Abstract
Developing a platform for in vitro cartilage formation would enhance the study of cartilage development, pathogenesis, and regeneration. To improve neocartilage formation, our group developed a novel self-assembly process for articular chondrocytes, which has been improved in this study using a novel combination of catabolic and anabolic agents. TGF-β1 was applied in conjunction with the enzyme chondroitinase-ABC (C-ABC) to additively increase tensile properties and synergistically enhance collagen content. Additionally, microarray analysis indicated that TGF-β1 up-regulated MAPK signaling in contrast to C-ABC, which did not enrich genetic pathways. The lack of genetic signaling spurred investigation of the biophysical role of C-ABC, which showed that C-ABC treatment increased collagen fibril diameter and density. After four weeks of culture in nude mice, neocartilage exhibited stability and maturation. This study illustrated an innovative strategy for improving in vitro and in vivo articular cartilage formation and elucidated mechanisms underlying TGF-β1 and C-ABC treatment.
Keywords: cartilage tissue engineering, collagen, glycosaminoglycan, soft tissue biomechanics
Introduction
Articular cartilage is crucial to proper joint function, providing a wear-resistant, low-friction surface covering the articulating surfaces of bones. Cartilage is largely acellular and relies on its extracellular matrix, which is predominantly composed of collagen and glycosaminoglycans (GAGs), to provide mechanical integrity. The matrix of cartilage contains aggrecan complexes with GAGs and an organized collagen network, both of which contribute to the tissue's biomechanics. Recapitulating the biomechanical and biochemical properties of native tissue has remained elusive despite decades of research. An effective method for in vitro grown cartilage would greatly advance the study of cartilage disease, regeneration, and development.
A self-assembly approach, which uses high density cell culture to promote chondrocyte aggregation, recapitulates cartilage development and produces robust neocartilage [1, 2]. Various strategies have been employed to overcome deficiencies of de novo cartilage such as overabundance of GAGs [3, 4] and tensile properties below those of native tissue [5]. For instance, applying exogenous stimuli such as growth factors [6], hydrostatic pressure [7, 8], and combinations of these stimuli [9] has improved the functional properties of self-assembled neocartilage. These developments highlight the potential of employing exogenous stimuli to enhance the self-assembly process.
The enzyme chondroitinase-ABC (C-ABC), which depletes GAGs, provides a method for inducing maturational growth. Because in vitro cartilage generally overproduces GAGs [3, 4], C-ABC has been investigated as a method of promoting matrix maturation. For example, treating cartilage explants with C-ABC and culturing for an additional 2 weeks increased tensile properties [10]. C-ABC treatment of chondrocytes seeded on agarose hydrogels increased the collagen concentration and tensile properties of constructs [11]. Furthermore, both single [12] and multiple [5] C-ABC treatments have been employed to increase the tensile properties of self-assembled neotissue without compromising compressive properties. These results suggest that C-ABC is an exciting method for improving in vitro cartilage growth.
TGF-β1 has also been investigated for its beneficial effects on cartilage growth. For instance, administering TGF-β increased GAG deposition in three-dimensional cultures of equine chondrocytes [13], rabbit chondrocytes [14], and bovine articular chondrocytes [15]. Additionally, administering TGF-β1 to self-assembled cartilage enhanced GAG and collagen production and concomitantly increased compressive and tensile properties [16]. TGF-β1 has also been employed in conjunction with hydrostatic pressure [9] and direct compression [17] to enhance the functional properties of neocartilage. These studies illustrate the ability of TGF-β1 to enhance both the matrix composition and biomechanical properties of in vitro cartilage.
This study used a combination of C-ABC and TGF-β1 treatments to integrate anabolic and catabolic strategies for enhancing cartilage formation. The objectives of this study were to 1) assess the effects of combining TGF-β1 and C-ABC treatment on self-assembled neocartilage, 2) investigate potential mechanisms underlying the response to these stimuli, and 3) evaluate the in vivo response of neotissue treated with TGF-β1 and C-ABC. This study tested the hypotheses that 1) TGF-β1 administration will act synergistically with C-ABC to increase tensile properties and collagen content of self-assembled neotissue, 2) C-ABC and TGF-β1 will up-regulate functionally-relevant molecular signaling pathways, and 3) self-assembled neocartilage will exhibit stability and maturation in vivo. To test these hypotheses, we employed a full factorial design of factor C-ABC (no treatment, treatment at 2 weeks) and factor TGF-β1 (no treatment, treatment during weeks 1&2 (continuous), treatment during weeks 1&3 (intermittent). The biomechanics, biochemical content, gene expression, collagen ultrastructure, and in vivo properties of neotissue were assessed.
Materials and Methods
Neocartilage culture
Immature bovine chondrocytes were harvested and cultured as described previously [2]. For C-ABC treatment, constructs were treated with C-ABC (Sigma) at an activity of 2 U/ml in chondrogenic medium for 4 hours [10]. TGF-β1 (Peprotech) was administered daily media at 30 ng/ml. TGF-β1 was administered during wks 1&2 (continuous) or during wks 1&3 (intermittent) based on previous work applying TGF-β1 to self-assembled cartilage [16]. At 4 weeks, constructs were prepared for biochemistry, histology, and biomechanics [5].
Quantitative biochemistry
Samples were frozen at −20°C for 24h and then lyophilized for dry weight determination. Subsequently, a pepsin and elastase protocol [5] was used to digest each sample. A Blyscan Glycosaminoglycan Assay kit (Accurate Chemical and Scientific Corp.) was used to quantify sulfated GAG content. Collagen abundance was determined using chloramine-T based assay [18].
Histology
Samples were cryoembedded and sectioned at 12 μm. Histology samples were fixed in formalin and then stained with Safranin-O/fast green and Picrosirius Red as described previously [5]. For IHC, samples were fixed in 4°C acetone and stained for collagen II or collagen I [5].
Mechanical testing
A creep indentation apparatus [19] was used to quantify compressive properties. Samples were tested as described previously [5] using a semi-analytical, semi-numeric, biphasic model [20] and finite element optimization [21] to yield each sample's aggregate modulus. For tensile testing, dermal punches were used to create dog-bone shaped samples [22]. A material testing system (Instron Model 5565) was used to apply uniaxial tension [5]. The slope of the linear portion of each stress-strain curve was reported as the Young's modulus (EY).
Scanning electron microscopy (SEM)
SEM was performed for control, intermittent TGF-β1, C-ABC, and combined intermittent TGF-β1 and C-ABC groups. Samples were fixed in 3% glutaraldehyde for 12h at 4°C and then dehydrated in an ascending series of ethanol. Samples were subsequently critical point dried and sputter coated with gold prior to imaging. Three locations were used for each sample and n=3. For each image, a 4×4 grid was drawn and three squares were randomly chosen from each image for diameter quantification using ImageJ. To quantify density, the ImageJ threshold function was used to set threshold limits. The measure function was then used to compute the area percentage corresponding to fibrils, which was reported as density.
RNA isolation and microarray hybridization
RNA was isolated at 2 wks for control, C-ABC, continuous TGF-β1, and C-ABC combined with continuous TGF-β1 (n=3). Isolation was performed 4h following the administration of C-ABC and/or TGF-β1 using a Qiagen RNeasy Lipid Tissue Mini Kit. RNA was analyzed using a nanodrop to determine RNA purity and concentration; an Agilent bioanalyzer was employed to ensure RNA was not degraded. 400ng of RNA was hybridized to bovine microarrays (Agilent) based on the manufacturer's one color gene expression protocol. Arrays were imaged using an Agilent high-resolution C scanner; images were processed using Agilent's Feature Extraction Software 10.5.
Microarray analysis
Entrez Gene IDs were cross-referenced with HomoloGene build 65 (ftp://ftp.ncbi.nih.gov/pub/HomoloGene/build65/) to determine human orthologs. Normalized intensities of each gene were used to determine differentially expressed genes relative to control samples (n=3 for each group). The Benjamini-Hochberg multiple testing correction was applied to generate adjusted p-values; data were filtered based on adjusted p-values < 0.005 and a minimal 2 fold change. Enriched pathways were determined based on p < 0.001.
PCR
For PCR analysis, RNA was isolated from the same samples used for the microarray assay. The primer for GAPDH was synthesized as described previously [23] with a forward sequence of GGTGATGCTGGTGCTGAGTA (5'-3') and reverse sequence of ATGCCAAAGTGGTCATGGAT (5'-3'). Other primers including MAP3K5, MAPK13, protein tyrosine phosphatase (PTP), and MAP2K3 were purchased from Invitrogen. RT was performed by incubating 500 ng of RNA with SuperScript III (Invitrogen) as recommended by the manufacturer. Real-time PCR was done using SYBR Green mastermix and 1 μM primers on a Rotor-gene 3000 real-time PCR machine (Corbett Research). A 10 min denaturing step was employed, followed by 45 cycles of 95°C (15s) and 60°C (60s). The take-off cycle (CT) for each gene of interest (GOI) was compared to the housekeeping gene GAPDH. Relative gene expressions were calculated using the 2−ΔΔCT method.
In vivo study
Four male athymic mice, age 6–8 weeks were utilized under the approval of the Animal Use and Care Administrative Advisory Committee, University of California, Davis. Under general anesthesia, one construct (treated with C-ABC and intermittent TGF-β1) and one cartilaginous bovine explant were implanted in a subcutaneous pouch, one in each side of the thorax, in a random fashion. The mice were humanely sacrificed at 4 weeks and the implanted tissues were harvested.
Statistical analysis
Biochemistry and mechanical testing data (n=6) were analyzed using a two-factor ANOVA of the factors C-ABC treatment or TGF-β1 treatment with p<0.05. Post-sacrifice and pre-implantation data were compared using an unpaired, one-tail t-test. PCR data (n=3) were analyzed using a one-factor ANOVA with p<0.05. SEM diameters were analyzed using repeated measures ANOVA, with 9 data points per sample (n=3). Student Newman–Keuls post-hoc test was applied when warranted. The interaction term of a two-factor ANOVA was used to test for additive or synergistic effects as described previously [24]. All data are shown as mean ± standard deviation.
Results
Gross morphology and histology
Neocartilage was assessed at 4 weeks via histology and morphology to assess the gross properties and composition of the neotissue (Fig. 1). All constructs, which originated from 5 mm wells, showed no contraction and exhibited the following diameters: 5.97±0.16, 5.10±0.08, 5.32±0.11, and 5.03±0.09 mm for control, C-ABC, TGF-β1, and combined treatments, respectively. Both treated and control neotissue exhibited uniform staining for GAGs and total collagen. Additionally, IHC showed that all constructs stained positive for collagen II but negative for collagen I. The presence of collagen II, GAGs, and total collagen but not collagen I demonstrates that both control and treated groups maintained normal cartilage phenotype.
Figure 1.
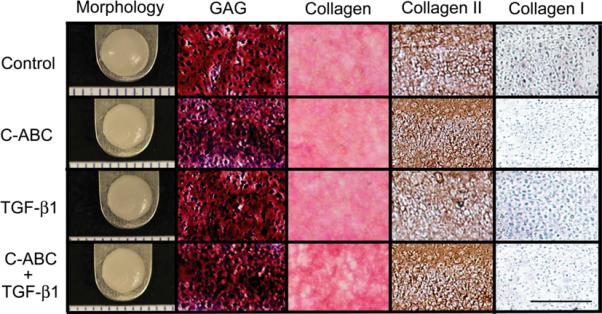
Histology and gross morphology at 4 weeks. Neocartilage treated with chondroitinase-ABC (C-ABC), intermittent TGF-β1, or C-ABC combined with TGF-β1. Histology showed that control and treated constructs had uniform distributions of glycosaminoglycans (GAGs), total collagen, and collagen II without exhibiting collagen I staining. Picrosirius Red was used to stain total collagen and Saf-O/fast green was used for GAG staining. IHC was employed to assess collagen I and collagen II distributions. Scale bar is 200 μm for histology images and gross morphology markings are 1 mm.
Biochemical properties
To investigate maturational growth of the matrix, the amount of collagen and GAGs was quantified at 4 weeks. GAG content (Fig. 2D) was highest for growth factor only groups, which exhibited values of 7.0±0.8%, 8.5±0.5%, and 9.2±0.8% for control, continuous TGF-β1, and intermittent TGF-β1, respectively. Groups treated with C-ABC had lower GAG contents except for the intermittent TGF-β1 group, which was not statistically different than the control value. Combined treatment synergistically increased collagen content (Fig. 2C), reaching 18.6±1.9% and 16.2±1.1% for continuous and intermittent TGF-β1 treatment, respectively. These results showed that combining TGF-β1 and C-ABC synergistically promotes maturation of matrix composition.
Figure 2.
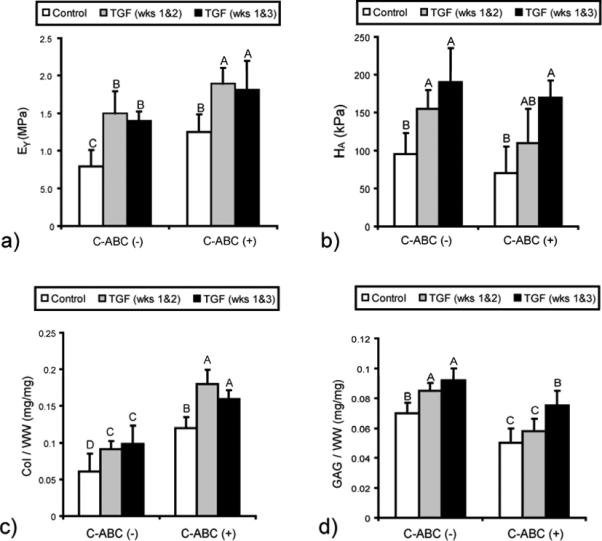
Biomechanical and biochemical properties at 4 weeks. Neocartilage was treated with chondroitinase-ABC (C-ABC), TGF-β1, or C-ABC and TGF-β1. (a) Tensile stiffness was enhanced by C-ABC and TGF-β1 treatments and additively increased for combined treatment groups. (b) Compressive stiffness was highest for groups treated with intermittent TGF-β1. (c) Combined C-ABC and TGF-β1 treatment synergistically increased collagen content. (d) GAG content was highest for groups treated with TGF-β1. Bars labeled with different letters exhibit significant differences (p < 0.05).
Biomechanical properties
To quantify the influence of C-ABC and TGF-β1 on biomechanics, the aggregate modulus in compression and Young's modulus (EY) in tension were determined. The compressive stiffness was highest for groups treated with intermittent TGF-β1 (Fig. 2B), which attained values of 170±22 kPa and 190±45 kPa when treated with and without CABC, respectively. The aggregate modulus was lower for continuous TGF-β1 treatment and lowest for groups receiving no TGF-β1 treatment. The higher compressive stiffness for intermittent TGF-β1 treatment suggested that growth factor administration increased compressive properties following C-ABC treatment, which initially results in negligible residual compressive stiffness due to GAG depletion. Enhanced mechanical properties reflected the observed changes in biochemical composition.
Tensile testing showed that combining C-ABC and TGF-β1 treatments additively increased the tensile stiffness. Tensile stiffnesses were 0.89±0.22, 1.33±0.23, 1.51±0.29, and 1.40±0.12 MPa for control, C-ABC, continuous TGF-β1, and intermittent TGF-β1 treatments, respectively. Young's moduli were highest for combined treatments, which reached 1.95±0.21 MPa and 1.83±0.38 MPa for continuous and intermittent treatment, respectively (Fig. 2A). The tensile stiffnesses for C-ABC in combination with TGF-β1 showed additive increases when compared to individually applied stimuli.
Comparison to native cartilage
A functionality index (FI) was developed to quantify the similarity between neocartilage and native tissue, which was also tested as a positive control. As shown in Eq. 1, the FI equally weights the compressive stiffness (EC), tensile stiffness (ET), GAG content (G), and collagen content (C). The subscripts `nat' and `sac' represent values for native and self-assembled cartilage, respectively.
| (1) |
Native values were 12.1 MPa, 0.2 MPa, 5.5%, and 15% for Young's modulus, aggregate modulus, GAG/ww, and collagen/ww, respectively. The FI yields a score of 1 when neocartilage properties are equivalent to those of native tissue. FI values were 0.41, 0.52, 0.78, and 0.84 for control, C-ABC, intermittent TGF-β1, and combined treatments, respectively. These FI values suggest that combined treatment produces neotissue closest to native cartilage.
Microarray analysis
To elucidate the mechanisms underlying the effects of C-ABC and TGF-β1 on cartilage formation, microarray analysis was conducted at 2 weeks for control, C-ABC, continuous TGF-β1, and combined treatment. The number of differentially expressed genes relative to control neotissue was 191, 548, and 537 for C-ABC, TGF-β1, and combined treatments, respectively. To understand gene expression changes on a more functional level, we used these differentially expressed gene lists to determine if any genetic pathways were enriched.
For both TGF-β1 treatments, the only significantly enriched pathway was MAPK signaling, which was observed for TGF-β1 treatment (19 genes, p = 0.0003) and combination treatment (19 genes, p = 0.00005). There was substantial overlap between the MAPK genes differentially regulated by TGF-β1 and combination treatments, with each group only having one distinct gene (Table 1). Both groups showed up-regulation of MAPK intermediates relating to p38 signaling including MAPKK3, MAP3K5, MAP3K6, and MAPK13. In addition, in both groups receiving TGF-β1 treatment TGF-β1 was up-regulated, while TGF-β3 and TGF-β receptor type II were down-regulated. The differential regulation of TGF-β1 and its receptor could suggest a feedback mechanism in response to TGF-β1 treatment. The enriched MAPK signaling observed for both groups receiving growth factor treatment could play a role in the observed increases in matrix production, which could subsequently enhance the mechanical properties of the neocartilage.
Table 1.
Differentially expressed genes in the MAPK pathway. Microarray analysis was used to determine genes that were differentially expressed in 2 week self-assembled neocartilage treated with TGF-β1 (during weeks 1&2) or with chondroitinase-ABC (C-ABC) and TGF-β1. These differentially expressed genes significantly enriched the MAPK pathway. Fold changes are relative to control values. Dashes represent no differential expression for that gene.
| TGF-β1 | C-ABC + TGF-β1 | |||
|---|---|---|---|---|
| Gene | FC | Regulation | FC | Regulation |
| RAS p21 activator 2 | 2.1 | up | 2.2 | up |
| Dual specificity phosphatase 4 | 2.8 | up | 2.5 | up |
| FGF 2 | 4.0 | up | 4.1 | up |
| MAPK 8 interacting protein 1 | 4.6 | up | 3.9 | up |
| MAP2K 3 | 4.1 | up | 3.9 | up |
| protein tyrosine phosphatase, type 7 (PTP) | 5.5 | up | 3.3 | up |
| TGF-β1 | 6.6 | up | 6.9 | up |
| calcium channel, β3 subunit | 2.2 | down | 2.5 | down |
| FGF 13 | 3.2 | up | 3.4 | up |
| FGF 18 | 2.3 | up | 3.2 | up |
| MAPK 13 | 3.2 | up | 2.1 | up |
| MAP3K 5 | 3.2 | down | 2.6 | down |
| MAP3K 6 | 5.1 | up | 5.4 | up |
| p21 protein-activated kinase 1 | 2.6 | up | 2.8 | up |
| PDGF receptor | 3.2 | down | 3.6 | down |
| tyrosine phosphatase - receptor type 5 | 14.4 | up | 13.7 | up |
| TGF-β receptor II | 2.6 | down | 2.7 | down |
| TGF-β3 | 2.4 | down | 2.1 | down |
| RAS guanyl releasing protein 4 | 4.5 | up | - | - |
| MAPK serine/threonine kinase 1 | - | - | 2.3 | up |
In contrast to TGF-β1 administration, C-ABC treatment did not significantly enrich genetic pathways. The number of differentially regulated genes was also substantially lower for C-ABC treatment, which only altered the expression of approximately 35% as many genes as TGF-β1 application. Furthermore, the TGF-β1 and combined treatments had nearly identical gene expression patterns, both in terms of the number of genes and specific genes that were differentially expressed. This trend was also observed for matrix genes (Table 2), which exhibited similar expression patterns for both growth factor treatments. The lower number of differentially expressed genes and lack of enriched pathways suggested that C-ABC did not influence gene expression significantly, in contrast to the response to the TGF-β1 treatments.
Table 2.
Differentially expressed matrix genes. Microarray analysis was used to determine genes that were differentially expressed in 2 week self-assembled neocartilage treated with chondroitinase-ABC (C-ABC), TGF-β1 (during weeks 1&2), or C-ABC and TGF-β1. Fold changes are relative to control values. TGF-β1 treatment up-regulated the expression of several cartilage matrix molecules including collagen II, collagen VI, collagen XI, and aggrecan while concomitantly decreasing the expression of collagen I.
| C-ABC | TGF-β1 | C-ABC + TGF-β1 | ||||
|---|---|---|---|---|---|---|
| Gene | FC | Regulation | FC | Regulation | FC | Regulation |
| collagen I | 1.1 | down | 4.5 | down | 5.0 | down |
| collagen II | 1.3 | up | 10.8 | up | 12.3 | up |
| collagen VI | 1.4 | up | 2.4 | up | 3.5 | up |
| collagen XI | 7.1 | up | 39.7 | up | 39.0 | up |
| aggrecan | 1.3 | up | 4.5 | up | 6.1 | up |
To validate microarray data, real time PCR was employed to quantify the expression of intermediates involved in MAPK signaling including MAP3K5, MAPK13, protein tyrosine phosphatase (PTP), and MAP2K3 (Fig. 3). Both TGF-β1 alone and combination treatments increased the expression of MAPK13, protein tyrosine phosphatase (PTP), and MAP2K3 while down-regulating MAP3K5. Additionally, the relative gene expression values were significantly lower for C-ABC, suggesting that TGF-β1 treatment altered the expression of MAPK intermediates more so than C-ABC. These expression trends mirrored the microarray data.
Figure 3.
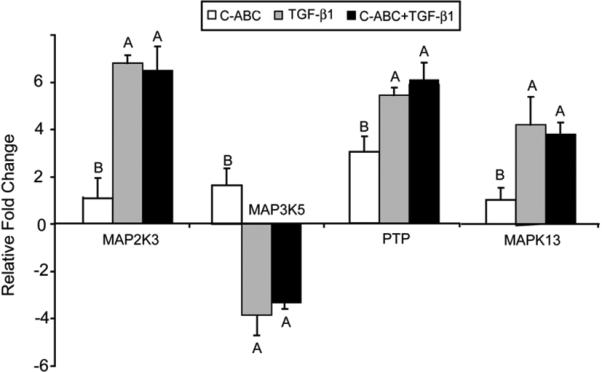
PCR of MAPK signaling genes in neocartilage treated with C-ABC and/or TGF-β1 at 2 weeks. Gene expression levels for MAP3K5, MAPK13, protein tyrosine phosphatase (PTP), and MAP2K3 were normalized to control values. Combined treatments and TGF-β1 treatments generally had gene expression levels that were not statistically distinct. Negative values reflect down-regulation in relation to control. Bars labeled with different letters exhibit significant differences (p < 0.05).
SEM analysis
The lack of genetic effects due to C-ABC treatment prompted further investigation of the role of C-ABC. It was hypothesized that C-ABC acted via a biophysical mechanism to influence tensile properties. Specifically, because collagen plays a major role in the tensile properties of cartilage, we considered that C-ABC was modulating the collagen network at the biophysical level, subsequently enhancing tensile properties. Several aspects of the collagen network contribute to its mechanics including fibril organization, fibril diameter, and post-translational modifications such as pyridinoline crosslinking. Polarized light microscopy showed that C-ABC did not produce any detectable collagen organization (data not shown). Because C-ABC did not appear to be promoting collagen orientation, we decided to also investigate collagen fibril diameters.
Our previous work showed that C-ABC treatment depletes the small proteoglycan decorin [5], which inhibits fibril growth, so it was hypothesized that C-ABC application would increase collagen fibril diameter. Control, intermittent TGF-β1, C-ABC, and combined treatments were imaged using SEM at 4 weeks (Fig. 4A). SEM image analysis showed that mean fibril diameters were 43.3±8.6 nm, 40.1±6.2 nm, 64.4±7.8 nm, and 59.2±4.3 nm for control, TGF-β1, C-ABC, and combined treatments, respectively (Fig. 4B). These values corresponded to a 50% increase in fibril diameter due to C-ABC treatment. TGF-β1 treatment alone did not significantly alter fibril diameter. Similar trends were observed for the density of fibrils, which was computed as the percent area occupied by collagen. Fibril density increased by 36% and 29% following C-ABC and combined treatments, respectively. These results indicated that CABC treatment was increasing fibril diameter and density, while TGF-β1 treatment did not have a significant influence.
Figure 4.
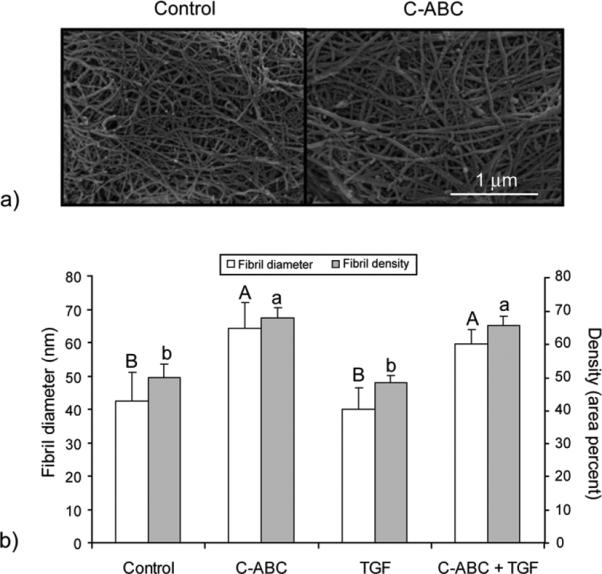
SEM analysis of neotissue at 4 weeks. (a) Representative SEM images of control neotissue and neocartilage treated with chondroitinase-ABC (C-ABC). (b) Quantification of fibril diameters based on image analysis of 3 locations on each neotissue sample with n=3. Bars labeled with different letters exhibit significant differences (p < 0.05).
In vivo maturation
To assess any changes in morphological, biochemical, or biomechanical properties following in vivo culture, neotissue (grown for 4 weeks in vitro and treated with C-ABC and intermittent TGF-β1) and native cartilage controls were implanted subcutaneously in athymic mice. Mice were sacrificed at 4 weeks after implantation (8 weeks total neotissue growth) and all samples were removed without complications. Both neocartilage and explants exhibited a cartilage phenotype as evidenced by positive staining for collagen II and negative staining for collagen I. Although both constructs and explants had the same diameters upon implantation, explants showed lower diameters and increased thicknesses post-sacrifice. Post-sacrifice diameters were 4.9±0.16 mm and 5.8±0.09 mm for explants and neocartilage, respectively. Respective thicknesses for explants and neocartilage were 1.82±0.14 mm and 1.25±0.07 mm.
Neocartilage constructs exhibited both biochemical and biomechanical maturation in vivo. Post-sacrifice explant tensile stiffness decreased (p=0.02) and the aggregate modulus remained unchanged (Fig. 5B, 5D). For constructs, tensile stiffness was significantly higher post-sacrifice (p=0.01), exhibiting 3.15±0.47 MPa compared to 1.95±0.62 MPa (Fig. 5B). Increased collagen content (Fig. 5C) paralleled the increased tensile properties: collagen/ww was 12.5±1.8% and 19.0±2.3% for pre-implantation post-sacrifice groups, respectively. Although compressive stiffness did not increase for explants, post-sacrifice neocartilage exhibited enhanced GAG content (Fig. 5E) and increased compressive stiffness (Fig. 5D). These results showed that self-assembled neocartilage matures in vivo, further enhancing the effects noted in vitro.
Figure 5.
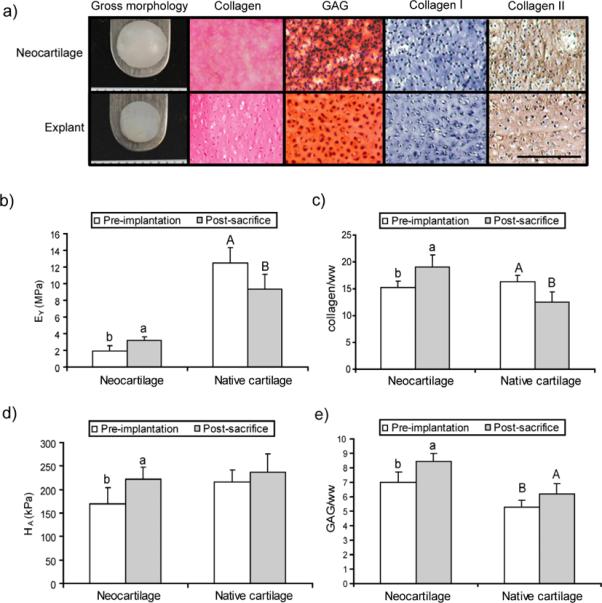
Properties of constructs post-sacrifice after in vivo culture in nude mice. Self-assembled articular cartilage was cultured for 4 weeks with TGF-β1 treatment during weeks 1&3 and chondroitinase-ABC (C-ABC) administration at day 14. Neocartilage was then implanted in nude mice for 4 weeks with condylar cartilage explants as controls (n=4). (a) Gross morphology and histology using Picrosirius Red for collagen, Safranin-O/fast green for glycosaminoglycans, and immunohistochemistry for collagen I and collagen II. Scale bar is 200 μm. (b) Post-sacrifice tensile stiffness increased for neocartilage while explant stiffness decreased. (c) Collagen content increased for constructs post-sacrifice while it decreased for native cartilage. (d) Compressive stiffness increased for constructs but did not significantly change for explants. (e) Glycosaminoglycan (GAG) content increased for both native tissue and neocartilage post-sacrifice. Bars labeled with different letters exhibit significant differences (p < 0.05).
Discussion
This study examined the mechanisms underlying dual anabolic and catabolic treatments for enhancing articular cartilage formation. Results confirmed our hypothesis that combining these agents (C-ABC and TGF-β1) would further enhance tensile properties compared to either treatment acting alone. This study demonstrated that 1) C-ABC and TGF-β1 can be applied to synergistically enhance neotissue formation, 2) TGF-β1 can be applied to aid the recovery of compressive stiffness following C-ABC treatment, 3) TGF-β1 increases MAPK signaling in self-assembled neocartilage, 4) C-ABC treatment increases collagen fibril diameter and density, and 5) neocartilage exhibits stability and maturation in vivo.
Neotissue treated with both TGF-β1 and C-ABC exhibited in vitro maturation, attaining biochemical and biomechanical properties approaching native values. This is particularly exciting for collagen content and tensile properties, which are typically lower for in vitro cartilage [25], For combined treatment groups, the collagen content spanned immature and adult values [3], indicating that TGF-β1 and C-ABC help recapitulate cartilage development. The additive increase of Young's modulus coupled with synergistic increases in collagen content showed the promise of applying TGF-β1 and C-ABC in combination. Collectively, these results indicated that dual treatment promoted in vitro cartilage development, producing neocartilage on par with native tissue, as evidenced by the higher functionality index.
Growth factor administration during week 3 also increased compressive stiffness following C-ABC treatment, which was a concern from prior work [5, 12]. In addition, intermittent TGF-β1 treatment combined with C-ABC treatment supplemented improvements to construct collagen content and tensile properties seen with C-ABC treatment alone. The ability of TGF-β1 to enhance both compressive and tensile properties following C-ABC treatment reinforced the benefits of combining anabolic and catabolic agents during neotissue formation.
Microarray analysis showed that TGF-β1 up-regulated MAPK signaling via p38, suggesting that this is the primary mechanism of TGF-β1 signaling in self-assembled neocartilage. Other work has shown that inhibiting p38 MAPK signaling in chondrocytes represses TGF-β1 gene expression in a dose-dependent manner. Similarly, inhibiting p38 MAPK activity reduced TGF-β-induced proteoglycan synthesis in articular chondrocytes [26], showing that p38 signaling influences biosynthesis. Various studies also showed the role of p38 signaling in chondrogenesis, which was suppressed by p38 inhibition [27]. Furthermore, inhibition of chondrogenesis using epidermal growth factor [28] or retinoic acid [29] both corresponded with reduced p38 activity, reinforcing the role of p38 in cartilage development. These studies highlight the developmental and biosynthetic role of p38 signaling in chondrocytes, which could explain the increased GAG and collagen contents observed in self-assembled neotissue treated with TGF-β1.
C-ABC has been employed to induce maturational growth during cartilage formation, [5, 11, 12] but its mechanism has not been previously interrogated. Unlike TGF-β1, C-ABC treatment does not appear to enrich functionally relevant pathways, suggesting that it did not significantly alter gene expression. The similarity between the gene expression profiles of TGF-β1 and combined treatments reinforced the minimal influence of C-ABC on genetic signaling. Although it is conceivable that C-ABC could indirectly alter gene expression by modulating the bioavailability of signaling molecules or reducing the abundance of matrix molecules that interact with chondrocytes, these effects did not appear to be predominant in self-assembled constructs. Increased tensile properties due to C-ABC treatment without gene expression changes suggested that CABC could have a biophysical mechanism.
Indeed, SEM analysis identified a potential biophysical mechanism underlying CABC treatment by illustrating increased collagen fibril diameters. The increased fibril diameters noted with C-ABC treatment, which parallel the fibril growth that occurs as cartilage matures [25], show that C-ABC promotes maturation of the collagen network. Fibril diameters of C-ABC treated constructs, which were 59±4.3 nm, are on par with the native tissue values of 30–80nm [30, 31]. In addition, fibril diameter has been shown to influence the stiffness of collagen fibers [32], which supports the hypothesis that the increased Young's modulus following C-ABC administration relates to fibril diameter, in addition to increased collagen content. This finding also suggests that strategies such as altering decorin expression could be employed to alter the collagen network. It is well known that small leucine-rich proteoglycans impact collagen fibrillogenesis, so they offer a rich area of research for improving neocartilage.
This study also showed that self-assembled cartilage can mature in vivo. Interestingly, explant tensile properties decreased while neocartilage properties increased. Other work has shown that cartilage explants can swell and subsequently demonstrate reduced tensile properties [33], which could explain why the explants were less stiff and had increased thicknesses. The improved neocartilage properties could result from the nutrient rich environment that in vivo environments provide, which has been shown to improve the properties of implanted engineered tissue [34]. Because the neocartilage was significantly more cellular than native tissue, it could have had a more pronounced response to in vivo nutrients and growth factors. The increased biochemical and biomechanical properties, without concomitant swelling or contraction, suggest that self-assembled cartilage can develop in vivo.
Conclusions
This study showed how anabolic and catabolic agents can be combined to promote in vitro and in vivo neocartilage maturation. TGF-β1 application increased MAPK signaling, which could be responsible for increased biosynthesis and the observed increases in matrix composition. Additionally, this study identified a potential mechanism for the effects of C-ABC on enhanced cartilage formation. The two distinct mechanisms underlying C-ABC and TGF-β1 treatments could help explain the synergistic effects of combining these agents. Based on the maturation that constructs displayed when implanted in mice, in vivo culture presents an exciting opportunity for improving de novo cartilage formation. Future experiments could further investigate the mechanisms underlying the effects of these stimuli and examine the response of self-assembled neocartilage in larger animal models.
Acknowledgements
The authors acknowledge funding support from NIAMS R01 AR053286 and thank Kevin Lockwood, James Cravotta, and Jamie Amaral for assistance during in vivo procedures. The funding source did not have a role in the collection, analysis and interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hoben GM, Hu JC, James RA, Athanasiou KA. Self-assembly of fibrochondrocytes and chondrocytes for tissue engineering of the knee meniscus. Tissue Eng. 2007;13:939–46. doi: 10.1089/ten.2006.0116. [DOI] [PubMed] [Google Scholar]
- [2].Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue Eng. 2006;12:969–79. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- [3].Williamson AK, Chen AC, Masuda K, Thonar EJ, Sah RL. Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–80. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- [4].Williamson AK, Masuda K, Thonar EJ, Sah RL. Growth of immature articular cartilage in vitro: correlated variation in tensile biomechanical and collagen network properties. Tissue Eng. 2003;9:625–34. doi: 10.1089/107632703768247322. [DOI] [PubMed] [Google Scholar]
- [5].Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. Journal of Orthopaedic Research. 2009;27:949–56. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue Eng Part A. 2008;15:1151–8. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hu JC, Athanasiou KA. The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 2006;12:1337–44. doi: 10.1089/ten.2006.12.1337. [DOI] [PubMed] [Google Scholar]
- [9].Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188–98. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- [11].Bian L, Crivello KM, Ng KW, Xu D, Williams DY, Ateshian GA, et al. Influence of temporary chondroitinase ABC-induced glycosaminoglycan suppression on maturation of tissue-engineered cartilage. Tissue Eng Part A. 2009;15:2065–72. doi: 10.1089/ten.tea.2008.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Natoli R, Revell CM, Athanasiou K. Chondroitinase ABC Treatment Results in Increased Tensile Properties of Self-Assembled Tissue Engineered Articular Cartilage. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2008.0478. doi:10.1089/ten.TEA.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fortier LA, Nixon AJ, Mohammed HO, Lust G. Altered biological activity of equine chondrocytes cultured in a three-dimensional fibrin matrix and supplemented with transforming growth factor beta-1. Am J Vet Res. 1997;58:66–70. [PubMed] [Google Scholar]
- [14].van Osch GJ, van der Veen SW, Buma P, Verwoerd-Verhoef HL. Effect of transforming growth factor-beta on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998;17:413–24. doi: 10.1016/s0945-053x(98)90101-9. [DOI] [PubMed] [Google Scholar]
- [15].van Osch GJ, van den Berg WB, Hunziker EB, Hauselmann HJ. Differential effects of IGF-1 and TGF beta-2 on the assembly of proteoglycans in pericellular and territorial matrix by cultured bovine articular chondrocytes. Osteoarthritis Cartilage. 1998;6:187–95. doi: 10.1053/joca.1998.0111. [DOI] [PubMed] [Google Scholar]
- [16].Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis Cartilage. 2009;17:114–23. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- [18].Almarza AJ, Athanasiou KA. Seeding techniques and scaffolding choice for tissue engineering of the temporomandibular joint disk. Tissue Eng. 2004;10:1787–95. doi: 10.1089/ten.2004.10.1787. [DOI] [PubMed] [Google Scholar]
- [19].Athanasiou KA, Agarwal A, Dzida FJ. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. J Orthop Res. 1994;12:340–9. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- [20].Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- [21].Athanasiou KA, Niederauer GG, Schenck RC., Jr. Biomechanical topography of human ankle cartilage. Ann Biomed Eng. 1995;23:697–704. doi: 10.1007/BF02584467. [DOI] [PubMed] [Google Scholar]
- [22].Aufderheide AC, Athanasiou KA. Assessment of a bovine co-culture, scaffold-free method for growing meniscus-shaped constructs. Tissue Eng. 2007;13:2195–205. doi: 10.1089/ten.2006.0291. [DOI] [PubMed] [Google Scholar]
- [23].Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23:425–32. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [24].Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30:723–31. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- [25].Responte DJ, Natoli RM, Athanasiou KA. Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng. 2007;35:363–411. doi: 10.1615/critrevbiomedeng.v35.i5.20. [DOI] [PubMed] [Google Scholar]
- [26].Studer RK, Chu CR. p38 MAPK and COX2 inhibition modulate human chondrocyte response to TGF-beta. J Orthop Res. 2005;23:454–61. doi: 10.1016/j.orthres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- [27].Oh CD, Chang SH, Yoon YM, Lee SJ, Lee YS, Kang SS, et al. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613–9. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- [28].Yoon YM, Oh CD, Kim DY, Lee YS, Park JW, Huh TL, et al. Epidermal growth factor negatively regulates chondrogenesis of mesenchymal cells by modulating the protein kinase C-alpha, Erk-1, and p38 MAPK signaling pathways. J Biol Chem. 2000;275:12353–9. doi: 10.1074/jbc.275.16.12353. [DOI] [PubMed] [Google Scholar]
- [29].Weston AD, Chandraratna RA, Torchia J, Underhill TM. Requirement for RAR-mediated gene repression in skeletal progenitor differentiation. J Cell Biol. 2002;158:39–51. doi: 10.1083/jcb.200112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Langsjo TK, Hyttinen M, Pelttari A, Kiraly K, Arokoski J, Helminen HJ. Electron microscopic stereological study of collagen fibrils in bovine articular cartilage: volume and surface densities are best obtained indirectly (from length densities and diameters) using isotropic uniform random sampling. J Anat. 1999;195(Pt 2):281–93. doi: 10.1046/j.1469-7580.1999.19520281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weiss C, Rosenberg L, Helfet AJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am. 1968;50:663–74. doi: 10.2106/00004623-196850040-00002. [DOI] [PubMed] [Google Scholar]
- [32].Christiansen DL, Huang EK, Silver FH. Assembly of type I collagen: fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000;19:409–20. doi: 10.1016/s0945-053x(00)00089-5. [DOI] [PubMed] [Google Scholar]
- [33].Asanbaeva A, Tam J, Schumacher BL, Klisch SM, Masuda K, Sah RL. Articular cartilage tensile integrity: modulation by matrix depletion is maturation-dependent. Arch Biochem Biophys. 2008;474:175–82. doi: 10.1016/j.abb.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Calve S, Lytle IF, Grosh K, Brown DL, Arruda EM. Implantation increases tensile strength and collagen content of self-assembled tendon constructs. J Appl Physiol. 2010;108:875–81. doi: 10.1152/japplphysiol.00921.2009. [DOI] [PubMed] [Google Scholar]


