Abstract
Nucleus accumbens-1 (NAC1), a nuclear factor belonging to the BTB/POZ gene family, is known to play important roles in proliferation and growth of tumor cells and in chemotherapy resistance. Yet, the mechanisms underlying how NAC1 contributes to drug resistance remain largely unclear. We reported here that autophagy was involved in NAC1-mediated resistance to cisplatin, a commonly used chemotherapeutic drug in the treatment of ovarian cancer. We found that treatment with cisplatin caused an activation of autophagy in ovarian cancer cell lines, A2780, OVCAR3, and SKOV3. We further demonstrated that knockdown of NAC1 by RNAi or inactivation of NAC1 by inducing the expression of a NAC1 deletion mutant that contains only the BTB/POZ domain significantly inhibited the cisplatin-induced autophagy, resulting in increased cisplatin cytotoxicity. Moreover, inhibition of autophagy and sensitization to cisplatin by NAC1 knockdown or inactivation were accompanied by induction of apoptosis. To confirm that the sensitizing effect of NAC1 inhibition on the cytotoxicity of cisplatin was attributed to suppression of autophagy, we assessed the effects of the autophagy inhibitors, 3-MA and chloroquine, and siRNAs targeting beclin 1 or Atg5, on the cytotoxicity of cisplatin. Treatment with 3-MA, chloroquine or beclin 1 and Atg5-targeted siRNA also enhanced the sensitivity of SKOV3, A2780 and OVCAR3 cells to cisplatin, indicating that suppression of autophagy indeed renders tumor cells more sensitive to cisplatin. Regulation of autophagy by NAC1 was mediated via high mobility group box1 (HMGB1), as the functional status of NAC1 was associated with the expression, translocation and release of HMGB1. The results of our study not only revealed a new mechanism determining cisplatin sensitivity, but also identified NAC1 as a novel regulator of autophagy. Thus, the NAC1- mediated autophagy may be exploited as a new target for enhancing the efficacy of cisplatin against ovarian cancer and other types of malignancies.
Keywords: NAC1, autophagy, apoptosis, HMGB1, cisplatin, ovarian cancer
Introduction
Nucleus accumbens-1 (NAC1), a transcription repressor that belongs to the BTB/POZ gene family, has been found to be overexpressed in several types of human carcinomas arising from ovary, cervix, endometrium, breast, and colon (Nakayama et al., 2006a; Nakayama et al., 2006b; Yeasmin et al., 2008). The highly conserved BTB/POZ domain is required for NAC1 homodimerization, and the formation of the homodimer complex participates in various biological functions, such as transcription regulation, protein degradation via ubiquitination, cell proliferation, apoptosis. It has been observed in patients with ovarian cancer that expression of NAC1 is significantly higher in recurrent post-treatment tumors than in untreated specimens (Davidson et al., 2007; Nakayama et al., 2006a; Nakayama et al., 2006b), and the up-regulation of NAC1 in human cancers contributes to tumor growth and survival, and to resistance of tumor cells to chemotherapeutic drug, paclitaxel (Jinawath et al., 2009; Nakayama et al., 2006b). Additionally, NACC1 that encodes NAC1 is amplified in many ovarian high-grade serous carcinomas. These studies suggest that NAC1 not only possesses oncogenic potential, but is also involved in modulation of drug resistance.
Cisplatin is a platinum compound commonly used in the treatment of ovarian cancer, one of the most lethal malignancies in women. However, development of resistance to cisplatin during the therapy often limits the effectiveness of this drug in treating patients with ovarian cancer. A diverse array of mechanisms of cisplatin resistance have been reported, including decreased intracellular accumulation of the drug, increased repair of DNA damage, reduced apoptosis (Borst et al., 2008), and involvement of certain enzymes such as mitogen-activated protein kinase phosphatase (Ren et al.; Wang et al., 2007). Recently, induction of autophagy, an evolutionarily conserved response to nutrient deprivation found in yeast, plants, worms, flies, mice and man, has been suggested to be potentially involved in cellular resistance to cisplatin (Harhaji-Trajkovic et al., 2009; Liu et al., 2009; Periyasamy-Thandavan et al., 2008). Autophagy is a process of self-digestion of cytoplasm and organelles through which cellular components are recycled for energy utilization. Recent studies have shown that autophagy is also a response to certain forms of therapeutic stress including cytotoxic chemotherapy and radiation therapy (Livesey et al., 2009; White and DiPaola, 2009). Nevertheless, the exact roles of autophagy in determining sensitivity of tumor cells to various treatments remain undefined, and many studies imply that it is context-dependent. For cisplatin, how autophagy induced by this chemotherapeutic drug affects its efficacy and the mechanism by which autophagy is regulated in cancer cells treated with this agent are largely unknown.
Based on the pro-survival role of NAC1 and its involvement in resistance to chemotherapy, in this study we sought to investigate: 1) the effect of NAC1 expression or functional status on cisplatin sensitivity; 2) whether NAC1 was involved in regulation of autophagy; 3) whether the pro-survival function of NAC1 was attributed to induction of autophagy. We demonstrate that NAC1 is a regulator of autophagy in cancer cells, and the regulation of autophagy by NAC1 is mediated through its effects on the expression, translocation, and release of high-mobility group box 1 (HMGB1), an important regulator of autophagy (Tang et al.). Our study also reveals that cisplatin-activated autophagy protects tumor cells against the cytotoxicity of this agent, and that inhibition of NAC1 and autophagy can enhance sensitivity of ovarian cancer cells to cisplatin. Thus, NAC1 may represent a novel therapeutic target for reinforcing the efficacy of cancer chemotherapy.
Results
Cisplatin induces a cyto-protective autophagy in ovarian cancer cells
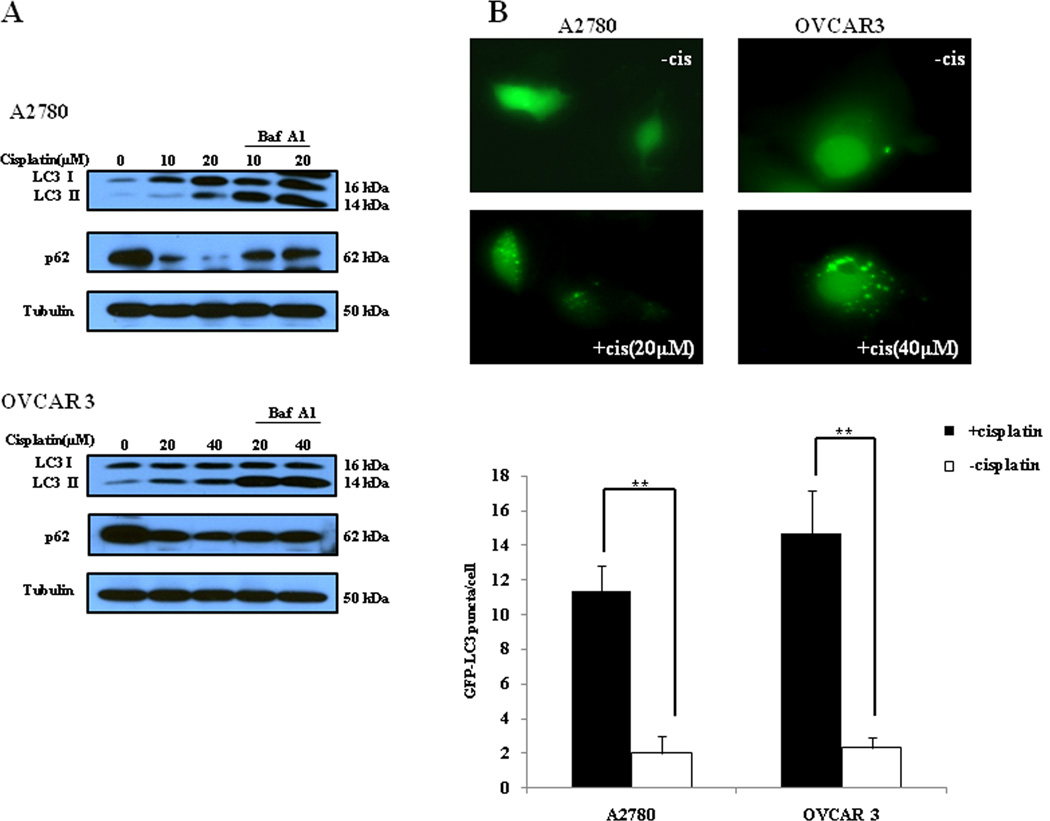
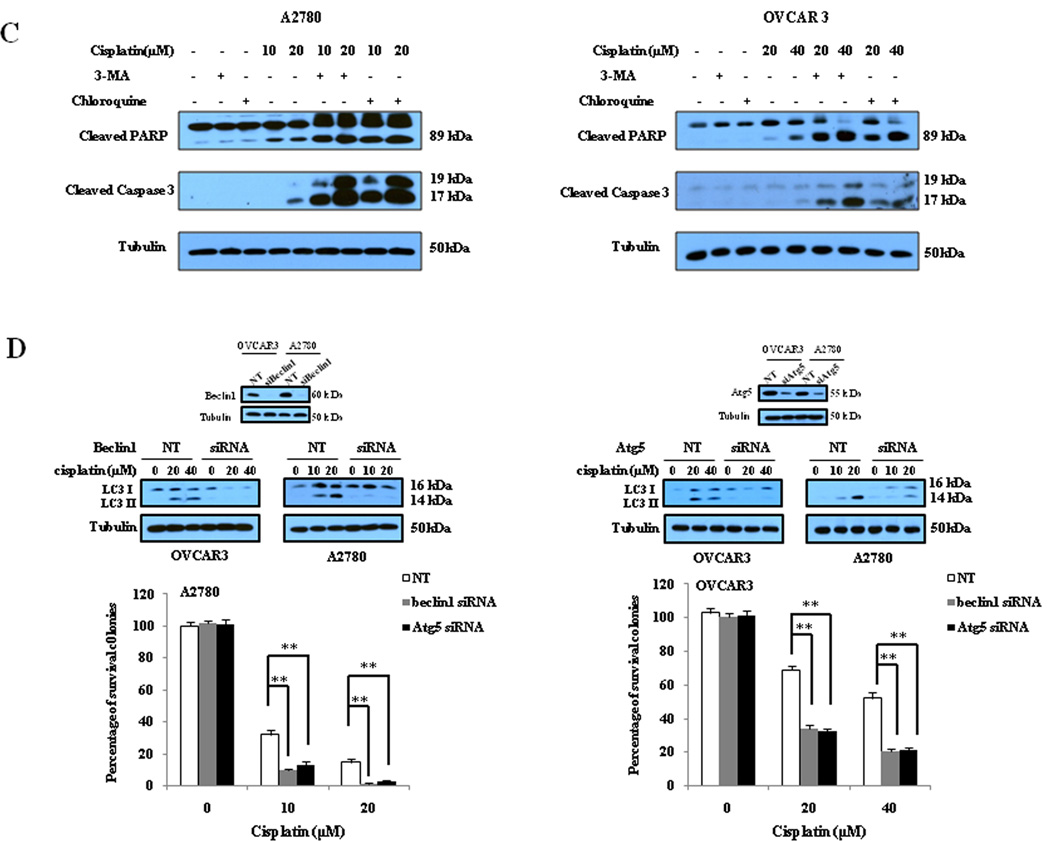
To determine the effect of cisplatin on autophagy and the role of autophagy in determining the sensitivity of cancer cells to this drug, we first examined the activity of autophagy in ovarian cancer cells treated with cisplatin. As shown in Fig. 1A, treatment of the human ovarian cancer cell lines A2780 and OVCAR3 with cisplatin caused a dose-dependent activation of autophagy, as evidenced by the increases in the amount of LC3 II and decreases in the amount of p62, two selective markers of autophagy. Fig. 1A also shows that LC3 II levels were further elevated in the presence of bafilomycin A1, an inhibitor of autophagosomelysosome fusion and LC3 II degradation, indicating an increase of autophagic flux in the cisplatin-treated cancer cells. The stimulative effect of cisplatin on autophagy was verified by a GFP-LC3 puncta formation assay, which demonstrated an increase in the number of GFP-LC3 puncta in the tumor cells treated with cisplatin (Fig. 1B). Next, we determined whether the cisplatin-stimulated autophagy played a protective or sensitizing role in tumor cells treated with this drug. We found that in comparison to treatment with cisplatin alone, co-treatment of ovarian cancer cells with cisplatin and inhibitors of autophagy, 3-MA or chloroquine, enhanced the cytotoxicity (Fig. 2A) and apoptosis (Fig. 2B and Fig. 2C) induced by cisplatin. To further prove the role of autophagy in determining sensitivity of tumor cells to cisplatin, we suppressed autophagy by silencing the expression of autophagy-related genes beclin 1 or Atg5, and then measured the effects of inhibiting autophagic activity on clonogenicity of the tumor cells. Fig. 2D shows that knockdown of beclin 1 or Atg5 in A2780 and OVCAR3 cells significantly reinforced the colony-inhibitory effect of cisplatin. These results suggest that cisplatin induces a canonical autophagy, and induction of autophagy plays a protective role in tumor cells subjected to the cytotoxicity of cisplatin.
Figure 1. Cisplatin induces autophagy in ovarian cancer cells.
(A) A2780 and OVCAR3 cells were treated with the indicated concentrations of cisplatin for 24 h in the absence or presence of 10 nM of bafilomycin A1. At the end of treatment, cell lysates were prepared, resolved by SDS-PAGE, and subjected to Western blot analysis using anti-LC3, anti-p62 or anti-tubulin antibodies, respectively. Tubulin was used as a loading control. (B) A2780 and OVCAR3 cells were transfected with a GFP-LC3 plasmid, followed by treatment with the indicated concentrations of cisplatin for 24 h. At the end of treatment, the cells were inspected under a fluorescence microscope. Quantitation of the GFP-LC3 puncta was performed by counting 20 cells for each sample, and average numbers of puncta per cell were shown. The bars are the mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. ** p < 0.01, t-test, cisplatin vs. vehicle.
Figure 2. Inhibition of autophagy by 3-MA and chloroquine enhances sensitivity of ovarian cancer cells to cisplatin.
(A) Upper panels: A2780 and OVCAR3 cells were treated with the indicated concentrations of cisplatin for 48 h in the presence or absence of 3-MA (2mM) or chloroquine (2.5µM). At the end of treatment, cell viability was measured by MTT assay; Lower panels: A2780 and OVCAR3 cells were treated with the indicated concentrations of cisplatin for 24 h in the presence or absence of 3-MA (2mM) or chloroquine (2.5µM), then plated in 35-mm cell culture dishes and incubated for 10 days at 37°C in a humidified atmosphere containing 5% CO2/95% air. At the end of incubation, colonies were stained with 1% methylene blue in 50% methanol for 30 min, washed with water, and colonies counted. Each point or bar represents mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. *p<0.05; **p<0.01; (B and C) A2780 and OVCAR3 cells were treated with the indicated concentrations of cisplatin for 24 h in the presence or absence of 3-MA (2 mM) or chloroquine (2.5 µM). Apoptosis was determined by: (B) flow cytometric analysis of Annexin V staining; and (C) Western blot analysis of PARP and cleaved caspase-3. (D) A2780 and OVCAR3 cells were transfected with a beclin 1-targeted or Ag5-targeted siRNA; forty-eight h later, the cells were treated with the indicated concentrations of cisplatin for 24 h, then plated in 35-mm cell culture dishes and incubated for 10 days at 37°C in a humidified atmosphere containing 5% CO2/95% air. At the end of incubation, colonies were stained with 1% methylene blue in 50% methanol for 30 min, washed with water, and colonies counted. The bars are the mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. *p<0.05; **p<0.01, vs. control, t-test.
NAC1 is essential for the cisplatin-activated autophagy
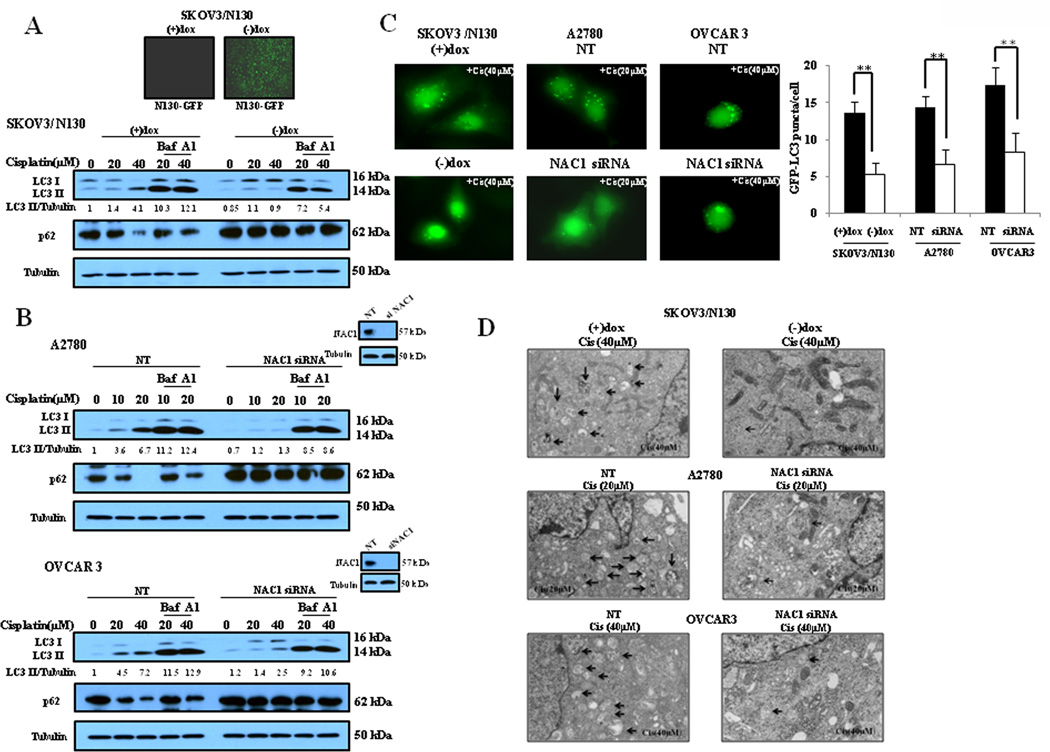
Overexpression of NAC1 in ovarian cancer and several other types of carcinomas has been reported to be correlative with tumor recurrence and resistance to chemotherapy. Nevertheless, the mechanisms by which NAC1 promotes survival of tumor cell and confers resistance to chemotherapy remain largely unclear. As autophagy was shown to play a prosurvival role in tumor cells treated with cisplatin (Fig. 1 and Fig. 2), we asked whether or not there was an association between the function of NAC1 and activity of autophagy. We first used a SKOV3 cell line in which an inducible (Tet-Off) expression construct of a NAC1 deletion mutant (N130) was introduced (Nakayama et al., 2006b). In this cell line (SKOV3/N130), expression of the NAC1 mutant is repressed when doxycycline is present; however, upon removal of doxycycline, the expression of this mutant is activated (Supplementary Information, Fig. S1; Fig. 3A, upper panel), and when expressed on its own the first 130 amino acid of NAC1 have the ability to exert a dominant negative effect and inactivate the NAC1 protein, since NAC1 needs to homodimerize through the BTB/POZ domain to be functionally active. Fig. 3A shows that in the cisplatin-treated SKOV3/N130 cells, activation of the expression of the NAC1 deletion mutant by removal of doxycycline led to suppression of autophagic response, as compared to autophagy in the cells with deactivation of the expression of NAC1 mutant in the presence of doxycycline. To further prove the role of NAC1 in inducing autophagy, we silenced the expression of NAC1 in A2780 and OVCAR3 cells followed by treatment with cisplatin, and then examined the level of autophagy. Fig. 3B demonstrates that silencing of NAC1 expression partially blocked the autophagic response activated by cisplatin, as compared to the non-targeted control. The effect of NAC1 on cisplatin-activated autophagy was also demonstrated by a GFP-LC3 puncta formation assay and by electron microscopy, showing that inactivation or silencing of NAC1 expression decreased the numbers of GFP-LC3 puncta (Fig. 3C) and the numbers of autophagosomes (Fig. 3D). These results indicate that NAC1 plays an essential role in mediating the autophagy induction by cisplatin treatment.
Figure 3. Inactivation or silencing of NAC1 expression blunts autophagy in ovarian cancer cells treated with cisplatin.
(A) SKOV3/N130 cells cultured in the presence or absence of doxycycline were treated with the indicated concentrations of cisplatin for 24 h. At the end of treatment, cell lysates were prepared, resolved by SDS-PAGE, and subjected to Western blot analysis of LC3 and p62, respectively. Tubulin was used as a loading control. (B) A2780 and OVCAR3 cells were transfected with a non-targeted RNA or siRNA targeting NAC1, followed by treatment with the indicated concentrations of cisplatin for 24 h. At the end of treatment, LC3 and p62 were examined by Western blot. (C) SKOV3/N130 cells cultured in the presence or absence of doxycycline, or A2780 and OVCAR3 cells with or without silencing of NAC1 expression, were transfected with a GFP-LC3 plasmid, followed by treatment with the indicated concentrations of cisplatin for 24h. Quantitation of GFP-LC3 puncta was performed as described in Fig. 1. The bars are the mean ± S.D. of triplicate; results shown are the representative of three identical experiments. **p < 0.01, t-test. (D) SKOV3/N130 cells cultured in the presence or absence of doxycycline, or A2780 and OVCAR3 cells with or without silencing of NAC1 expression, were treated with the indicated concentrations of cisplatin or vehicle for 24h. At the end of treatment, the cells were harvested by trypsinization, fixed and embedded in spur resin. Ninety nm thin sections were cut and examined at 80 Kv with a JEOL 1200EX transmission electron microscope. Arrows indicate autophagic vacuoles.
Inhibition of NAC1 enhances sensitivity of ovarian cancer cells to cisplatin
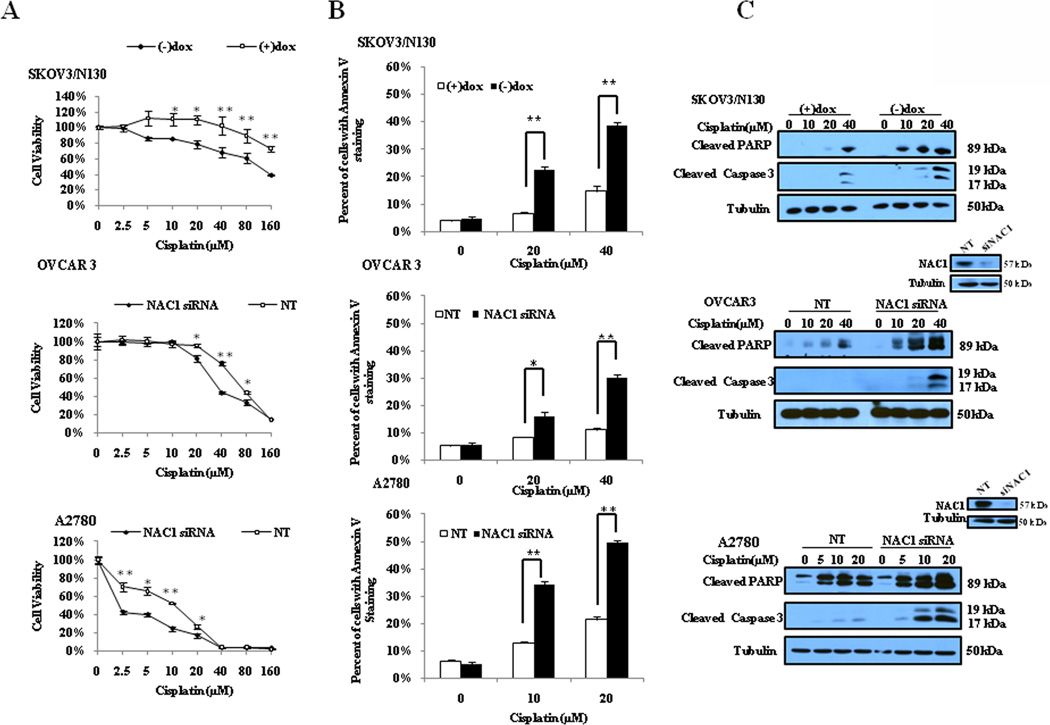
To determine the effect of NAC1 on cellular sensitivity to cisplatin, we treated the ovarian cancer cell lines harboring different status of NAC1 with a series of concentrations of cisplatin. At the end of 48 h treatment, cellular viability was measured using a MTT assay. Fig. 4A shows that inactivation of NAC1 either by inducing the expression of a NAC1 deletion mutant or by silencing of NAC1 expression by siRNA significantly enhanced the cytotoxicity of cisplatin in tumor cells, as compared to that in the cells expressing wild-type NAC1 or non-targeting siRNA. Furthermore, we demonstrated that inactivation or silencing of NAC1 significantly augmented the cisplatin-induced apoptosis, as indicated by increases in Annexin V staining and in the amounts of cleaved caspase-3 and PARP (Fig. 4B and Fig. 4C). Suppression of autophagy and activation of apoptosis by inhibiting NAC1 in the cisplatin-treated cells suggest a regulatory role for NAC1 in the cross-talk between autophagy and apoptosis.
Figure 4. Inactivation or silencing of NAC1 expression increases sensitivity of ovarian cancer cells to cisplatin.
SKOV3/N130 cells cultured in the presence or absence of doxycycline, or A2780 and OVCAR3 cells with or without silencing of NAC1 expression, were treated with the indicated concentrations of cisplatin for 48h. At the end of treatment, (A) cell viability was measured by MTT assay. Each point represents mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. *p<0.05; **p<0.01; (B and C) apoptosis was determined by flow cytometric analysis of Annexin V staining (B) and Western blot analysis of PARP and cleaved caspase-3 (C). Tubulin was used as a loading control. *p < 0.05; **p < 0.01, t-test.
Regulation of autophagy by NAC1 is mediated by HMGB1
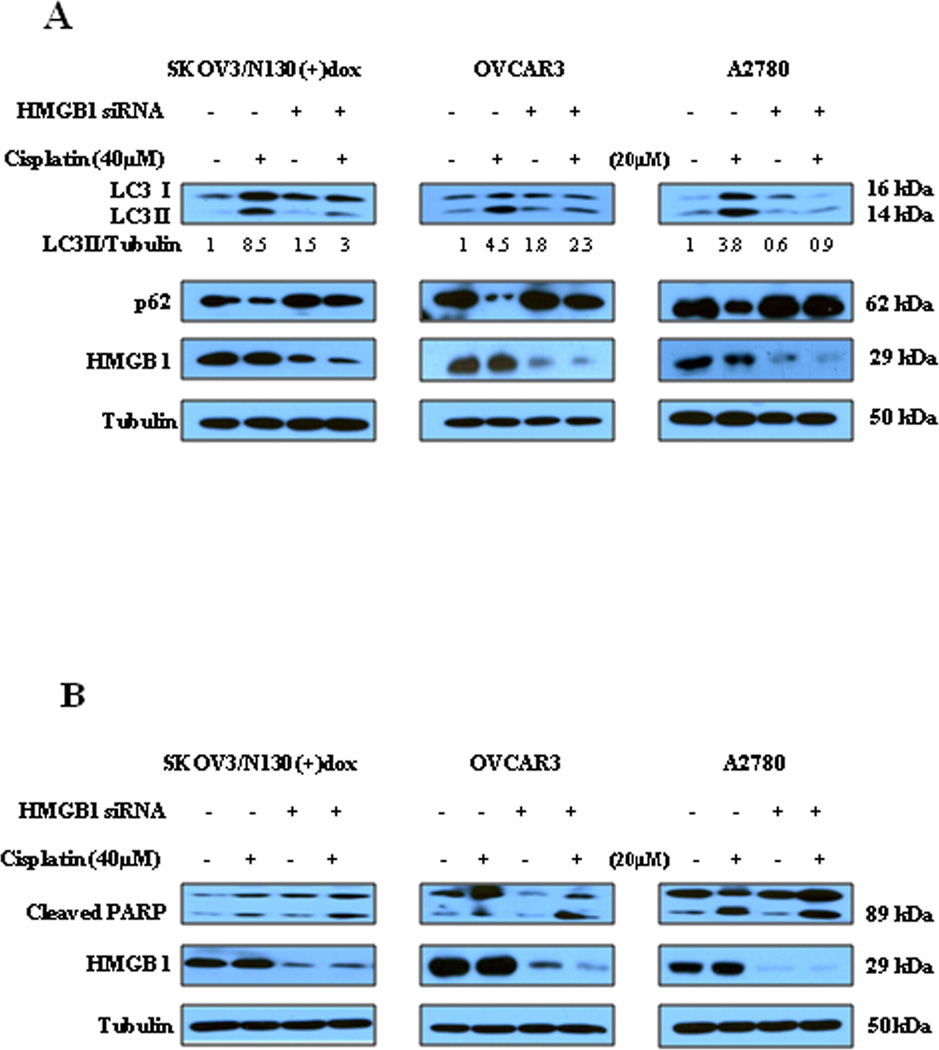
In quantitative proteomic analyses of proteins that are potentially regulated by NAC1, we observed that when NAC1 status was switched from inactive state to active state in SKOV3/N130 cells, there was a ~2-fold increase in the amount of HMGB1 protein, an important regulator of autophagy. Thus, we next explored whether or not HMGB1 could be a mediator of the NAC1-activated autophagy. In these experiments, we knocked down the expression of HMGB1 in ovarian cancer cells with active NAC1 function, and then treated these cells with cisplatin. Knockdown of HMGB1 either in SKOV3/N130 cells cultured in the presence of doxycycline or in the parental OVCAR3 and A2780 cells resulted in blunting of cisplatin-induced autophagy, as demonstrated by a decrease in the amount of LC3 II and an increase in p62, in comparison to that in the cells without knockdown of HMGB1 (Fig. 5A). Knock-down of HMGB1 in these cells also led to increased apoptosis in the cisplatin-treated cells, as indicated by an elevated level of cleaved PARP (Fig. 5B). These results suggest a key role for HMGB1 in mediating the NAC1-activated autophagy.
Figure 5. Silencing of HMGB1 decreases autophagy but increases apoptosis in the cisplatin-treated ovarian cancer cells harboring active NAC1.
SKOV3/N130 cells cultured in the presence of doxycycline, or parental A2780 and OVCAR3 cells, were transfected with a non-targeted RNA or a siRNA targeting HMGB1, followed by treatment with 40 µM of cisplatin or vehicle for 24h. At the end of treatment, cell lysates were prepared, resolved by SDS-PAGE, and subjected to Western blot analysis of HMGB1, LC3, p62 (A) and PARP (B). Tubulin was used as a loading control. Results shown are the representative of three similar experiments.
NAC1 modulates the expression, translocation, and release of HMGB1
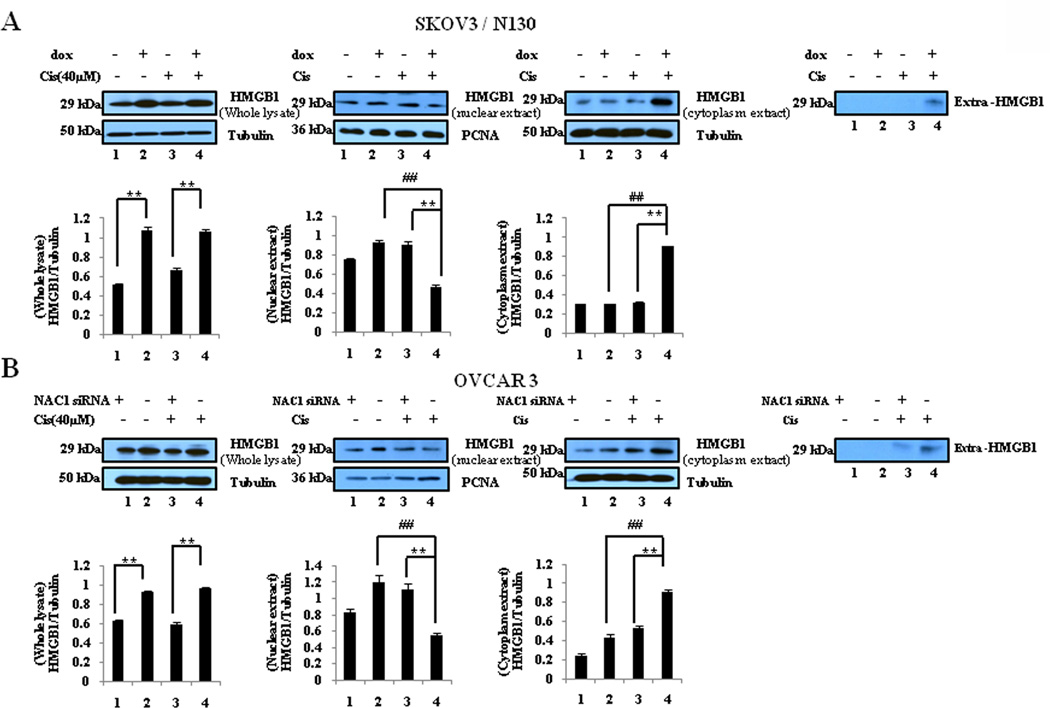
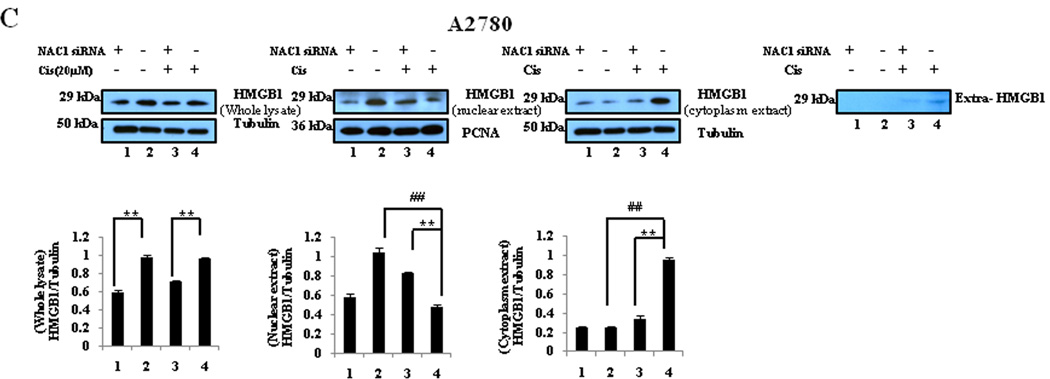
Since HMGB1 was shown to be required for the NAC1-regulated autophagy in the cisplatin-treated cells (Fig. 5), we next determined the effects of NAC1 on the expression, translocation, and release of HMGB1. Fig. 6 shows that in both the cisplatin-treated and non-treated cells, inactivation or silencing of NAC1 led to a decrease in the expression of HMGB1. Cisplatin treatment reduced the amount of HMGB1 in the nuclei but increased HMGB1 protein in the cytoplasm of the cells with active NAC1 function (Fig. 6), indicating that this agent causes cytosolic translocation of HMGB1. Notably, inhibition of NAC1 blocked the cytosolic translocation of HMGB1 in these ovarian cancer cells treated with cisplatin (Fig. 6). Cisplatin-induced release of HMGB1 was also suppressed by NAC1 inhibition (Fig. 6). These results suggest that the functional status of NAC1 impacts the expression, translocation, and release of HMGB1 in tumor cells under stresses such as cytotoxic insults.
Figure 6. Effects of NAC1 status on the expression, translocation, and release of HMGB1.
SKOV3/N130 cells cultured in the presence or absence of doxycycline (A), or A2780 (B) and OVCAR3 (C) cells with or without silencing of NAC1 expression, were treated with 40 µM of cisplatin for 24h. At the end of treatment, total cell lysates, nuclear extracts, cytoplasmic fractions, and concentrated conditioned media, were prepared, resolved by SDS-PAGE, and subjected to Western blot analysis of HMGB1. Tubulin was used as a loading control for whole cell lysates and cytoplasmic extracts, and PCNA was used as a loading control for nucleic extracts. Results shown are the representative of three similar experiments. **P < 0.01 versus the NAC1 mutant or siRNA treatment; ## P < 0.01 versus vehicle treatment.
Discussion
In this study we report a previously unrecognized role of NAC1 in regulating autophagy, and provide a possible mechanistic role for how NAC1 up-regulation, as observed in several types of human cancer, contributes to tumor progression and early recurrence in patients after chemotherapy as previously reported. We show that treatment with the chemotherapeutic drug cisplatin activates autophagy in ovarian cancer cells (Fig. 1), and inactivation and gene silencing of NAC1 blocks the activation of autophagy by cisplatin (Fig. 3). We further demonstrate that regulation of autophagy by NAC1 is mediated through its effect on HMGB1, as the functional status of NAC1 affects the expression, translocation, and release of this critical autophagy regulator (Fig. 6), which is known to induce autophagy by disrupting the interaction between beclin 1 and bcl-2 (Tang D et al 2010).
Because tumor recurrence as a result of resistance to chemotherapeutic drugs remains a formidable problem in managing cancer patients, the results from this study should illuminate fundamental properties of chemo-resistance and provide new therapeutic targets to treat advanced recurrent neoplastic diseases. The role of NAC1 in activation of autophagy, a cellular process of self-digestion in response to various stresses, may account, at least in part, for the prosurvival function of this cancer-associated protein. Indeed, autophagy has been appreciated as an important mechanism for conferring resistance to chemotherapy. For instance, it has been shown that induction of autophagy promotes resistance of leukemic cells to adriamycin and vincristine (Liu et al.). Similarly, we demonstrate here that cisplatin-induced autophagy protects ovarian cancer cells from the cytotoxic effects of cisplatin (Fig. 2). It has been known that cisplatin induces autophagy in various types of cells (Fanzani et al.; Kaushal et al., 2008; Ren et al.), but how autophagy contributes to cellular sensitivity to this drug remains to be clarified. Several studies have shown that activation of autophagy in the cisplatin-treated cells inhibited apoptosis and protected cells from cisplatin toxicity (Harhaji-Trajkovic et al., 2009; Liu et al., 2009; Periyasamy-Thandavan et al., 2008; Ren et al.). In contrast, there are also studies reporting that forced expression of Beclin 1, a key autophagy gene, activates both autophagy and apoptosis, and potentiates the cytotoxic effect of cisplatin on tumor cells (Sun et al.). We demonstrate here that autophagy induced by cisplatin is cyto-protective, as suppression of autophagy by inhibiting NAC1 or by using small molecule inhibitors of autophagy augments the cytotoxic effect of cisplatin (Fig. 2 and Fig. 4). Therefore, it is likely that the functional consequences of activating autophagy are context-dependent, and factors such as cell type and treatment regimen could all determine the fates of autophagic cells for their survival or death. Additionally, we observed a similar effect of NAC1 on autophagy that was induced by other stimuli (nutrient depletion, hypoxia or rapamycin treatment) in ovarian cancer cells (Supplementary Information, Fig. S2) and in other types of cells treated with cisplatin (Supplementary Information, Fig. S3, Fig. S4 and Fig. S5), suggesting that NAC1 may act as an autophagy regulator in various types of tumor cells responding to multiple forms of stress.
Regulation of autophagy by NAC1 appears to be mediated through HMGB1, a highly conserved chromatin-associated nuclear protein and an extracellular signaling molecule that is now appreciated as a critical regulator of autophagy and apoptosis by acting as an inducer of autophagy and a suppressor of apoptosis (Ellerman et al., 2007; Tang et al.). The expression or function of HMGB1 has been reported to be closely linked to cancer development and progression (Ellerman et al., 2007). Although the expression of HMGB1 is activated in cisplatin-resistant cancer cells (Nagatani et al., 2001), the actual effects of this protein on cellular sensitivity to cisplatin vary in different cell types (He et al., 2000; Sharma et al., 2009; Wei et al., 2003). We show that the expression, translocation, and release of HMGB1 are altered by the functional status of NAC1 (Fig. 6), which is also demonstrated to modulate the sensitivity of ovarian cancer cells to cisplatin via autophagy (Fig. 4 and Fig. 5). Thus, it is likely that the effect of HMGB1 on cisplatin sensitivity could be dependent on the functional status of NAC1 in an individual cell type. At present, the precise mechanism by which NAC1 controls the expression, translocation and release of HMGB1 is unknown; however, considering that NAC1 predominantly localizes in the nuclei (Supplementary Information, Fig. S6) and its role as a transcription factor, it is likely that NAC1 activates the transcription of HMGB1, leading to increased expression, translocation and release of HMGB1. The possibility that NAC1 assists HMGB1 in nucleo-cytoplasmic shuttling is also worth investigating. Note worthily, the release of HMGB1 can also be regulated by autophagy, as reported by Thorburn et al. (Thorburn et al., 2009a; Thorburn et al., 2009b). It is conceivable that the increased amount of cytoplasmic HMGB1 activates autophagy, since HMGB1 is known to modulate autophagy by disrupting the interaction between beclin 1 and bcl-2 in the cytoplasm (Tang D et al 2010). In addition, consistent with the role of HMGB1 in balancing autophagy and apoptosis, our results show that silencing of HMGB1 not only inhibits autophagy but also activates apoptosis in ovarian cancer cells subjected to cisplatin treatment (Fig. 5). As reduced apoptosis is believed to be one of the mechanisms of cisplatin resistance, the converse relationship between autophagy and apoptosis in tumor cells treated with cisplatin, as shown in this study, might also help explain why apoptosis is suppressed in cisplatin-resistant cells.
Development of drug resistance is a complex process involving multiple genes and the pathways they control. It is most likely that NAC1 participates in chemo-resistance through several other mechanisms besides mediating autophagy. For example, NAC1 has been reported to inactivate the Gadd45 tumor suppressor pathway that is activated by chemotherapeutic compounds (Jinawath et al., 2009; Nakayama et al., 2007). Thus, NAC1-expressing tumor cells should have a survival advantage in the presence of cytotoxic drugs. Moreover, NAC1 has been found to play a role in maintaining pluripotency in embryonic stem cells by transcriptionally regulating gene expression of other pluripotency-associated factors including Nanog, Oct4, Sox2, Klf4, Sall1 and Sall4 (Kim et al., 2008; Muller et al., 2008; Wang et al., 2006). As exportation of foreign compounds such as cytotoxic drugs is an inherent feature of stem cells, it is possible that cancer cells with NAC1 up-regulation may adopt a similar strategy as embryonic stem cells to survive during chemotherapy.
In summary, our study identified NAC1 as a novel regulator of autophagy and its involvement in modulation of cisplatin sensitivity, and demonstrated that inhibition of NAC1 can enhance sensitivity of ovarian cancer cells to this chemotherapeutic drug. These findings may expand current knowledge of the autophagic protein networks and provide new insight into how autophagy is regulated in tumor cells. Thus, NAC1 might be explored as a novel therapeutic target for prevention of tumor cells from becoming resistance to chemotherapy and for reinforcement of the efficacy of cisplatin against malignant tumors.
Materials and Methods
Cell lines and culture
The human ovarian cancer cell lines SKOV3, A2780 and OVCAR3 were purchased from American Type Culture Collection (Manassas, VA, USA). SKOV3/N130 line was created by introduction of an inducible (Tet-Off) expression construct of a NAC1 deletion mutant (N130). A2780 and SKOV3/N130 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum; OVCAR3 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum. All of the cell culture media contain 100 units/ml penicillin and 100 µg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air.
Reagents and antibodies
Cisplatin was purchased from ENZO Life Science (Plymouth Meeting, PA, USA); geneticin (G418) was purchased from Invitrogen (Carlsbad, CA, USA); bafilomycin A1, doxycycline, 3-methyladenosine (3-MA), chloroquine disphosphate, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA). Nuclear and Cytoplasmic Extraction Reagents and Western blot reagents were obtained from Pierce Biotechnology (Rockford, IL, USA). The antibodies to LC3, Atg5, α-tubulin, PARP, caspase3, and HMGB1 were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-NAC1 antibody was obtained from Novus (Littleton, CO, USA); antip62 (SQSTM1) antibody was purchased from ENZO Life Science; anti-PCNA and anti-beclin 1 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All of the cell culture media and other reagents were from Invitrogen.
siRNA transfection
siRNA duplexes were prepared by QIAGEN (Valencia, CA, USA). Transfection of siRNA was performed according to the manufacturer’s protocol. Briefly, cells in exponential phase of growth were plated in six-well cell culture plates at 1 × 105 cells per well, grown for 24 h, and then transfected with siRNA using Oliofectamine and OPTI-MEM I-reduced serum medium. The concentrations of siRNA were chosen based on dose-response studies.
Western blot
Cells were lysed in M-PER mammalian protein extraction reagent (Pierce Biotechnology) supplemented with a protease inhibitor cocktail from Roche (Indianapolis, IN, USA), followed by centrifugation at 14,000× g for 10 minutes. At the end of centrifugation, cell lysates were collected and protein concentration of cell lysates were measured. Protein (10–20µg) were resolved by SDS-PAGE and transferred to PVDF membrane from Bio-Rad (Hercules, CA, USA). The blots were then incubated with primary antibodies in 3% BSA/TBST at 4°C overnight, followed by incubation with secondary antibodies at room temperature for 1 h. The protein signals were detected by ECL method.
Autophagy assays
Autophagy was measured by the following methods: 1) Western blot analysis of LC3 and p62; 2) microscopic observation of GFP-LC3 puncta; 3) electron microscopic examination of double or multi-membrane vacuoles in the cytoplasm. These methods were reported previously (Cheng et al.; Wu et al., 2009).
Apoptosis assays
Apoptosis was examined by: 1) flow cytometric analysis of Annexin V and 7-AAD staining. Briefly, 100 µl Guava Nexin reagent from Millipore (Bedford, MA, USA) was added to 1 × 105 cells (in 100 µl) and the cells were incubated with the reagent for 20 minutes at room temperature in the dark. At the end of incubation, the cells were analyzed by a Guava EasyCyte™ Plus FlowCytometry System (Millipore); 2) Western blot analysis of the cleaved PARP and caspase-3.
Cell viability assay
Cell viability was measured by MTT assay. Briefly, cells were plated at a density of 5 × 103 cells per well on 96-well plates and subjected to different treatment. Following 48 h incubation at 37°C in a humidified atmosphere containing 5% CO2/95% air, the samples were incubated for another 4 h with MTT reagent. The formazan product was dissolved in DMSO and read at 570 nm on a Victor3 Multi Label plate reader (PerkinElmer, Boston, MA).
Clonogenic Assay
Tumor ells were subjected to different treatments; at the end of treatments, a fixed number of cells (determined experimentally to generate single colonies) were plated in 35-mm tissue culture dishes. Following incubation at 37°C in a humidified atmosphere containing 5% CO2/95% air for 10 days, cells were stained with 1% methylene blue in 50% methanol for 45 min, washed with water, and colonies (≥50 cells) were counted.
Preparation of cellular fractions
Cells subjected to different treatment were harvested, washed with cold PBS, and centrifuged. The cell-pellets were re-suspended in 200 µl of CER I extraction buffer, vortexed vigorously for 15 s and incubated on ice for 10 minutes. Then, the samples were mixed with 11µl of CER II extraction buffer, vortexed for 5 s, and centrifuged at 16, 000 × g for 5 minutes. Following collection of the cytoplasmic proteins, the nuclear pellets were re-suspended in 100 µl of NER buffer and incubated for 40 min on ice, with vortexing for 15 s every 10 minutes. At the end of incubation, the samples were centrifuged at 16,000× g for 10 minutes to collect the nuclear proteins.
Measurement of HMGB1 release
Conditioned cell culture media were collected following different treatment, and then filtered through Millex-GP membranes (Millipore, Bedford, MA) to remove cell debris. Samples were concentrated 20-fold with Amicon Centricon YM 3 columns (Millipore), according to the manufacturer’s instructions. Concentrated conditioned media were analyzed by Western blot for HMGB1.
Supplementary Material
Footnotes
Supported by grants from the US Public Health Service (R01CA135038 and RO1CA103937) and from the Penn State College of Medicine
Conflict of interest
The authors declare no conflict of interest.
References
- Borst P, Rottenberg S, Jonkers J. How do real tumors become resistant to cisplatin? Cell Cycle. 2008;7:1353–1359. doi: 10.4161/cc.7.10.5930. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Li H, Ren X, Niu T, Hait WN, Yang J. Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition. PLoS One. 5:e9715. doi: 10.1371/journal.pone.0009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Berner A, Trope CG, Wang TL, Shih Ie M. Expression and clinical role of the bric-a-brac tramtrack broad complex/poxvirus and zinc protein NAC-1 in ovarian carcinoma effusions. Hum Pathol. 2007;38:1030–1036. doi: 10.1016/j.humpath.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Ellerman JE, Brown CK, de Vera M, Zeh HJ, Billiar T, Rubartelli A, et al. Masquerader: high mobility group box-1 and cancer. Clin Cancer Res. 2007;13:2836–2848. doi: 10.1158/1078-0432.CCR-06-1953. [DOI] [PubMed] [Google Scholar]
- Fanzani A, Zanola A, Rovetta F, Rossi S, Aleo MF. Cisplatin triggers atrophy of skeletal C2C12 myotubes via impairment of Akt signalling pathway and subsequent increment activity of proteasome and autophagy systems. Toxicol Appl Pharmacol. doi: 10.1016/j.taap.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Harhaji-Trajkovic L, Vilimanovich U, Kravic-Stevovic T, Bumbasirevic V, Trajkovic V. AMPK-mediated autophagy inhibits apoptosis in cisplatin-treated tumour cells. J Cell Mol Med. 2009;13:3644–3654. doi: 10.1111/j.1582-4934.2009.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liang CH, Lippard SJ. Steroid hormones induce HMG1 overexpression and sensitize breast cancer cells to cisplatin and carboplatin. Proc Natl Acad Sci U S A. 2000;97:5768–5772. doi: 10.1073/pnas.100108697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinawath N, Vasoontara C, Yap KL, Thiaville MM, Nakayama K, Wang TL, et al. NAC-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating Gadd45 pathway. Oncogene. 2009;28:1941–1948. doi: 10.1038/onc.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal GP, Kaushal V, Herzog C, Yang C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy. 2008;4:710–712. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2009 doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- Liu L, Yang M, Kang R, Wang Z, Zhao Y, Yu Y, et al. HMGB1-induced autophagy promotes chemotherapy resistance in leukemia cells. Leukemia. doi: 10.1038/leu.2010.225. [DOI] [PubMed] [Google Scholar]
- Livesey KM, Tang D, Zeh HJ, Lotze MT. Autophagy inhibition in combination cancer treatment. Curr Opin Investig Drugs. 2009;10:1269–1279. [PubMed] [Google Scholar]
- Muller FJ, Laurent LC, Kostka D, Ulitsky I, Williams R, Lu C, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405. doi: 10.1038/nature07213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatani G, Nomoto M, Takano H, Ise T, Kato K, Imamura T, et al. Transcriptional activation of the human HMG1 gene in cisplatin-resistant human cancer cells. Cancer Res. 2001;61:1592–1597. [PubMed] [Google Scholar]
- Nakayama K, Nakayama N, Davidson B, Katabuchi H, Kurman RJ, Velculescu VE, et al. Homozygous deletion of MKK4 in ovarian serous carcinoma. Cancer Biol Ther. 2006a;5:630–634. doi: 10.4161/cbt.5.6.2675. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2006b;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Nakayama N, Wang TL, Shih Ie M. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007;67:8058–8064. doi: 10.1158/0008-5472.CAN-07-1357. [DOI] [PubMed] [Google Scholar]
- Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–640. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- Ren JH, He WS, Nong L, Zhu QY, Hu K, Zhang RG, et al. Acquired cisplatin resistance in human lung adenocarcinoma cells is associated with enhanced autophagy. Cancer Biother Radiopharm. 25:75–80. doi: 10.1089/cbr.2009.0701. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ramanjaneyulu A, Ray R, Rajeswari MR. Involvement of high mobility group B proteins in cisplatin-induced cytotoxicity in squamous cell carcinoma of skin. DNA Cell Biol. 2009;28:311–318. doi: 10.1089/dna.2009.0851. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, et al. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 294:204–210. doi: 10.1016/j.canlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene. 29:5299–5310. doi: 10.1038/onc.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 190:881–892. doi: 10.1083/jcb.200911078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J, Frankel AE, Thorburn A. Regulation of HMGB1 release by autophagy. Autophagy. 2009a;5:247–249. doi: 10.4161/auto.5.2.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2009b;16:175–183. doi: 10.1038/cdd.2008.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- Wei M, Burenkova O, Lippard SJ. Cisplatin sensitivity in Hmbg1−/− and Hmbg1+/+ mouse cells. J Biol Chem. 2003;278:1769–1773. doi: 10.1074/jbc.M210562200. [DOI] [PubMed] [Google Scholar]
- White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel R, et al. Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-D-glucose against human glioma cells through blunting of autophagy. Cancer Res. 2009;69:2453–2460. doi: 10.1158/0008-5472.CAN-08-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeasmin S, Nakayama K, Ishibashi M, Katagiri A, Iida K, Purwana IN, et al. Expression of the bric-a-brac tramtrack broad complex protein NAC-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin Cancer Res. 2008;14:1686–1691. doi: 10.1158/1078-0432.CCR-07-4085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.