkun AN. Canadian credentialing guidelines for colonoscopy. Can J Gastroenterol. 2008;22:17–22. doi: 10.1155/2008/837347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enns R, Romagnuolo J, Ponich T, Springer J, Armstrong D, Barkun AN. Canadian credentialing guidelines for flexible sigmoidoscopy. Can J Gastroenterol. 2008;22:115–9. doi: 10.1155/2008/874796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer . 1st edn. Luxembourg: World Health Organization; 2010. European guidelines for quality assurance in colorectal cancer screening and diagnosis. [Google Scholar]
- 10.Global Rating Scale. 2008. < http://www.grs.nhs.uk/> (Accessed on August 25, 2011).
- 11.The AGREE collaboration Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12:18–23. doi: 10.1136/qhc.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. 2008.
- 14.Lu Y, Jones D, Barkun A, Enns R, Sinclair P, Martel M. Is it ethical to silence expertise in the development of clinical practice guidelines? Report and analysis from an international consensus conference. Can J Gastroenterol. 2011;25(Suppl A):A167. [Google Scholar]
- 15.Agre P, Kurtz RC, Krauss BJ. A randomized trial using videotape to present consent information for colonoscopy. Gastrointest Endosc. 1994;40:271–6. doi: 10.1016/s0016-5107(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 16.Luck A, Pearson S, Maddern G, Hewett P. Effects of video information on precolonoscopy anxiety and knowledge: A randomised trial. Lancet. 1999;354:2032–5. doi: 10.1016/s0140-6736(98)10495-6. [DOI] [PubMed] [Google Scholar]
- 17.Jabbar A, Wright R. Effects of video information on pre-colonoscopy anxiety and knowledge: A randomized trial. Gastrointest Endosc. 2001;53:140–2. [PubMed] [Google Scholar]
- 18.Shaw MJ, Beebe TJ, Tomshine PA, Adlis SA, Cass OW. A randomized, controlled trial of interactive, multimedia software for patient colonoscopy education. J Clin Gastroenterol. 2001;32:142–7. doi: 10.1097/00004836-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Huntley JS, Shields DA, Stallworthy NK. Consent obtained by the Junior House Officer – is it informed? J Royal Soc Med. 1998;91:528–30. doi: 10.1177/014107689809101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segarajasingam DS, Pawlik J, Forbes GM. Informed consent in direct access colonoscopy. J Gastroenterol Hepatol. 2007;22:2081–5. doi: 10.1111/j.1440-1746.2007.04461.x. [DOI] [PubMed] [Google Scholar]
- 21.Dafnis G, Ekbom A, Pahlman L, Blomqvist P. Complications of diagnostic and therapeutic colonoscopy within a defined population in Sweden. Gastrointest Endosc. 2001;54:302–9. doi: 10.1067/mge.2001.117545. [DOI] [PubMed] [Google Scholar]
- 22.Tran DQ, Rosen L, Kim R, Riether RD, Stasik JJ. Actual colonoscopy: What are the risks of perforation? Am Surg. 2001;67:845–8. [PubMed] [Google Scholar]
- 23.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 24.Bilotta JJ, Floyd JL, Waye JD. Arterial oxygen desaturation during ambulatory colonoscopy: Predictability, incidence, and clinical insignificance. Gastrointest Endosc. 1990;36(3 Suppl):S52–8. [PubMed] [Google Scholar]
- 25.Eckardt AJ, Swales C, Bhattacharya K, et al. Open access colonoscopy in the training setting: Which factors affect patient satisfaction and pain? Endoscopy. 2008;40:98–105. doi: 10.1055/s-2007-995469. [DOI] [PubMed] [Google Scholar]
- 26.Elfant AB, Korn C, Mendez L, Pello MJ, Peikin SR. Recall of informed consent after endoscopic procedures. Dis Colon Rectum. 1995;38:1–3. doi: 10.1007/BF02053848. [DOI] [PubMed] [Google Scholar]
- 27.Kimmery MB, Burnett DA, Carr-Locke DL, et al. Transmission of infection by gastrointestinal endoscopy. Gastrointest Endosc. 1993;39:885–8. [Google Scholar]
- 28.US Food and Drug Administration . 2009. Preventing cross-contamination in endoscope processing: safety communication from FDA, CDC, and the VA. < www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm190273.htm> (Accessed on October 2, 2011). [Google Scholar]
- 29.American Society for Gastrointestinal Endoscopy Appropriate use of gastrointestinal endoscopy: A consensus statement from the American Society for Gastrointestinal Endoscopy. Gastrointest Endosc. 2000;52:831–7. [PubMed] [Google Scholar]
- 30.Vader J-P, Froehlich F, Dubois RW, et al. European Panel on the Appropriateness of Gastrointestinal Endoscopy (EPAGE): Conclusion and WWW Site. Endoscopy. 1999;31:687–694. doi: 10.1055/s-1999-72. [DOI] [PubMed] [Google Scholar]
- 31.Froehlich F, Repond C, Mullhaupt B, et al. Is the diagnostic yield of upper GI endoscopy improved by the use of explicit panel-based appropriateness criteria? Gastrointest Endosc. 2000;52:333–41. doi: 10.1067/mge.2000.107906. [DOI] [PubMed] [Google Scholar]
- 32.Morini S, Hassan C, Meucci G, Toldi A, Zullo A, Minoli G. Diagnostic yield of open access colonoscopy according to appropriateness. Gastrointest Endosc. 2001;54:175–9. doi: 10.1067/mge.2001.116565. [DOI] [PubMed] [Google Scholar]
- 33.Quine MA, Bell GD, McCloy RF, Devlin HB, Hopkins A. Appropriate use of upper gastrointestinal endoscopy – a prospective audit. Steering Group of the Upper Gastrointestinal Endoscopy Audit Committee. Gut. 1994;35:1209–14. doi: 10.1136/gut.35.9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John BJ, Irukulla S, Pilgrim G, Swift I, Abulafi AM. Surveillance colonoscopies for colorectal polyps – too often, too many! An audit at a large district general hospital. Colorectal Dis. 2008;10:898–900. doi: 10.1111/j.1463-1318.2008.01516.x. [DOI] [PubMed] [Google Scholar]
- 35.Pickard M, Dewar EP, Kapadia RC, Khan RB, Hutchinson IF, Nejim A. Follow up of patients with colorectal polyps: Are the BSG guidelines being adhered to? Colorectal Dis. 2007;9:203–6. doi: 10.1111/j.1463-1318.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 36.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–71. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 37.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: Results of a national survey. J Clin Gastroenterol. 2009;43:554–8. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 38.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–13. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 39.Dellon ES, Lippmann QK, Sandler RS, Shaheen NJ. Gastrointestinal endoscopy nurse experience and polyp detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1342–7. doi: 10.1016/j.cgh.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harewood GC, Chrysostomou K, Himy N, Leong WL. A “time-and-motion” study of endoscopic practice: Strategies to enhance efficiency. Gastrointest Endosc. 2008;68:1043–50. doi: 10.1016/j.gie.2008.03.1116. [DOI] [PubMed] [Google Scholar]
- 41.Buchan J, Dal Poz MR. Skill mix in the health care workforce: Reviewing the evidence. Bull World Health Organ. 2002;80:575–80. [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–54. doi: 10.1161/CIRCULATIONAHA.106.183095. [DOI] [PubMed] [Google Scholar]
- 43.Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58:869–80. doi: 10.1136/gut.2007.136580. [DOI] [PubMed] [Google Scholar]
- 44.Anderson MA, Ben-Menachem T, Gan SI, et al. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060–70. doi: 10.1016/j.gie.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 45.Joshi GP, Chung F, Vann MA, et al. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111:1378–87. doi: 10.1213/ANE.0b013e3181f9c288. [DOI] [PubMed] [Google Scholar]
- 46.Vann MA. Perioperative management of ambulatory surgical patients with diabetes mellitus. Curr Opin Anaesthesiol. 2009;22:718–24. doi: 10.1097/ACO.0b013e3283310f51. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenstein DR, Jagannath S, Baron TH, et al. Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815–26. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 48.Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–9. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 49.Smith L, Rembacken BJ. What are the characteristics of a good colonoscopist? Endoscopy. 2007;39(Suppl 1):A369. [Google Scholar]
- 50.Rex DK. Still photography versus videotaping for documentation of cecal intubation: A prospective study. Gastrointest Endosc. 2000;51:451–9. doi: 10.1016/s0016-5107(00)70447-0. [DOI] [PubMed] [Google Scholar]
- 51.Thomas-Gibson S. The caecum or not the caecum? Eur J Gastroenterol Hepatol. 2008;20:500–2. doi: 10.1097/MEG.0b013e3282f519a2. [DOI] [PubMed] [Google Scholar]
- 52.Ball JE, Osbourne J, Jowett S, Pellen M, Welfare MR. Quality improvement programme to achieve acceptable colonoscopy completion rates: Prospective before and after study. BMJ. 2004;329:665–7. doi: 10.1136/bmj.329.7467.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rabeneck L, Rumble RB, Axler J, et al. Cancer Care Ontario Colonoscopy Standards: standards and evidentiary base. Can J Gastroenterol. 2007;21(Suppl D):5D–24D. [PMC free article] [PubMed] [Google Scholar]
- 54.Denis B, Weiss A-M, Peter A, Bottlaender J, Chiappa P. Quality assurance and gastrointestinal endoscopy: An audit of 500 colonoscopic procedures. Gastroenterol Clin Biol. 2004;28:1245–55. doi: 10.1016/s0399-8320(04)95218-9. [DOI] [PubMed] [Google Scholar]
- 55.Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: The key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122–30. doi: 10.1111/j.1572-0241.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 56.Pitetti R, Davis PJ, Redlinger R, White J, Wiener E, Calhoun KH. Effect on hospital-wide sedation practices after implementation of the 2001 JCAHO procedural sedation and analgesia guidelines. Arch Pediatr Adolesc Med. 2006;160:211–6. doi: 10.1001/archpedi.160.2.211. [DOI] [PubMed] [Google Scholar]
- 57.Conigliaro R, Rossi A. Implementation of sedation guidelines in clinical practice in Italy: Results of a prospective longitudinal multicenter study. Endoscopy. 2006;38:1137–43. doi: 10.1055/s-2006-944842. [DOI] [PubMed] [Google Scholar]
- 58.Kim WH, Cho YJ, Park JY, Min PK, Kang JK, Park IS. Factors affecting insertion time and patient discomfort during colonoscopy. Gastrointest Endosc. 2000;52:600–5. doi: 10.1067/mge.2000.109802. [DOI] [PubMed] [Google Scholar]
- 59.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–6. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 60.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–5. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 62.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–8. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Lugay M, Otto G, Kong M, Mason DJ, Wilets I. Recovery time and safe discharge of endoscopy patients after conscious sedation. Gastroenterol Nurs. 1996;19:194–200. doi: 10.1097/00001610-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 65.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 66.Spodik M, Goldman J, Merli K, Walker C, Alpini B, Kastenberg D. Providing an endoscopy report to patients after a procedure: A low-cost intervention with high returns. Gastrointest Endosc. 2008;67:103–11. doi: 10.1016/j.gie.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 67.Alberta. Evidence Act, RSA, 2000, c.A-18 s.19.
- 68.British Columbia. Evidence Act, RSBC, 1996, c.124 s.51.
- 69.Manitoba. Evidence Act, RSM. 1987, c.E150 ss.9&10.
- 70.New Brunswick. Evidence Act, RSNB, 1973, c.E-11 s.43.
- 71.Newfoundland and Labrador. Evidence Act, RSNL, 1990, c.E-16, s.8.1.
- 72.Northwest Territories Evidence Act, RSNWT, 1988, c.E-18, ss.13&14.
- 73.Nova Scotia . Evidence Act, RSNS, 1989, c.154 s.60. [Google Scholar]
- 74.Nunavut. Evidence Act, RSNWT, 1988, c.E-8 s.14.
- 75.Ontario. Quality of Care Information Protection Act, SO, 2004, c.3 Schedule B, ss. 1&5.
- 76.Quebec. An Act Respecting Health Services and Social Services, RSQ, c.S-4.2 ss.183.1, 183.2 and 183.3.
- 77.Yukon. Evidence Act, RSY, 2002, c.78 s.13.
- 78.American College of Surgeons ACS National Surgical Quality Improvement Program. 2006 [Google Scholar]
- 79.ICH Harmonised tripartite guideline for good clinical practice. Federal Register; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use; May 9, 1997; pp. 25961–709. [PubMed] [Google Scholar]
- 80.Faigel DO. Quality, competency and endosonography. Endoscopy. 2006;38(Suppl 1):S65–9. doi: 10.1055/s-2006-946657. [DOI] [PubMed] [Google Scholar]
- 81.Spier BJ, Durkin ET, Walker AJ, Foley E, Gaumnitz EA, Pfau PR. Surgical resident’s training in colonoscopy: Numbers, competency, and perceptions. Surg Endosc. 2010;24:2556–61. doi: 10.1007/s00464-010-1002-5. [DOI] [PubMed] [Google Scholar]
- 82.Spier BJ, Benson M, Pfau PR, Nelligan G, Lucey MR, Gaumnitz EA. Colonoscopy training in gastroenterology fellowships: Determining competence. Gastrointest Endosc. 2010;71:319–24. doi: 10.1016/j.gie.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 83.Armstrong D, Barkun AN, Chen Y, et al. Access to specialist gastroenterology care in Canada: The Practice Audit in Gastroenterology (PAGE) Wait Times Program. Can J Gastroenterol. 2008;22:155–60. doi: 10.1155/2008/292948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Armstrong D, Hollingworth R, Gardiner T, et al. Practice Audit in Gastroenterology (PAGE) program: A novel approach to continuing professional development. Can J Gastroenterol. 2006;20:405–10. doi: 10.1155/2006/685960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Armstrong D, Hollingworth R, Macintosh D, et al. Point-of-care, peer-comparator colonoscopy practice audit: The Canadian Association of Gastroenterology Quality Program – Endoscopy. Can J Gastroenterol. 2011;25:13–20. doi: 10.1155/2011/320904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leddin D, Armstrong D, Barkun AN, et al. Access to specialist gastroenterology care in Canada: Comparison of wait times and consensus targets. Can J Gastroenterol. 2008;22:161–7. doi: 10.1155/2008/479684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grassini M, Verna C, Battaglia E, Niola P, Navino M, Bassotti G. Education improves colonoscopy appropriateness. Gastrointest Endosc. 2008;67:88–93. doi: 10.1016/j.gie.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 88.Coriat R, Pommaret E, Chryssostalis A, et al. Quality control of colonoscopy procedures: A prospective validated method for the evaluation of professional practices applicable to all endoscopic units. Gastroenterol Clin Biol. 2009;33:103–8. doi: 10.1016/j.gcb.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 89.Taylor KM, Arajs K, Rouse T, Harris AW. Prospective audit of colonoscopy quality in Kent and Medway, UK. Endoscopy. 2008;40:291–5. doi: 10.1055/s-2007-995528. [DOI] [PubMed] [Google Scholar]
- 90.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 91.de Lange T, Moum BA, Tholfsen JK, Larsen S, Aabakken L. Standardization and quality of endoscopy text reports in ulcerative colitis. Endoscopy. 2003;35:835–40. doi: 10.1055/s-2003-42619. [DOI] [PubMed] [Google Scholar]
- 92.Robertson DJ, Lawrence LB, Shaheen NJ, et al. Quality of colonoscopy reporting: a process of care study. Am J Gastroenterol. 2002;97:2651–6. doi: 10.1111/j.1572-0241.2002.06044.x. [DOI] [PubMed] [Google Scholar]
- 93.Soekhoe JK, Groenen MJ, van Ginneken AM, et al. Computerized endoscopic reporting is no more time-consuming than reporting with conventional methods. Eur J Intern Med. 2007;18:321–5. doi: 10.1016/j.ejim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 94.Groenen MJ, Ajodhia S, Wynstra JY, et al. A cost-benefit analysis of endoscopy reporting methods: Handwritten, dictated and computerized. Endoscopy. 2009;41:603–9. doi: 10.1055/s-0029-1214852. [DOI] [PubMed] [Google Scholar]
- 95.American Society of Anesthesiologists ASA Physical Status Classification System. < www.asahq.org/clinical/physicalstatus.htm> (Accessed on October 2, 2011).
- 96.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32:429–34. doi: 10.1007/BF03011357. [DOI] [PubMed] [Google Scholar]
- 97.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172–80. doi: 10.1136/gut.45.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: A progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 99.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology. 2006;131:1392–9. doi: 10.1053/j.gastro.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 100.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest Endosc. 2004;60:505–12. doi: 10.1016/s0016-5107(04)01878-4. [DOI] [PubMed] [Google Scholar]
- 101.D’Haens G, Sandborn WJ, Feagan BG, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. 2007;132:763–86. doi: 10.1053/j.gastro.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 102.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–9. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 103.Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394–7. doi: 10.1016/s0140-6736(74)91770-x. [DOI] [PubMed] [Google Scholar]
- 104.The Italian Liver Cirrhosis Project Reliability of endoscopy in the assessment of variceal features. J Hepatol. 1987;4:93–8. doi: 10.1016/s0168-8278(87)80015-6. [DOI] [PubMed] [Google Scholar]
- 105.Garcia-Tsao G, Sanyal A, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 106.Yacavone RF, Locke GR, III, Gostout CJ, Rockwood TH, Thieling S, Zinsmeister AR. Factors influencing patient satisfaction with GI endoscopy. Gastrointest Endosc. 2001;53:703–10. doi: 10.1067/mge.2001.115337. [DOI] [PubMed] [Google Scholar]
- 107.Ko HH, Zhang H, Telford JJ, Enns R. Factors influencing patient satisfaction when undergoing endoscopic procedures. Gastrointest Endosc. 2009;69:883–91. doi: 10.1016/j.gie.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 108.Yanai H, Schushan-Eisen I, Neuman S, Novis B. Patient satisfaction with endoscopy measurement and assessment. Dig Dis. 2008;26:75–9. doi: 10.1159/000109392. [DOI] [PubMed] [Google Scholar]
- 109.Bytzer P, Lindeberg B. Impact of an information video before colonoscopy on patient satisfaction and anxiety – a randomized trial. Endoscopy. 2007;39:710–4. doi: 10.1055/s-2007-966718. [DOI] [PubMed] [Google Scholar]
- 110.Denberg TD, Coombes JM, Byers TE, et al. Effect of a mailed brochure on appointment-keeping for screening colonoscopy: A randomized trial. Ann Intern Med. 2006;145:895–900. doi: 10.7326/0003-4819-145-12-200612190-00006. [DOI] [PubMed] [Google Scholar]
- 111.Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol. 2001;96:1786–90. doi: 10.1111/j.1572-0241.2001.03872.x. [DOI] [PubMed] [Google Scholar]
- 112.Hackett ML, Lane MR, McCarthy DC. Upper gastrointestinal endoscopy: Are preparatory interventions effective? Gastrointest Endosc. 1998;48:341–7. doi: 10.1016/s0016-5107(98)70001-x. [DOI] [PubMed] [Google Scholar]
- 113.Coombes JM, Steiner JF, Bekelman DB, Prochazka AV, Denberg TD. Clinical outcomes associated with attempts to educate patients about lower endoscopy: A narrative review. J Community Health. 2008;33:149–57. doi: 10.1007/s10900-007-9081-5. [DOI] [PubMed] [Google Scholar]
- 114.de Jonge V, Sint Nicolaas JS, Lalor EA, et al. A prospective audit of patient experiences in colonoscopy using the Global Rating Scale: A cohort of 1187 patients. Can J Gastroenterol. 2010;24:607–13. doi: 10.1155/2010/724924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawley ME, Javaid B. The impact of patient questionnaires in improving endoscopy services. Endoscopy. 2008;40(Suppl 1):A173. [Google Scholar]