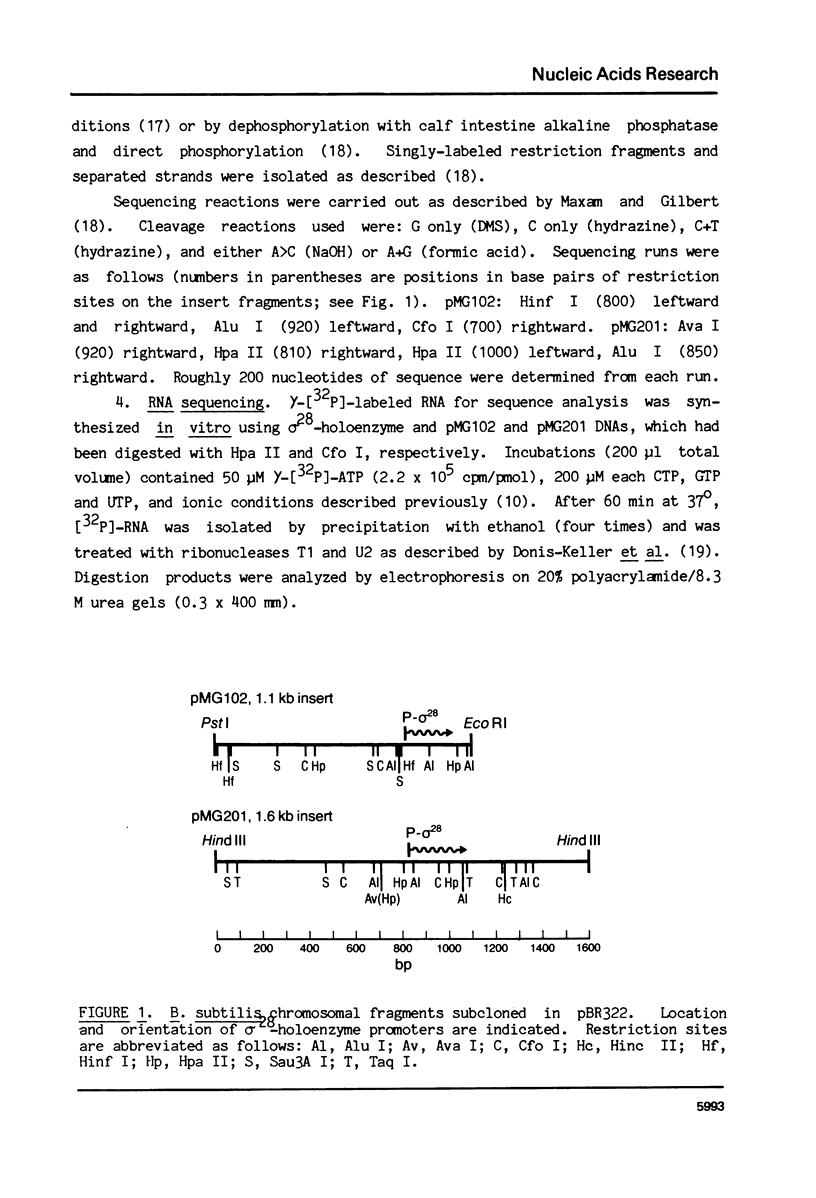
Abstract
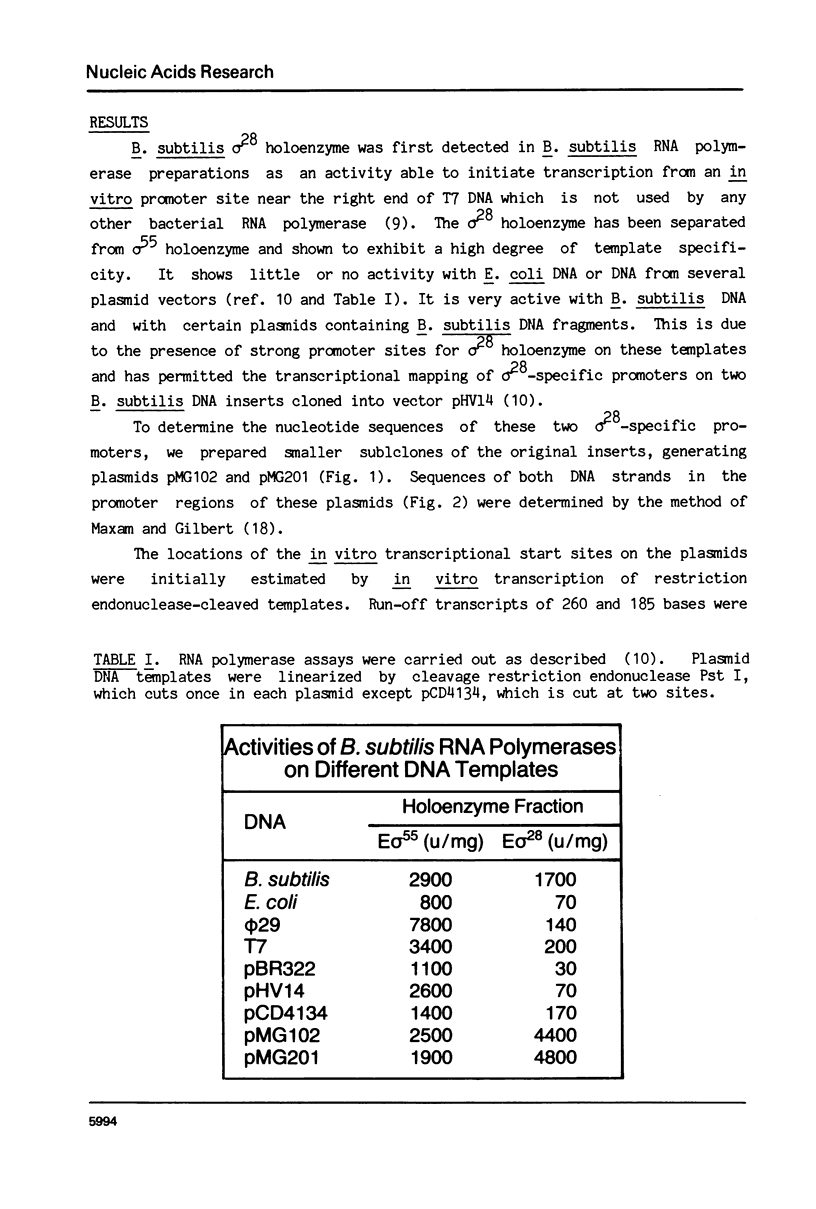
RNA polymerase holoenzymes from many bacterial species share a common promoter recognition specificity since they use the same promoter sites on a variety of templates. These promoters generally include sequences homologous to the average sequences -TTGACA- and -TATAATA-, located -35 and -10 base pairs, respectively, upstream of the transcriptional state site. We have isolated a minor form of B. subtilis RNA polymerase in which the normal sigma subunit (sigma 55) is replaced by a smaller polypeptide (sigma 28) and which is highly specific for a class of promoter sites not used by the sigma 55-holoenzyme. Sequencing of two B. subtilis promoter sites used by the sigma 28-holoenzyme reveals identical sequences at -35 and -10 base pairs from the start site, which are -CTAAA- and -CCGATAT-, respectively. These results confirm that sigma subunit plays a major direct role in promoter sequence recognition, and support a model in which sigma interacts sequentially with -35 and -10 regions, respectively.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chenchick A., Beabealashvilli R., Mirzabekov A. Topography of interaction of Escherichia coli RNA polymerase subunits with lac UV5 promoter. FEBS Lett. 1981 Jun 1;128(1):46–50. doi: 10.1016/0014-5793(81)81076-9. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Murray C. L., Rabinowitz J. C. Specificity of promoter site utilization in vitro by bacterial RNA polymerases on Bacillus phage phi 29 DNA. Transcription mapping with exonuclease III. J Biol Chem. 1980 Sep 25;255(18):8819–8830. [PubMed] [Google Scholar]
- DeRiemer L. H., Meares C. F. Early steps in the path of nascent ribonucleic acid across the surface of ribonucleic acid polymerase, determined by photoaffinity labeling. Biochemistry. 1981 Mar 17;20(6):1612–1617. doi: 10.1021/bi00509a031. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. Purification of a positive regulatory subunit from phage SP01-modified RNA polymerase. Nature. 1977 Nov 3;270(5632):28–32. doi: 10.1038/270028a0. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Lang N., Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981 Feb;23(2):615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. A modified RNA polymerase transcribes a cloned gene under sporulation control in Bacillus subtilis. Nature. 1979 Nov 15;282(5736):256–260. doi: 10.1038/282256a0. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7000–7004. doi: 10.1073/pnas.77.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaehning J. A., Wiggs J. L., Chamberlin M. J. Altered promoter selection by a novel form of Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5470–5474. doi: 10.1073/pnas.76.11.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Pausing and termination of transcription within the early region of bacteriophage T7 DNA in vitro. J Biol Chem. 1981 Mar 25;256(6):2777–2786. [PubMed] [Google Scholar]
- Kudo T., Jaffe D., Doi R. H. Free sigma subunit of Bacillus subtilis RNA polymerase binds to DNA. Mol Gen Genet. 1981;181(1):63–68. doi: 10.1007/BF00339006. [DOI] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Murray C. L., Rabinowitz J. C. RNA polymerase from Clostridium acidi-urici. Characterization of a naturally occurring rifampicin-resistant bacterial enzyme. J Biol Chem. 1981 May 25;256(10):5153–5161. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Simpson R. B. The molecular topography of RNA polymerase-promoter interaction. Cell. 1979 Oct;18(2):277–285. doi: 10.1016/0092-8674(79)90047-3. [DOI] [PubMed] [Google Scholar]
- Stender W. Sequence-specific interaction of sigma subunit of E. coli RNA polymerase with DNA. Nucleic Acids Res. 1980 Aug 11;8(15):3459–3466. doi: 10.1093/nar/8.15.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington C., Pero J. Distinctive nucleotide sequences of promoters recognized by RNA polymerase containing a phage-coded "sigma-like" protein. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5465–5469. doi: 10.1073/pnas.76.11.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington C., Pero J. Promoter recognition by phage SP01-modified RNA polymerase. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1185–1189. doi: 10.1073/pnas.75.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijan R., Pero J. Bacteriophage SP01 regulatory proteins directing late gene transcription in vitro. Nature. 1976 Aug 26;262(5571):753–757. doi: 10.1038/262753a0. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Bush J. W., Chamberlin M. J. Utilization of promoter and terminator sites on bacteriophage T7 DNA by RNA polymerases from a variety of bacterial orders. Cell. 1979 Jan;16(1):97–109. doi: 10.1016/0092-8674(79)90191-0. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Gilman M. Z., Chamberlin M. J. Heterogeneity of RNA polymerase in Bacillus subtilis: evidence for an additional sigma factor in vegetative cells. Proc Natl Acad Sci U S A. 1981 May;78(5):2762–2766. doi: 10.1073/pnas.78.5.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]