Abstract
Roles of complement factors in prion infection of the central nervous system remain unclear. In this study, we assessed the strain-dependent reactivity of complement factors in prion infections of Neuro2a (N2a) cells and mouse brains. N2a cells persistently infected with either Chandler or 22L scrapie strains were cultured in the presence of normal mouse serum (NMS), followed by staining with the phosphatidylserine binding protein and early apoptosis marker Annexin V. The proportion of Annexin V positive cells was increased both in Chandler- and 22L-infected cells. Preincubation of NMS with anti-C1q, C3 and/or C9 antibodies reduced Annexin V positive cells in Chandler-infected cells, while only anti-C3 antibodies were effective on 22L-infected cells. The immunohistochemistry showed that deposition of C1q and C3 was different between Chandler- and 22L-infected mouse brains. These results indicate that the reactivity of complement factors differs between prion strains both in vitro and in vivo.
Keywords: prion, scrapie, complement factors, strain difference
Introduction
The complement system plays key roles in the immune system including regulation of immune reactions and the elimination of phagocytosed antigens, immune complexes, tumor cells and apoptotic cells. Complement factors also have multiple functions for synapse remodeling (Stevens et al., 2007), neurogenesis (Shinjo et al., 2009), cell survival (Soan et al., 1999; 2001; Dashiell et al., 2000) and cell death (Ren et al., 2008). Complement factors also seem to be involved in pathogenesis of neurodegenerative disease such as Alzheimer’s disease (AD). Previous studies showed that β-amyloid, the major constituent of senile plaques, binds C1q and induces complement activation, which may promote either neuroprotection or neurotoxicity (Guan et al., 1994; Webster et al., 1997; Sarvari et al., 2003).
Prion diseases are fatal neurodegenerative disorders including scrapie in sheep and goats, bovine spongiform encephalopathy in cattle, chronic wasting disease in cervids and Creutzfeldt-Jakob disease in humans. These diseases are characterized in the central nervous system (CNS) by deposition of abnormal forms of prion protein (e.g. PrPSc), vacuolation of neural tissue, astrocytosis and microglial activation. Previous studies using C1q, factor B/C2 or C3 depleted mice (Klein et al., 2001; Mabbott et al., 2001) have implicated the involvement of these complement factors in the spread of prions from peripheral tissues to CNS. Klein et al. (2001) and Zabel et al. (2007) showed that complement receptor CD21/35 on follicular dendritic cells has an important role in lymphoid prion accumulation and neuroinvasion of prion. Flores-Langarica et al. (2009) demonstrated that C1q is involved in PrPSc uptake into conventional dendritic cells, which have an important role in the prion propagation from the peripheral tissue to the CNS. Direct binding of C1q to amyloid fibrils, beta-oligomers prepared from human or mouse recombinant PrP and purified PrPSc, resulting in activation of the classical complement pathway, has been demonstrated in vitro, suggesting that prion infection induces complement activation (Blanquet-Grossard et al., 2005; Dumestre-Perard et al., 2007; Mitchell et al., 2007; Sim et al., 2007; Sjoberg et al., 2008; Erlich et al., 2010).
Klein et al. (2001) and Mabbott et al. (2001) suggested that complement factors seem to be less important in the CNS than the periphery, because depletion of either C1q, factor B/C2 or C3 did not affect the survival period of mice intracerebrally infected with Chandler and ME7 scrapie. Mabbott and Bruce (2004) showed that the incubation periods of C5 deficient mice infected with ME7 and 79A scrapie via intracerebral or peripheral route were similar to those of wild type mice. However, there still remains the possibility that complement factors are involved in neuropathogenesis of prion diseases. Association of complement factors with amyloid plaques of human prion disease was demonstrated by immunohistochemistry (Ishii et al., 1984; Kovacs et al. 2004). mRNA levels of C1q and C3 increase in the brains of mice intracerebrally infected with Chandler, 22L or ME7 strains in the pre-clinical phase of the disease, indicating that expression of complement factors are altered in the early stage of the neuropathogenesis in some prion strains (Dandoy-Dron et al., 1998; Skinners et al., 2006; Hwang et al., 2009).
In this study, we have further assessed the possible involvement of complement factors in the neuropathogenesis of prion disease using murine neuroblastoma (N2a) cells and mice infected with Chandler and 22L scrapie strains. Our data suggest that complement factors induce translocation of phosphatidylserine in the plasma membrane of prion-infected N2a cells and that the reaction of complement components varies with prion strain.
Results
Normal mouse serum treatment induces degenerative change in scrapie-infected N2a cells
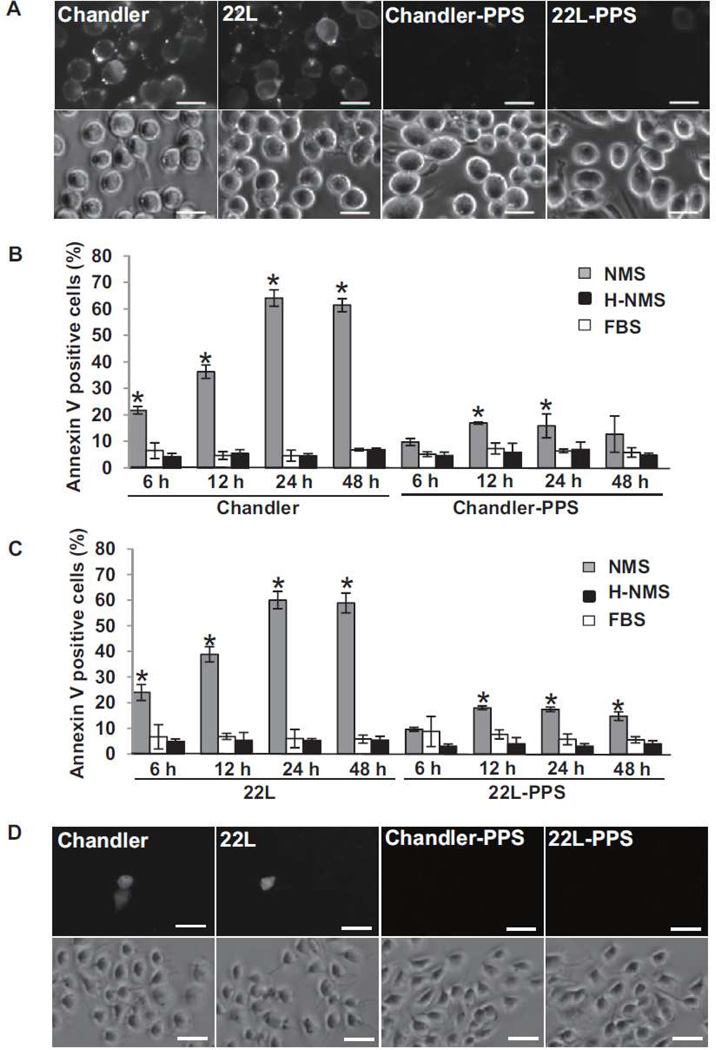
To assess the possibility that complement factors react on scrapie-infected cells, we used N2a cells persistently infected with Chandler or 22L strains. For uninfected negative controls, we cured the scrapie infection in these cell lines using pentsan polysulfate (PPS). The cells were treated with normal mouse serum (NMS), heat-inactivated NMS (H-NMS) or fetal bovine serum (FBS) for 6, 12, 24 and 48 h (Fig 1). NMS contains almost all murine complement components, whereas these factors are inactivated in H-NMS and absent in FBS. After these treatments, the cells were stained with Annexin V, a protein that labels phosphatidylserine in the outer leaflet of the plasma membrane as a marker of an early stage of apoptosis (Koopman et al, 1994). Time dependent increases in Annexin V-positive cells were observed only in the cultures treated with NMS, culminating in much higher percentages of positive cells in the Chandler- and 22L-infected N2a cultures (60–64%) than in the PPS-cured cultures (14–18%). These results indicated that NMS treatment induced degenerative change in Chandler- and 22L-infected N2a cells to an extent that was enhanced ~4-fold by scrapie infection. Less than 10% of the cells treated with H-NMS and FBS were Annexin V positive, indicating that the NMS factors mediating the increase in Annexin V positivity were heat sensitive, as is known to be true for complement factors. Moreover, a similarly low percentage of FBS-treated cells were Annexin V-positive, indicating that bovine serum factors cannot be substituted for murine factors in mediating the observed degenerative changes. To assess if cell death occurs in these cells or not, the cells were incubated with propidium iodide (PI) at 24 h after NMS treatment (Fig. 1D). Although PI uptake was slightly increased in the Chandler- and 22L-infected N2a cells compared to PPS-cured cells, less than 10% of the cells were positive for PI, suggesting that translocation of phosphatidylserine hardly resulted in cell death.
Fig. 1.
Effects of treatment of scrapie-infected N2a cells with NMS. (A) Annexin V staining on N2a cells treated with NMS. Chandler-infected, 22L-infected or PPS-cured (from Chandler or 22L infection, respectively) N2a cells were seeded in 24 well plates and cultured for 2 days. The cells were treated with 10% NMS for 24 h and stained with Annexin V. Bars show 20 µm. (B) Time course of the percentage of Annexin V positive cells in Chandler-infected and PPS-cured N2a cells. The cells were treated with NMS, H-NMS or FBS for 6, 12, 24 and 48 h and stained with Annexin V. The graphs show the means of the proportion of Annexin V positive cells in three fields. The error bars show standard deviation (SD). Gray bars, NMS treated cells. White bars, HS treated cells. Black bars, FBS treated cells. *p<0.01 vs H-NMS or FBS-treated cells (Student’s t test). The results are representative of 3 independent experiments. (C) Time course of the percentage of Annexin V positive cells in 22L-infected and PPS-cured N2a cells. The graphs show the mean of the proportion of Annexin V positive cells in three fields. The error bars show SD. Gray bars, NMS treated cells. White bars, H-NMS treated cells. Black bars, FBS treated cells. *p<0.01 vs HS or FBS-treated cells (Student’s t test). The results are representative of 3 independent experiments. (D) PI uptake into N2a cells treated with NMS. Chandler-infected, 22L-infected or PPS-cured (from Chandler or 22L infection, respectively) N2a cells were seeded in 24 well plates and cultured for 2 days. The cells were treated with 10% NMS for 24 h and stained with PI. Bars show 50 µm.
Different complement factors are involved in NMS-induced degenerative changes in Chandler- and 22L-infected N2a cells
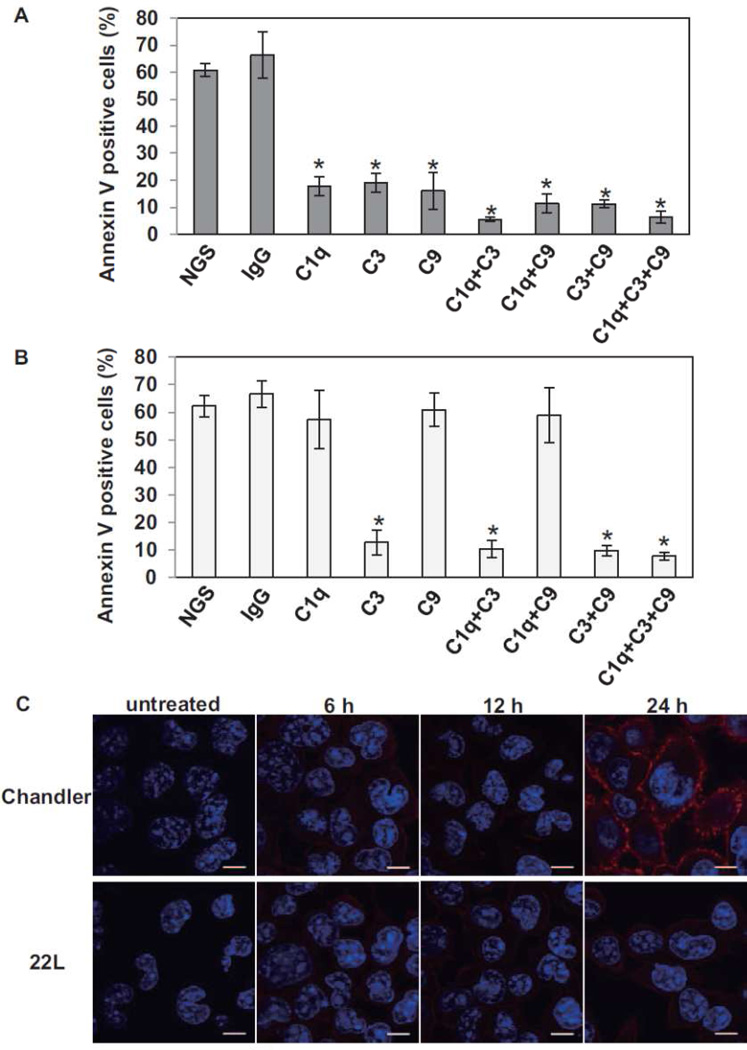
To further examine the role of murine complement factors in the NMS-induced degenerative changes in Chandler- and 22L- infected N2a cells, the NMS was pretreated with anti-C1q, C3, C9 antibodies or mixture of these antibodies. Because all of the antibodies were derived from goats, we used normal goat serum (NGS) and anti-mouse IgG goat serum as controls. In Chandler-infected N2a (Fig. 2A), the pretreatment with individual anti-C1q, C3 or C9 antibodies reduced the proportion of Annexin V positive cells (p<0.01 vs pretreatment with NGS or anti-mouse IgG, Student’s t test). The mixtures of anti-C1q+C3 and anti-C1q+C3+C9 were even more effective than the individual anti-C1q, C3 or C9 antibodies (p<0.01, Student’s t test), suggesting that multiple complement components are involved in the degeneration of Chandler-infected N2a cells. In contrast, in 22L-infected cells (Fig. 2B), the anti-C1q and anti-C9 antibodies were ineffective, while the anti-C3 antibody alone reduced the percentage of Annexin V positive cells as effectively as combinations of antibodies to C1q+C3, C3+C9 and C1q+C3+C9. These results suggested that only C3 was involved in the degenerative change of 22L-infected N2a cells. The involvement of multiple complement factors in the induction of Annexin V positivity in Chandler-infected N2a cultures (Fig. 2A) raised the possibility that membrane attack complex (MAC) formation occurs on the plasma membrane on these cells. To assess this possibility, we performed immunocytochemistry for MAC on Chandler-, 22L-infected (Fig. 2C) or PPS-cured N2a cells (data not shown). In Chandler-infected N2a cells, MAC was detected at 24 h after treatment, suggesting that MAC formation occurred on Chandler-infected N2a cells, but not on 22L-infected and PPS-cured N2a cells.
Fig. 2.
Effects of pre-incubation of NMS with anti-complement antibodies on the number of Annexin V positive cells in Chandler- (A) and 22L-infected N2a cells (B). NMS were incubated with anti-C1q, C3 and/or C9 goat serum for 3 h at 4 °C. Then Chandler- or 22L-infected cells were treated with the NMS. For the control, NMS were treated with normal goat serum (NGS) or anti-mouse IgG goat serum (IgG). The graphs show the means of the proportions of Annexin V positive cells in three fields. The error bars show SD. *p<0.01 vs NGS or IgG-treated NMS (Student’s t test). The results are representative of 3 independent experiments. (C) MAC formation on Chandler- and 22L-infected N2a cells after NMS treatment. The cells were treated with NMS for 6, 12 and 24 h, fixed and subjected to immunocytochemistry for MAC. Red, MAC. Blue, DAPI. Bars show 10 µm.
C1q colocalizes with PrP in Chandler-infected N2a cells
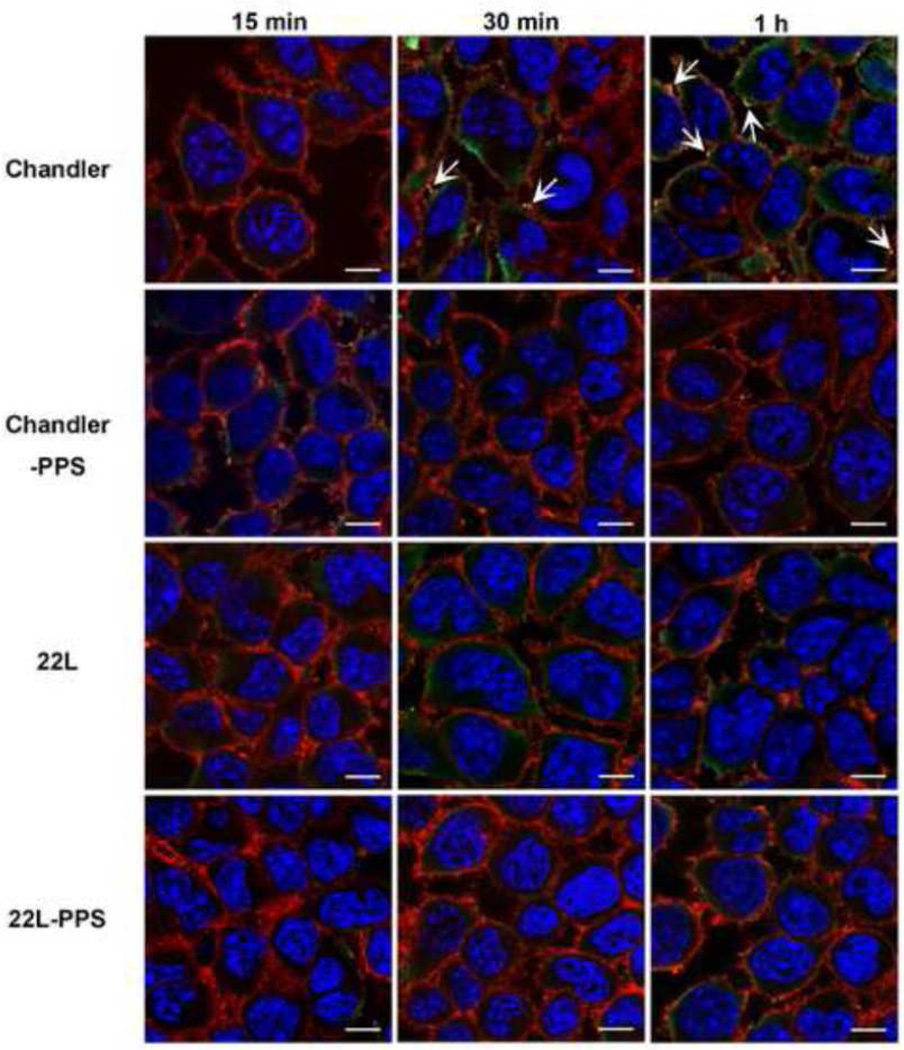
To examine if C1q is associated with PrP in scrapie-infected or PPS-cured N2a cells after exposure to NMS, we performed double staining of C1q (green) and PrP (red) under non-denaturing condition (Fig. 3). In Chandler-infected N2a cells, colocalization of C1q with PrP (yellow) was detected at 30 min and 1 h. In 22L-infected cells, colocalization was not detected at any time point and C1q staining seemed localized in the cytoplasm more than on the cell surface. C1q staining was hardly detected in PPS-cured cells at any time point. No C1q staining was detected in untreated cells (data not shown). These results suggest that PrP on Chandler-infected N2a cells was associated with C1q on the cell surface.
Fig. 3.
Localization of PrP and C1q in scrapie-infected N2a cells treated with NMS. The cells were treated with NMS for 15, 30 min or 1h at 37°C and fixed with 4% PFA 4% sucrose for double immunostaining of PrP (red) and C1q (green). The nuclei were counterstained with DAPI (blue). Bars show 10 µm. Arrows show merged signals.
C3 colocalizes with PrP in 22L-infected N2a cells
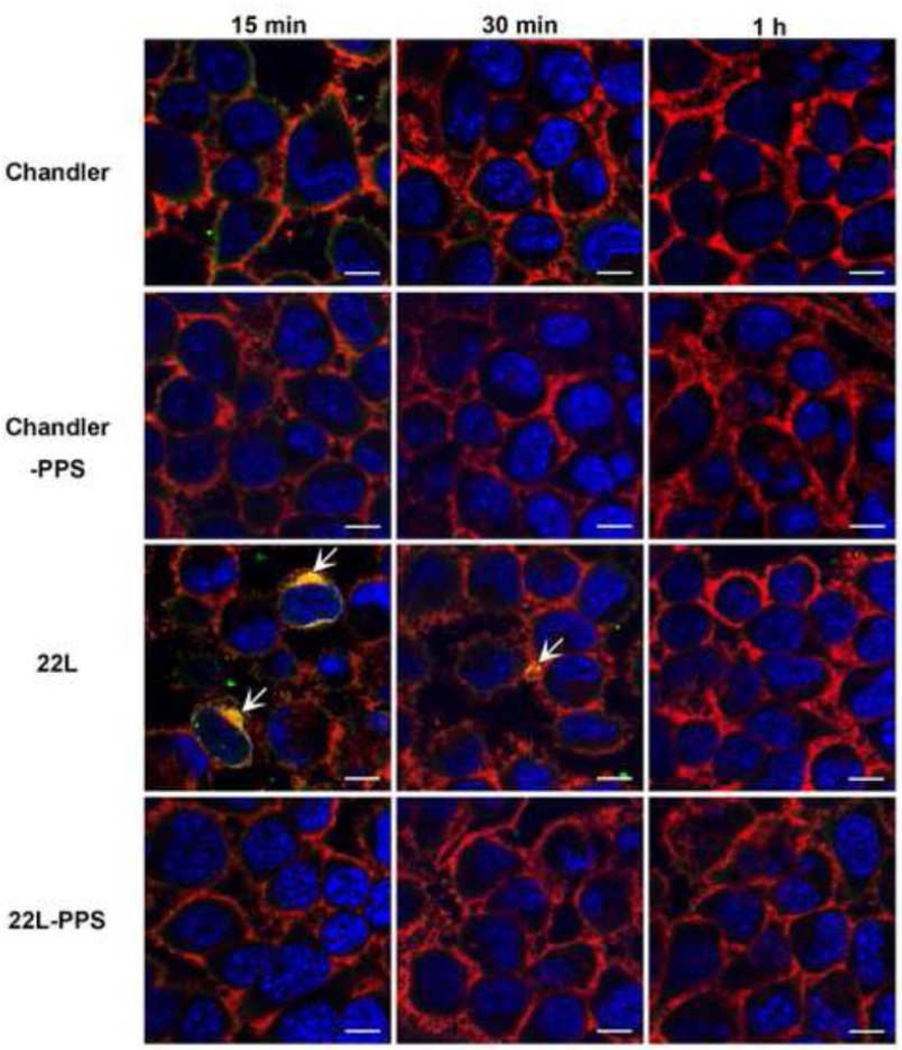
Next we analyzed the association of C3 with PrP in scrapie-infected or PPS-cured N2a cells (Fig. 4). In 22L-infected cells, colocalization of C3 and PrP was detected in the cytoplasm and on the cell surface at 15 and 30 min after NMS treatment. Although C3 staining was observed rarely in Chandler-infected or PPS-cured N2a cells, no colocalization was detected. No staining was detected in untreated cells (data not shown). These results suggest that PrP on 22L-infected N2a cells was associated with C3 on the cell surface and in the cytoplasm.
Fig. 4.
Localization of PrP and C3 in scrapie-infected N2a cells treated with NMS. The cells were treated with NMS for 15, 30 min, or 1h and fixed with 4% PFA 4% sucrose in PBS for double immunostaining of PrP (red) and C3 (green). The nuclei were counterstained with DAPI (blue). Bars show 10 µm. Arrows show merged signals.
Distribution of C1q and C3 in scrapie-infected mouse brain
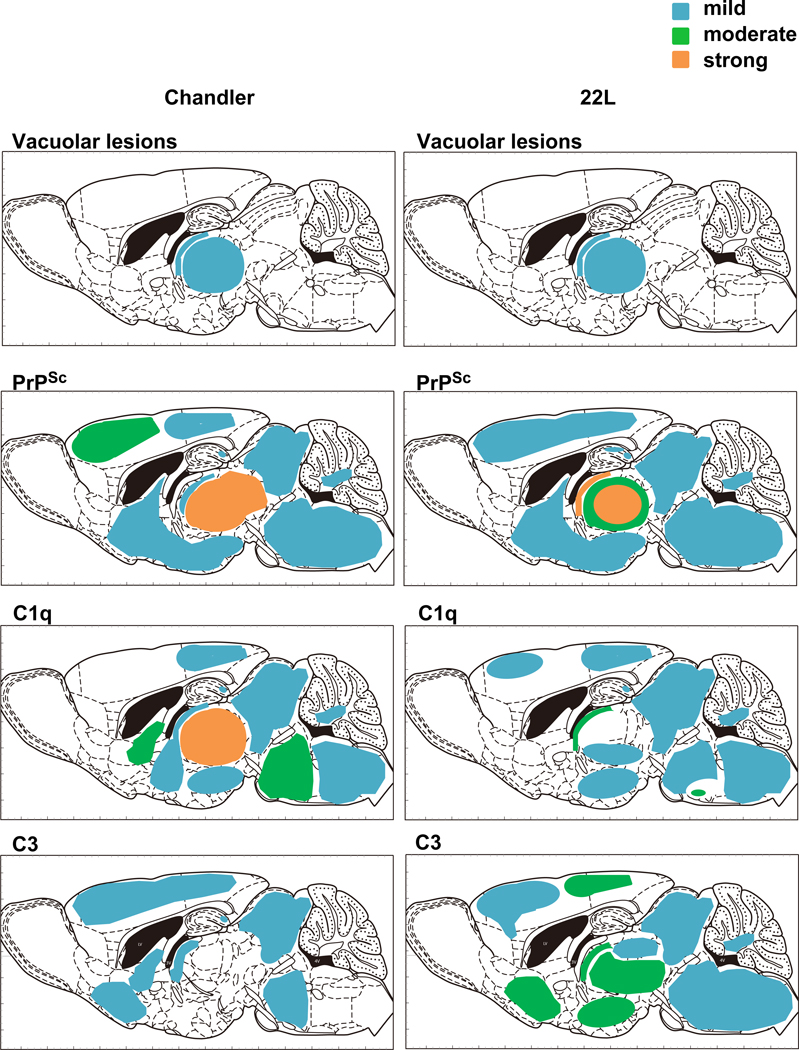
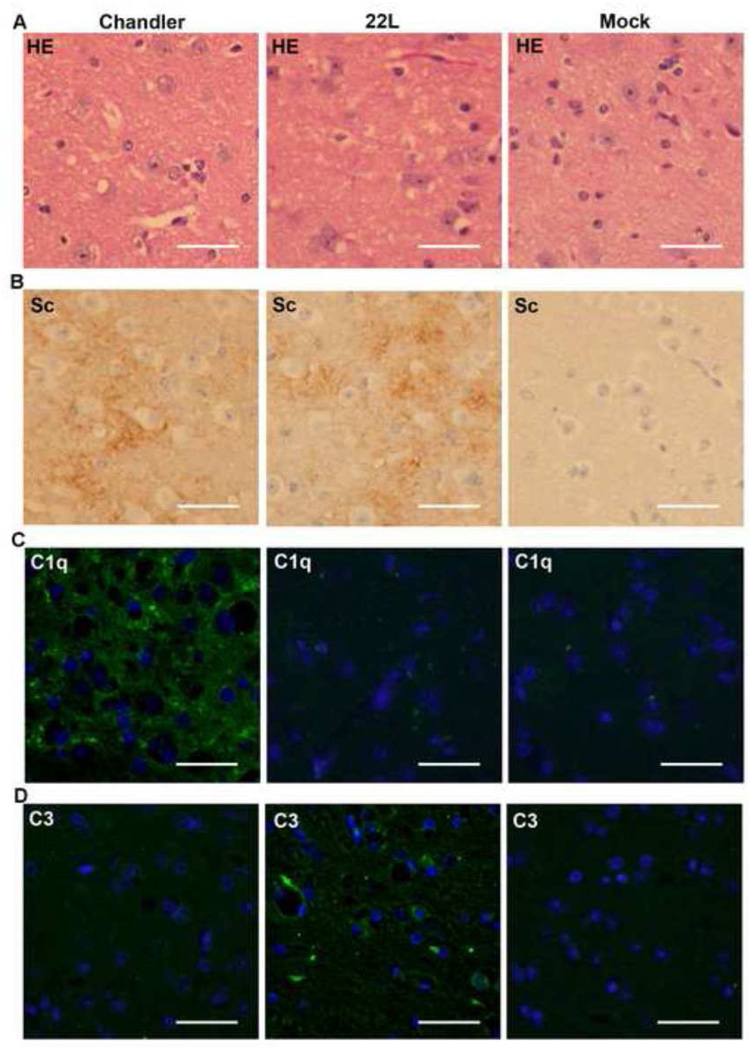
We also analyzed the distributions of vacuolar lesions, PrPSc, C1q and C3 in Chandler-, 22L- or mock-infected mouse brains (Figs. 5 & 6). At 90 dpi (pre-clinical stage of the disease) in Chandler-infected mouse brains, mild vacuolar degeneration was observed in the thalamus. PrPSc was strongly detected in thalamus, moderately in secondary motor cortex and mildly in retrosplenial agranular cortex, septum, CA1 of hippocampus, midbrain, pons and medulla of cerebellum. The C1q distribution was almost consistent with PrPSc except in the secondary motor cortex. Mild deposition of C3 was detected in cerebral cortex, septum, stria medullaris of thalamus, midbrain and pons. Vacuolar lesions in the major part of thalamus lacked deposition of C3 (Fig.6). In 22L-infected mouse brains at 90 dpi, mild vacuolar lesions and PrPSc were distributed similar to those in Chandler-infected mice. However, in contrast to the Chandler-infected mice, the dorsal part of thalamus lacked C1q immunoreactivity, despite the presence of vacuolar lesions and PrPSc (Fig. 6). Moreover, C3 staining was much more pronounced in the thalamus (Fig. 6) and also was distributed widely throughout other regions including the hypothalamus, stria terminalis, septum, cerebral cortex, midbain, pons, medulla oblongata and medulla of cerebellum (Fig. 5). These results suggested that there were differences in complement activation between Chandler- and 22L-infected mouse brains at 90 dpi. At 133 and 166 dpi, C1q and C3 signals were widely distributed both in Chandler- and 22L-infected infected mouse brains and there was no substantial difference between the two scrapie strains (data not shown). Neither C1q nor C3 signal was detected in mock-infected mouse brains at any time points (Fig. 6).
Fig. 5.
Summary of distribution of vacuolation, PrPSc, C1q and C3 of Chandler- or 22L-infected mouse at 90 dpi. The colors indicate the severity of vacuolation or relative intensity of PrPSc, C1q or C3 deposition. Blue, mild; Green, moderate; Orange, strong; Graphics of the sagittal section of mouse brain are from “The Mouse Brain 2nd edition” (Academic Press).
Fig. 6.
Histopathology of the dorsal part of thalamus at 90 dpi. The slides from Chandler-, 22L- or mock-infected mouse brains were subjected to HE staining (A) and immunohistochemistry for the detection of PrPSc (B), C1q (C) and C3 (D). Green, C1q or C3. Blue, DAPI. Bars show 50 µm.
Discussion
Our current data suggest that the reaction of complement factors differs between Chandler and 22L scrapie strains. There are multiple distinct prion strains distinguished by biological and biochemical typing, even though the amino acid sequence of the constituent protein is identical. Strains can differ in incubation period, neuropathology, cellular tropism, the size of protease-resistant PrPSc fragments, PrPSc glycosylation ratios, and PrPSc conformation (Bruce et al., 1989; Bessen and Marsh, 1992; 1994; Caughey et al., 1998; Safar et al., 1998; Kuczius and Groschup, 1999; Mahal et al., 2007). In this study, we used two mouse-adapted scrapie strains, Chandler and 22L. These two strains can be distinguished by biochemical typing, such as PrPSc glycoform profiles, replication efficiency in cell culture, and conformation (Nishida et al., 2000; Atarashi et al., 2006; Mahal et al., 2007; Sim and Caughey, 2009; Baron et al., 2011). In contrast, biological properties of these strains, such as incubation period, distribution of pathological lesions and deposition of PrPSc, are similar, although the vacuolar lesions in the cerebellum are more prominent in Chandler-infected mice (data not shown). Our study provides evidence that differential reactivity with complement factors is a biological feature that discriminates the Chandler and 22L strains. Accordingly, it will also be of interest to compare the involvement of complement factors in infections with other mouse-adapted scrapie strains, such as Obihiro, G1 and ME7, that have distinct biological properties (i.e., incubation period, distribution of pathological lesions, glial activation).
The reason for the difference in complement factor reactivity between the Chandler and 22L strains remains unclear. Our confocal microscopy data indicate that PrP was colocalized with C1q in Chandler-infected N2a cells, whereas PrP in 22L-infected N2a cells was colocalized with C3. The resolution of confocal microscopy does not allow us to conclude unequivocally that complement factors bind directly to PrP or PrPSc. However, one of the possible explanations for different complement reactivities of Chandler and 22L PrPSc is that they directly bind to C1q and C3 with different relative affinities. Another possibility is that these strains have different affinities for inhibitors of C5 convertase and MAC formation, such as C4b binding protein, clusterin, CD59 and fibronectin (Speth et al., 2008), resulting in differences in MAC formation on Chandler and 22L-infected N2a cells. Recombinant PrP, fibrils, oligomers and purified PrPSc directly bind C1q in vitro and induce complement activation (Blanquet-Grossard et al., 2005; Dumestre-Perard et al., 2007; Mitchell et al, 2007; Sim et al., 2007; Sjoberg et al., 2008). Moreover Veerhuis et al., (2005) reported that C1q enhanced PrP-peptide fibril formation. C4b binding protein also binds recombinant PrP in vitro (Sjoberg et al., 2008). However, further study is needed to demonstrate whether pathogenic forms of PrP bind complement factors and/or their inhibitors in prion infections in vivo and in cultured cells.
We didn’t detect colocalization of C3 and PrP in Chandler-infected N2a cells, which seems to be conflicting with respect to involvement of C3 for the Chandler strain in translocation of phosphatidylserine in N2a cells. We suggest two possibilities for the reason. One is that C3 may be involved in the process of MAC formation through classical and/or lectin pathways without directly binding to PrP/PrPSc. Another possibility is that C3 may bind PrP only in the absence of C1q because of the former’s lower affinity to PrP/PrPSc. Extensive additional experiments would be required to discriminate between these possibilities.
Annexin V has a high affinity for phosphatidylserine (Koopman et al., 1994), which is exposed from the inner layer to the outer layer of the plasma membrane in the early stages of cell death by apoptosis and necrosis (Fadok et al., 1992). In the current study, we found that infected N2a cells were stained with Annexin V after NMS treatment. However, the cells had not progressed to either full-blown apoptosis or necrosis because less than 10% of the cells were stained with PI at 24 h after treatment. In addition, the cells were negative for cleaved caspase-3 at 24 h (data not shown). A similar phenomenon has been reported when human B cells are treated with C3 (Løbner et al., 2009); i.e., the cells were positive for Annexin V, but were negative for cleaved caspase-3. Segmentation of nuclei was not observed. Therefore, our data suggest that complement factors induced translocation of phosphatidylserine, without cell death. The other factors may be required for cell death in prion infections.
The relatively strong deposition of PrPSc in the thalami of Chandler- and 22L-infected mice at the preclinical 90 dpi time point seems likely to account for the mild vacuolation and deposition of either C1q or C3, respectively, in this region. Although we do not have any evidence that these complement factors were activated, there is a possibility that complement activation may occur relatively strongly in the thalamus at this time point, and be involved in neuropathogenesis. However, the functional/clinical consequences thalamic lesions in particular are not clear because by the onset of clinical signs, the accumulation of PrPSc, complement factors, gliosis and vacuolation are more evenly distributed throughout the brain.
The immunohistochemistry data showed widespread distribution of C1q both in Chandler- and 22L-infected mouse brains, although the dorsal part of thalamus in 22L-infected mice lacked C1q immunoreactivity. We suspect that signals stimulating C1q synthesis may not be different between Chandler and 22L infection, but that activation of C1q might be limited in 22L infections. Indeed, C1q in normal mouse serum was not involved in inducing Annexin V positivity in 22L-infected N2a cells. With respect to the immunohistochemistry data, we think that that reactivity of complement factors may be different in vivo as well as in N2a cells.
It is also unclear whether complement activation might work to alleviate or worsen disease because of multifunctionality of complement factors in vivo. Nonetheless, we suggest two possibilities for the roles of complement factors in prion infections from our current data. One possibility is that complement factors facilitate microglial phagocytosis of prion-infected neurons by exposing phosphatidylserine on the cell surface. Although phosphatidylserine itself works as an “eat-me” signal and promotes phagocytosis (Marguet et al., 1999), it has been reported that binding of C1q and C3b on the cell surface also facilitates phagocytosis. Because phosphatidylserine has been known as a C1q binding molecule (Païdassl et al., 2008), exposure of phosphatidylserine may result in further deposition of C1q, which in turn may accelerate phagocytosis of the target cells. Another possibility for the roles of complement factors is to cause degeneration of prion-infected neurons. Bordin & Whitfield (2003) showed that C1q induced apoptosis in human fibroblasts. In addition, it has been reported that C1q is involved in removal of excess synapses in development (Stevens et al., 2007). C3 is reported to induce translocation of phosphatidylserine from the inner to the outer of the plasma membrane in human B cells (Løbner et al., 2009). MAC is composed of C5b, C6, C7, C8 and multiple C9 (C5b-9), which form transmembrane channels on the plasma membrane resulting in lysis by fluid influx into the cells. When the number of the C5b-9 molecules on the target membrane is limited, cell lysis does not occur. However, the C5b-9 molecules in sublytic conditions have a pro-apoptotic effect by mediating cellular signaling pathways (Hughs et al., 2000). MAC formed on the Chandler-infected N2a cells could have been sub-lytic in our experiments because cell lysis was not observed. However, some previous studies have reported that complement factors C1q and C3 have anti-apoptotic and neuroprotective effects as well (Rus et al., 1996; Dashiell et al., 2000; Benoit and Tenner, 2011). Interestingly, Erlich et al. (2010) suggested that C1q binds small oligomers derived from murine recombinant PrP and inhibits cytotoxic effects of PrP oligomers. It is possible that complement factors have both neurotoxic and neuroprotective effects and that the role of the complement factors may be different depending on the stage of the disease.
In conclusion, our data provide evidence that the reaction of complement factors varies with the prion strains and that complement reactions can induce the translocation of phosphatidylserine in the membrane of prion-infected N2a cells. The roles of complement factors in prion infection might be further elucidated in the future using ex vivo systems such as slice cultures and mixed cultures of neurons and glial cells.
Materials and Methods
Antibodies
Anti-complement C1q, C3 and C9 goat polyclonal antibodies were purchased from Quidal Corporation. Anti-MAC rabbit polyclonal antibodies were purchased from Calbiochem. Anti-PrP mouse monoclonal antibody 6D11 and human monoclonal antibody D13 were purchased from Covance and InPro Biotechnology, respectively. Biotinylated anti-human IgG was purchased from Jackson ImmunoResearch. All Alexa-labeled secondary antibodies were purchased from Invitrogen.
Preparation of mouse serum
NMS was prepared from 6–8 weeks old RML mice. Blood was collected from the heart under inhalation anesthesia with isoflulane and coagulated at 4°C for 6 h. NMS was incubated at 56°C for 30 min for preparation of H-NMS. Rocky Mountain Laboratories is an AALAC-accredited facility, and all animal procedures were approved by the institution’s Animal Use and Care Committee.
Treatment of N2a cells with NMS, HS-NMS and FBS
N2a cells persistently infected with Chandler (Race et al., 1988) or 22L scrapie strains (Kocisko et al., 2003) were maintained at 37°C in a humidified atmosphere of 5% CO2 in Opti-MEM supplemented with 10% FBS, 2 mM L-glutamine (Invitrogen) and penicillin/streptomycin (Invitrogen). For the negative controls, the infected cells were treated with PPS to cure their prion infection. After 5 passages with PPS, PrPSc was not detected by Western Blotting (data not shown). The cells were seeded on 24 well plates at approximately 10% (for Chandler) or 5% confluence (for 22L) and were cultured for 2 days. Then the cells were cultured with media supplemented with 10% NMS, H-NMS or FBS for 6, 12, 24 and 48 h. To assess inhibitory effects of anti-complement antibodies on the cellular exposure of phosphatidylserine, NMS was treated with anti-C1q, C3 and/or C9 goat polyclonal antibodies at 150 µg/ml of total protein for each antibody. For controls, NMS was treated with NGS or anti-mouse IgG goat polyclonal antibodies at 150 and 450 µg/ml of total protein. Exposure of phosphatidylserine on the outer side of the plasma membrane was detected by Annexin V staining (Invitrogen) according to the manufacturer’s instructions. Three areas of each well were randomly chosen and Annexin V positive cells were counted. Proportions of Annexin V positive cells were calculated by dividing by the total number of cells. Three independent experiments were performed. Cell death was detected by PI uptake. The cells were incubated with PI (Invitrogen) at 5µg/ml for 20 min at 37°C and observed by fluorescent microscopy (Nikon).
Immunocytochemistry
For MAC detection, the cells were seeded on glass-bottomed Lab-Tek 8 well chamber slides (Nalge Nunc International). At 6, 12 or 24 h after treatment with NMS, the cells were fixed with cold methanol for 15 min, dried and blocked with 2% bovine serum albumin (BSA) in phosphate buffered saline with 0.01% Tween 20. The cells were incubated with anti-MAC antibodies at a 1:100 dilution at 4°C overnight, followed by incubation with Alexa568-anti rabbit goat polyclonal antibodies at a 1:800 dilution for 1 h at room temperature. The cells were counterstained with 4’6-diamidino-2-phenylindole (DAPI) at 1 µg/ml. For double staining of PrP and C1q/C3, the cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 5 min and blocked with 2% BSA for 15 min at room temperature. For the primary antibodies, the cells were incubated with anti-PrP (6D11) mouse monoclonal antibody at a 1:1000 dilution and anti-C1q or C3 goat polyclonal antibodies at a 1:100 dilution. For the secondary antibodies, Alexa488-labeled anti-goat IgG polyclonal antibodies and Alexa 568-labeled anti-mouse IgG polyclonal antibodies were used at a 1:800 dilution for each. The samples were observed with an LSM 510 microscope (Carl Zeiss Inc).
Histological analysis
C57BL/6 mice (8–10 weeks old) were infected intracerebrally with 0.5% Chandler-, 22L-, or normal mouse brain homogenates under inhalation anesthesia with isoflulane. At 90, 133 and 166 dpi, 4 mice for each strain were euthanized and brains were harvested. The samples were fixed with 10% phosphate-buffered formalin, dehydrated and embedded in paraffin. The paraffin blocks were cut at 4 µm and subjected to HE staining and immunohistochemistry for PrPSc, C1q and C3. PrPSc staining was performed using a Ventana automated Discovery XT stainer. For antigen retrieval, the slides were incubated in CC1 buffer (Ventana) containing Tris-borate-EDTA pH 8.0 for 180 min at 95°C. Then the cells were incubated with anti mouse PrP human antibody D13 at a dilution of 1:500 at 4°C for 16 h, followed by a biotinylated anti-human IgG at 1:500 and avidin-horseradish peroxidase. Then the slides were reacted with diaminobenzidine (Ventana) as chromogen and observed with an Olympus BX51 microscope. For C1q and C3 detection, the slides were pre-treated with 0.03% proteinase K at 37°C for 30 min, incubated with anti-C1q or C3 antibodies at 4°C overnight. Then the slides were incubated with Alexa488-labeled anti goat IgG for secondary antibody and counterstained with DAPI.
Highlights.
-
>
We assessed complement reaction in prion infections of N2a cells and mouse brains.
-
>
Complement factors induced translocation of phosphatidylserine in infected N2a cells.
-
>
The reactivity of complements differs between prion strains in vitro and in vivo.
Acknowledgements
The authors greatly appreciate Dr. David Dorward in Microscopy Unit, Dr. Dan Long, Ms. Rebecca Rosenke and Ms. Lori Lubke in Histology Section, Rocky Mountain Laboratories, NIH/NIAID for technical support. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health (project no. ZIA AI000580-20 & ZIA ZI000580-21). This work was also supported by Grand-in-Aid for Young Scientist (B) from the Ministry of Education, Culture, Sports, Science and Technology (grant no. 23780303) and by a grant for Research on Measures for Intractable Diseases from the Ministry of Health, Labour and Welfare of Japan (grant no. H23-Nanchi-Ippan-013).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atarashi R, Sim VL, Nishida N, Caughey B, Katamine S. Prion strain-dependent differences in conversion of mutant prion proteins in cell culture. J. Virol. 2006;80:7854–7862. doi: 10.1128/JVI.00424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron GS, Hughson AG, Raymond GJ, Offerdahl DK, Barton KA, Raymond LD, Dorward DW, Caughey B. Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry. 2011;50:4479–4490. doi: 10.1021/bi2003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit ME, Tenner AJ. Complement protein C1q-mediated neuroprotection is correlated with regulation of neuronal gene and microRNA expression. J. Neurosci. 2011;31:3459–3469. doi: 10.1523/JNEUROSCI.3932-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen RA, Marsh RF. Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J. Virol. 1992;66:2096–2101. doi: 10.1128/jvi.66.4.2096-2101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessen RA, Marsh RF. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 1994;68:7859–7868. doi: 10.1128/jvi.68.12.7859-7868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanquet-Grossard F, Thielens NM, Vendrely C, Jamin M, Arlaud GJ. Complement protein C1q recognizes a conformationally modified form of the prion protein. Biochemistry. 2005;44:4349–4356. doi: 10.1021/bi047370a. [DOI] [PubMed] [Google Scholar]
- Bordin S, Whitfield D. Cutting edge: proliferating fibroblasts respond to collagenous C1q with phosphorylation of p38 mitogen-activated protein kinase and apoptotic features. J. Immunol. 2003;170:667–671. doi: 10.4049/jimmunol.170.2.667. [DOI] [PubMed] [Google Scholar]
- Bruce ME, McBride PA, Farquhar CF. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci. Lett. 1989;102:1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- Caughey B, Raymond GJ, Bessen RA. Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J. Biol. Chem. 1998;273:32230–32235. doi: 10.1074/jbc.273.48.32230. [DOI] [PubMed] [Google Scholar]
- Dandoy-Dron F, Guillo F, Benboudjema L, Deslys JP, Lasmezas C, Dormont D, Tovey MG, Dron M. Gene expression in scrapie. Cloning of a new scrapie-responsive gene and the identification of increased levels of seven other mRNA transcripts. J Biol Chem. 1998;273:7691–7697. doi: 10.1074/jbc.273.13.7691. [DOI] [PubMed] [Google Scholar]
- Dashiell SM, Rus H, Koski CL. Terminal complement complexes concomitantly stimulate proliferation and rescue of Schwann cells from apoptosis. Glia. 2000;30:187–198. doi: 10.1002/(sici)1098-1136(200004)30:2<187::aid-glia8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Dumestre-Perard C, Osmundson J, Lemaire-Vieille C, Thielens N, Grives A, Favier B, Csopaki F, Jamin M, Gagnon J, Cesbron JY. Activation of classical pathway of complement cascade by soluble oligomers of prion. Cell Microbiol. 2007;9:2870–2879. doi: 10.1111/j.1462-5822.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- Erlich P, Dumestre-Perard C, Ling WL, Lemaire-Vieille C, Schoehn G, Arlaud GJ, Thielens NM, Gagnon J, Cesbron JY. Complement protein C1q forms a complex with cytotoxic prion protein oligomers. J. Biol. Chem. 2010;285:19267–19276. doi: 10.1074/jbc.M109.071860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Flores-Langarica A, Sebti Y, Mitchell DA, Sim RB, MacPherson GG. Scrapie pathogenesis: the role of complement C1q in scrapie agent uptake by conventional dendritic cells. J. Immunol. 2009;182:1305–1313. doi: 10.4049/jimmunol.182.3.1305. [DOI] [PubMed] [Google Scholar]
- Guan E, Robinson SL, Goodman EB, Tenner AJ. Cell-surface protein identified on phagocytic cells modulates the C1q-mediated enhancement of phagocytosis. J. Immunol. 1994;152:4005–4016. [PubMed] [Google Scholar]
- Hughes J, Nangaku M, Alpers CE, Shankland SJ, Couser WG, Johnson RJ. C5b-9 membrane attack complex mediates endothelial cell apoptosis in experimental glomerulonephritis. Am. J. Physiol. Renal. Physiol. 2000;278:F747–F757. doi: 10.1152/ajprenal.2000.278.5.F747. [DOI] [PubMed] [Google Scholar]
- Hwang D, Lee IY, Yoo H, Gehlenborg N, Cho JH, Petritis B, Baxter D, Pitstick R, Young R, Spicer D, Price ND, Hohmann JG, Dearmond SJ, Carlson GA, Hood LE. A systems approach to prion disease. Mol. Syst. Biol. 2009;5:252. doi: 10.1038/msb.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Haga S, Yagishita S, Tateishi J. The presence of complements in amyloid plaques of Creutzfeldt-Jakob disease and Gerstmann-Straussler-Scheinker disease. Appl. Pathol. 1984;2:370–379. [PubMed] [Google Scholar]
- Klein MA, Kaeser PS, Schwarz P, Weyd H, Xenarios I, Zinkernagel RM, Carroll MC, Verbeek JS, Botto M, Walport MJ, Molina H, Kalinke U, Acha-Orbea H, Aguzzi A. Complement facilitates early prion pathogenesis. Nat. Med. 2001;7:488–492. doi: 10.1038/86567. [DOI] [PubMed] [Google Scholar]
- Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. New inhibitors of scrapie-associated prion protein formation in a library of 2000 drugs and natural products. J. Virol. 2003;77:10288–10294. doi: 10.1128/JVI.77.19.10288-10294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. [PubMed] [Google Scholar]
- Kovacs GG, Gasque P, Strobel T, Lindeck-Pozza E, Strohschneider M, Ironside JW, Budka H, Guentchev M. Complement activation in human prion disease. Neurobiol. Dis. 2004;15:21–28. doi: 10.1016/j.nbd.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kuczius T, Groschup MH. Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol. Med. 1999;5:406–418. [PMC free article] [PubMed] [Google Scholar]
- Lόbner M, Leslie RG, Prodinger WM, Nielsen CH. Spontaneous complement activation on human B cells results in localized membrane depolarization and the clustering of complement receptor type 2 and C3 fragments. Immunology. 2009;128:e661–e669. doi: 10.1111/j.1365-2567.2009.03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott NA, Bruce ME. Complement component C5 is not involved in scrapie pathogenesis. Immunobiology. 2004;209:545–549. doi: 10.1016/j.imbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Mabbott NA, Bruce ME, Botto M, Walport MJ, Pepys MB. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat. Med. 2001;7:485–487. doi: 10.1038/86562. [DOI] [PubMed] [Google Scholar]
- Mahal SP, Baker CA, Demczyk CA, Smith EW, Julius C, Weissmann C. Prion strain discrimination in cell culture: the cell panel assay. Proc. Natl. Acad. Sci. USA. 2007;104:20908–20913. doi: 10.1073/pnas.0710054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marguet D, Luciani MF, Moynault A, Williamson P, Chimini G. Engulfment of apoptotic cells involves the redistribution of membrane phosphatidylserine on phagocyte and prey. Nat. Cell. Biol. 1999;1:454–456. doi: 10.1038/15690. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Kirby L, Paulin SM, Villiers CL, Sim RB. Prion protein activates and fixes complement directly via the classical pathway: implications for the mechanism of scrapie agent propagation in lymphoid tissue. Mol. Immunol. 2007;44:2997–3004. doi: 10.1016/j.molimm.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Nishida N, Harris DA, Vilette D, Laude H, Frobert Y, Grassi J, Casanova D, Milhavet O, Lehmann S. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 2000;74:320–325. doi: 10.1128/jvi.74.1.320-325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Païdassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, Arlaud GJ, Frachet P. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J. Immunol. 2008;180:2329–2338. doi: 10.4049/jimmunol.180.4.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- Ren G, Huynh C, Bijian K, Cybulsky AV. Role of apoptosis signal-regulating kinase 1 in complement-mediated glomerular epithelial cell injury. Mol. Immunol. 2008;45:2236–2246. doi: 10.1016/j.molimm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J. Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]
- Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- Sarvari M, Vago I, Weber CS, Nagy J, Gal P, Mak M, Kosa JP, Zavodszky P, Pazmany T. Inhibition of C1q-beta-amyloid binding protects hippocampal cells against complement mediated toxicity. J. Neuroimmunol. 2003;137:12–18. doi: 10.1016/s0165-5728(03)00040-7. [DOI] [PubMed] [Google Scholar]
- Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cells. 2009;27:2824–2832. doi: 10.1002/stem.225. [DOI] [PubMed] [Google Scholar]
- Sim RB, Kishore U, Villiers CL, Marche PN, Mitchell DA. C1q binding and complement activation by prions and amyloids. Immunobiology. 2007;212:355–362. doi: 10.1016/j.imbio.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Sim VL, Caughey B. Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol. Aging. 2009;30:2031–2042. doi: 10.1016/j.neurobiolaging.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Sjoberg AP, Nystrom S, Hammarstrom P, Blom AM. Native, amyloid fibrils and beta-oligomers of the C-terminal domain of human prion protein display differential activation of complement and bind C1q, factor H and C4b-binding protein directly. Mol. Immunol. 2008;45:3213–3221. doi: 10.1016/j.molimm.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Abbassi H, Chesebro B, Race RE, Reilly C, Haase AT. Gene expression alterations in brains of mice infected with three strains of scrapie. BMC Genomics. 2006;7:114. doi: 10.1186/1471-2164-7-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane L, Cho HJ, Niculescu F, Rus H, Shin ML. C5b-9 terminal complement complex protects oligodendrocytes from death by regulating Bad through phosphatidylinositol 3-kinase/Akt pathway. J. Immunol. 2001;167:2305–2311. doi: 10.4049/jimmunol.167.4.2305. [DOI] [PubMed] [Google Scholar]
- Soane L, Rus H, Niculescu F, Shin ML. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of bcl-2 and mediated by inhibition of caspase-3 activation. J. Immunol. 1999;163:6132–6138. [PubMed] [Google Scholar]
- Speth C, Prodinger WM, Wurzner R, Stoiber H, Dierich MP. Fundamental Immunology. 6th edition. Philadelphia: LIPPENCOTT WILLIAMS & WILKINS; 2008. Complement; pp. 1047–1060. [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Veerhuis R, Boshuizen RS, Morbin M, Mazzoleni G, Hoozemans JJ, Langedijk JP, Tagliavini F, Langeveld JP, Eikelenboom P. Activation of human microglia by fibrillar prion protein-related peptides is enhanced by amyloid-associated factors SAP and C1q. Neurobiol. Dis. 2005;19:273–282. doi: 10.1016/j.nbd.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Webster S, Bonnell B, Rogers J. Charge-based binding of complement component C1q to the Alzheimer amyloid beta-peptide. Am. J. Pathol. 1997;150:1531–1536. [PMC free article] [PubMed] [Google Scholar]
- Zabel MD, Heikenwalder M, Prinz M, Arrighi I, Schwarz P, Kranich J, von Teichman A, Haas KM, Zeller N, Tedder TF, Weis JH, Aguzzi A. Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J. Immunol. 2007;179:6144–6152. doi: 10.4049/jimmunol.179.9.6144. [DOI] [PubMed] [Google Scholar]