Abstract
We have developed a cellular system constituted of human telomerase immortalized fibroblasts that gradually underwent neoplastic transformation during propagation in culture. We exploited this cellular system to investigate gene and miRNA transcriptional programs in cells at different stages of propagation, representing five different phases along the road to transformation, from non-transformed cells up to tumorigenic and metastatic ones. Here we show that gene and miRNA expression profiles were both able to divide cells according to their transformation phase. We identified more than 1,700 genes whose expression was highly modulated in cells at at least one propagation stage and we found that the number of modulated genes progressively increased at successive stages of transformation. These genes identified processes significantly deregulated in tumorigenic cells, such as cell differentiation, cell movement and extracellular matrix remodeling, cell cycle and apoptosis, together with upregulation of several cancer testis antigens. Alterations in cell cycle, apoptosis, and cancer testis antigen expression were particular hallmarks of metastatic cells. A parallel deregulation of a panel of 43 miRNAs strictly connected to the p53 and c-Myc pathways and with oncogenic/oncosuppressive functions was also found. Our results indicate that cen3tel cells can be a useful model for human fibroblast neoplastic transformation, which appears characterized by complex and peculiar alterations involving both genetic and epigenetic reprogramming, whose elucidation could provide useful insights into regulatory networks underlying cancerogenesis.
Introduction
Normal cells have to accumulate successive genetic and epigenetic changes to become cancer cells (Hanahan and Weinberg, 2011). For some human tumors the hierarchy in mutation acquisition has been disclosed, such as for example in colorectal cancer, in which the most important genetic variations accompanying the transition from low tumorigenic adenocarcinoma to metastatic carcinoma have been identified (Michor et al., 2005), but for most cancers the sequence of genomic variations is still unknown.
We have set up a cellular system that, recapitulating neoplastic transformation of human fibroblasts, allowed gaining information on the stepwise acquisition of cellular and molecular variations leading to tumorigenicity. This cellular system, named cen3tel, was obtained after fibroblast immortalization by ectopic expression of the human telomerase catalytic subunit (hTERT) (Mondello et al., 2003). Reconstitution of telomerase activity made cells able to overcome cellular senescence and become immortal; however, the achievement of the indefinite replicative potential was accompanied by the acquisition of successive mutations in oncogenes and oncosuppressor genes leading to neoplastic transformation. In fact, cells formed tumors when inoculated under the skin of immunocompromised mice and cells at further stages of propagation in culture generated lung metastases when injected into the mouse caudal vein (Belgiovine et al., 2010; Zongaro et al., 2005).
Studying molecular and cellular variations during culture propagation of cen3tel cells, we identified five main phases in the road map to transformation (Belgiovine et al., 2010; Zongaro et al., 2005), each characterized by specific features. Briefly, in the first phase (early cen3tel cells), cells behaved similarly to normal parental fibroblasts. In the second phase (mid cen3tel cells), they showed the ability to grow in the absence of solid support and CDKN2A downregulation. In the third phase (phase I tumorigenic cells), cells became able to induce tumors in nude mice; in parallel, we found a mutation in TP53 and MYC overexpression. Cells in phase IV and V (phase II and III tumorigenic cells) induced tumors with a shorter latency compared to cells of tumorigenic phase I (about 8 and 2 days, respectively, versus about 30 days); moreover, phase III tumorigenic cells were metastatic. Histological analysis revealed that the tumors developed by cen3tel cells at tumorigenic phase I and II were pleomorphic sarcomas, those developed by phase III cen3tel cells showed a hemangiopericytoma-like vascular pattern, similar to human poorly differentiated, round-cell synovial sarcomas (Belgiovine et al., 2010). Characterizing the invasion mechanism of tumorigenic cells, we identified Rnd3, a GTPase involved in cytoskeletal organization and cell movement (Riou et al., 2010), as an important gene regulating invasion and metastasis formation in tumor cells (Belgiovine et al., 2010).
To get further insights into the transformation process, we performed a genome-wide analysis of the gene and miRNA expression profiles of cen3tel cells at different phases of transformation, relatively to normal parental fibroblasts. In this article, we present the main results of this analysis.
Materials and Methods
mRNA and miRNA microarray probe preparation, hybridization, and scanning
Total RNA extraction from actively dividing primary fibroblasts (Population Doubling, PD, 15) and from cen3tel cells representing the five phases of propagation (cells at PD 37, 97, 167, 618, and 1034), microarray probe preparation, hybridization, and scanning for gene expression analysis were performed as previously described (Belgiovine et al., 2010). The cells used for microarray analysis had just been tested for their specific characteristics or derived from batches of cells frozen in parallel to their characterization. For miRNA expression analysis, 100 ng of total RNA from actively dividing primary fibroblasts (PD 15) and from cen3tel cells at PDs 97, 167, 618, and 1034 were treated following the miRNA microarray protocol (Agilent Technologies, Placerville, CA). Briefly, RNA was dephosphorylated and denaturated; then a ligation and labeling step was performed. Samples were hybridized to oligonucleotide glass arrays containing 11,080 probes corresponding to 470 human and 64 human viral miRNAs, from the Sanger database release 9.1 (Human miRNA Microarray V1, Agilent Technologies). After hybridization, slides were washed following the Agilent procedure and scanned with the dual-laser microarray scanner version B (G2505B, Agilent Technologies).
Microarray image and data analysis
Images were analyzed using the Feature Extraction software (Agilent Technologies). Raw data elaboration was carried out with Bioconductor (www.bioconductor.org) (Gentleman et al., 2004), using R statistical language. For gene expression analysis, background correction was performed with the normexp method with an offset of 50, loess was used for the within-array normalization and A-quantile for the between-array normalization. For miRNA expression analysis, raw data were processed with the method of invariant selection and normalization (Pradervand et al., 2009). The LIMMA (LInear Models for Microarray Analysis) package was then used to identify differentially expressed genes/microRNAs in cen3 cells at different population doublings versus parental cen3 cells. The empirical Bayes method was used to compute a moderated t-statistics (Smyth, 2004). p-values were adjusted for multiple testing by using a false discovery rate (FDR) correction (Benjamini and Hochberg, 1995). For gene expression, transcripts with a log base twofold change (logFC) versus cen3 cells greater than 2 or lower than −2 and adjusted p-value less than 0.01 were considered as differentially expressed. On the other hand, miRNAs with log fold change vs. cen3 cells greater that 1 or lower than −1 and adjusted p-value <0.01 were considered as differentially expressed.
Real-time RT-PCR
Microarray results were confirmed by quantitative real-time reverse transcription PCR (RT-PCR) using dedicated sets of commercial primers (Qiagen, Chatsworth, CA) for each gene or miRNA analysed. Briefly, specific cDNA was generated in a single-step reaction from 1 μg of the same RNAs used for the microarray analysis, using the miScript Reverse Transcription Kit or the QuantiTec Reverse Transcription Kit (Qiagen), for miRNAs or genes, respectively, following the manufacturer's instructions. miRNA and gene expression levels were quantified by SYBR Green chemistry using either the commercial miScript Universal Primer together with the miScript Primer Assay or the QuantiTect SYBR Green Kit (Qiagen). Reactions were run in triplicate for each experimental point and each experiment was repeated twice. Real-time PCR was done on the Light Cycler 480 (Roche, Indianapolis, IN), using 96-well reaction plates. Raw data were normalized using snRNPU6 or GusB results for miRNA or gene expression, respectively.
Cluster analysis
MeV version 4.6.1 (Saeed et al., 2006) was used for unsupervised hierarchical clustering, performed on both the global expression profiles of cells at different PDs and on subsets of genes according to their ontological classification. Euclidean distance as similarity metrics and complete linkage as linkage method were used.
Gene Ontology and network analysis
In order to look for any overrepresented biological process-level 5 (BP5) of the Gene Ontology (GO), we used the functional annotation tool available within DAVID Website (http://david.abcc.ncifcrf.gov/), using the lists of differentially expressed genes at each PD. MetaCore version 6.5 (GeneGo Inc., St. Joseph, MI) was used for network analysis, that was applied to differentially expressed miRNAs in order to find out possible connections. In particular, a network consisting of shortest paths (i.e., having the smallest possible number of directed one-step interactions) was built between pairs of modulated miRNAs, in each direction. This tool uses standard Dijkstra's shortest paths algorithm.
Gene set enrichment analysis (GSEA)
GSEA was used to evaluate significant enrichment in predefined curated sets of genes from online pathway databases and publications in PubMed (Subramanian et al., 2005).
miRNA target prediction analysis
The seed-based algorithm from TargetScan was used for the identification of gene:miRNA pairs among the lists of differentially expressed mRNAs and genes (Lewis et al., 2005).
Results
Gene expression profiling of cen3tel cells at different stages of transformation
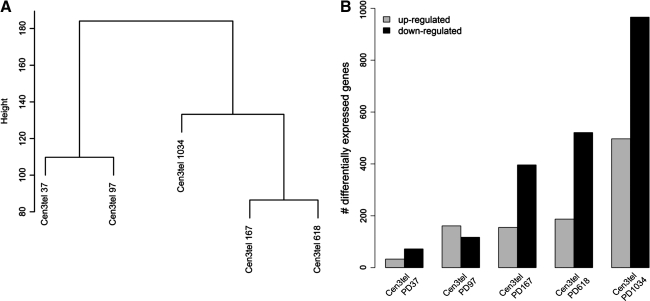
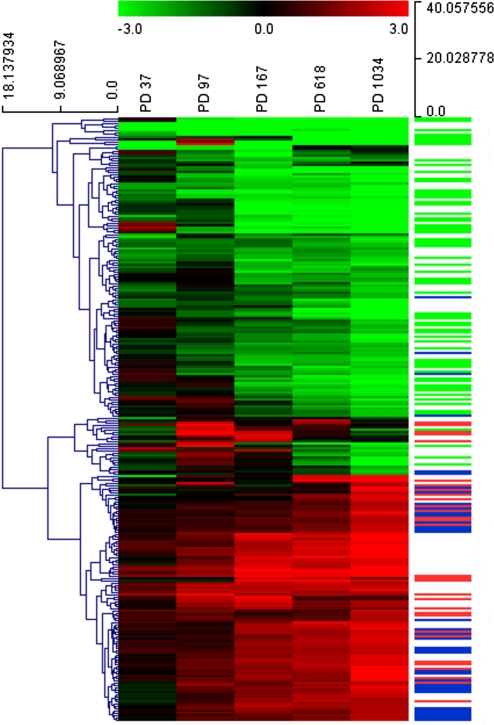
To analyze gene expression profiles of cen3tel cells at different stages of transformation, we used RNA samples prepared from cells at PD 37, 97, 167, 618, and 1034, representing early cen3tel, mid cen3tel and phase I, phase II and phase III tumorigenic cells, respectively. Gene expression profiling was performed using Agilent oligonucleotide glass arrays containing probes that correspond to ∼20,000 transcripts. RNA samples prepared from cen3tel cells at the different PDs were hybridized in duplicate against primary fibroblast RNA (cen3 cells at PD 15). The global expression profiles divided nontumorigenic from tumorigenic cen3tel cells. Cells at PDs 167 and 618 resulted to be the most close to one another, according to Euclidean distance as similarity metric. Cells at PD 1034 were closer to cells at PDs 167/618 than to cells at PDs 37 and 97, which formed a separate cluster, confirming their dissimilarity from cells at the other stages of propagation (Fig. 1A).
FIG. 1.
Global gene expression profiling of cen3tel cells at different stages of transformation. (A) Dendrogram obtained from unsupervised hierarchical clustering using Euclidean distance as similarity metrics and complete linkage as linkage method. Each branch of the dendrogram is represented by the global gene expression profile of cells at each PD. The vertical axis indicates the Euclidean distance between samples/clusters. (B) Histogram plot showing the number of genes up- or downregulated in cen3tel cells at different PDs relatively to parental fibroblasts.
Transcripts with logFC versus cen3 cells greater than 2 or lower than −2 and adjusted p-value less than 0.01 were considered as differentially expressed. As shown in Figure 1B, the total number of genes modulated increased during culture propagation, ranging from 104 in early cen3tel cells to 1,462 in phase III tumorigenic cells. All the variations in gene expression occurred spontaneously during cen3tel cell culture propagation. It is worth noticing that, in cells at all passages, the number of genes downregulated was significantly higher compared to those upregulated, except in cells at PD 97.
The number of genes that were exclusively modulated at one specific passage (from PD 37 to PD 1034) was 33, 81, 77, 59, and 754. A total of 772 genes were modulated in cells at at least two passages; among these, 17 were shared by cells at all PDs (Table 1); thus, also by cells at few passages after telomerase activation, which still behaved similarly to normal fibroblasts. A total of 94 modulated genes were shared by cells at PDs 97, 167, 618, and 1034, 353 by cells at PDs 167, 618, 1034, and 619 by cells at PDs 618 and 1034. As expected, many genes changing their expression at one PD maintained the modulation at later passages.
Table 1.
List of the 17 Genes Commonly Modulated in cen3tel Cells at Different PDs, Relative to Parental cen3 Fibroblastsa
| Gene name | Description | Cen3tel 37 | Cen3tel 97 | Cen3tel 167 | Cen3tel 618 | Cen3tel 1034 |
|---|---|---|---|---|---|---|
| PSAT1 | phosphoserine aminotransferase 1 | 2.59 | 3.32 | 3.27 | 3.08 | 3.50 |
| CCL20 | chemokine (C-C motif) ligand 20 | −2.65 | 2.31 | −2.69 | −2.48 | −3.16 |
| IL8 | interleukin 8 | −5.30 | 2.92 | −4.91 | −3.01 | −3.77 |
| C15orf48 | chromosome 15 open reading frame 48 | −2.66 | −2.56 | −3.36 | −3.14 | −4.08 |
| CXCL5 | chemokine (C-X-C motif) ligand 5 | −4.41 | −3.89 | −4.05 | −4.02 | −5.29 |
| HMOX1 | heme oxygenase (decycling) 1 | −3.06 | −3.57 | −2.95 | −2.66 | −3.16 |
| IGF2 | insulin-like growth factor 2 | −2.16 | −3.41 | −3.67 | −3.72 | −4.21 |
| IL13RA2 | interleukin 13 receptor, alpha 2 | −2.63 | −2.17 | −3.83 | −3.09 | −3.21 |
| KANK4 | KN motif and ankyrin repeat domains 4 | −2.46 | −3.20 | −3.18 | −3.10 | −3.64 |
| MMP12b | matrix metalloproteinase 12 (macrophage elastase) | −4.03 | −3.90 | −5.33 | −5.35 | −5.89 |
| MMP12b | matrix metalloproteinase 12 (macrophage elastase) | −4.11 | −4.05 | −5.77 | −5.75 | −5.99 |
| NPTX1b | neuronal pentraxin I | −2.53 | −4.05 | −4.69 | −4.48 | −5.40 |
| NPTX1b | neuronal pentraxin I | −2.22 | −3.23 | −3.46 | −3.36 | −4.01 |
| PRLR | prolactin receptor | −2.29 | −2.11 | −2.94 | −2.49 | −3.10 |
| PTGDS | prostaglandin D2 synthase 21 kDa (brain) | −2.32 | −3.34 | −3.41 | −3.44 | −3.93 |
| SPON1 | spondin 1, extracellular matrix protein | −2.16 | −4.60 | −4.52 | −4.50 | −5.28 |
| TES | testis derived transcript (3 LIM domains) | −2.10 | −2.54 | −2.60 | −2.57 | −3.51 |
| THBD | thrombomodulin | −2.11 | −2.76 | −2.85 | −2.86 | −3.46 |
| WISP2 | WNT1 inducible signaling pathway protein 2 | −2.01 | −3.01 | −3.91 | −4.05 | −4.40 |
The values are the log2 of the ratio.
Genes with more than one probe on the array.
Analyzing the 17 genes modulated at all PDs (Table 1), we found that their expression modulation tended to become greater with increasing PDs; however, the only gene with a statistically significant change in its expression between tumorigenic and non-tumorigenic stages was MMP12 (Student p-value <0.01). One of these genes was upregulated compared to parental fibroblasts; it encoded for phosphoserine amino-transferase 1 (PSAT1), whose overexpression has been reported to stimulate cell growth and increase chemoresistance of colon cancer cells (Vie et al., 2008). Two genes belonging to the cytokine families (CCL20, IL8) were downregulated in cells at each PD, except in cells at PD 97, where they showed a higher expression compared to cen3 primary fibroblasts.
All the other genes were downregulated in cells at each PD. Among the downregulated genes, one belonged to the cytokine family (CXCL5) and two were interleukin receptors (IL13RA2, PRLR). Other genes belonging to this family were found to be downregulated with further cen3tel propagation, although in many tumors overexpression of cytokines and cytokine receptors promotes tumor growth. Also, IGF2 and HMOX1 were found downregulated in our cellular system, despite positive effects on cancer cell growth and survival have been described for these genes (Tauber et al., 2010). SPOND1 and MMP12 are involved in extracellular matrix formation and remodeling, while KANK4 is a member of the KANK family, which has a role in the formation of actin stress fibers (Zhu et al., 2008). It is worth noticing that KANK1 has been found as a candidate tumor suppressor in renal carcinoma (Roy et al., 2005). Possible tumor suppressor functions have also been described for TES, C15orf48, and WISP2. The latter is downstream of the Wnt signalling pathway and is downregulated in colon tumors, where the expression of other WISP genes is increased. THBD seems to be a negative regulator of epithelial–mesenchymal transition. Finally, two genes with reduced expression are known to be expressed in the nervous system (NPTX1) or in the brain (PTGDS).
Considering the 17 genes as a whole, we can conclude that most genes, whose expression is modulated at early stages after ectopic telomerase expression and maintained throughout all cen3tel propagation, are associated with cancer. Their change in expression in early cen3tel cells is clearly not sufficient for neoplastic transformation, but might have contributed to this phenomenon together with the subsequent expression variations of many other cancer-associated genes.
Functional enrichment analysis of the genes differentially expressed in cen3tel cells at different stages of transformation
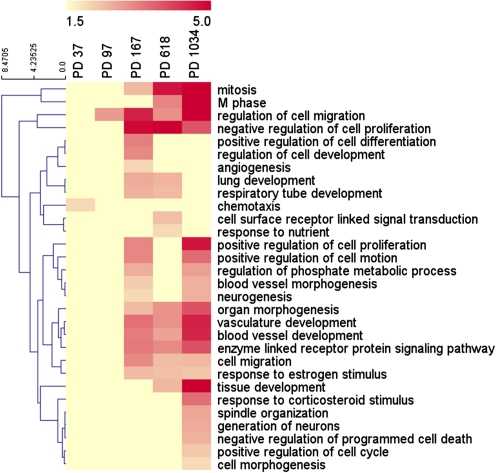
To investigate the possible functional meaning of the molecular changes associated with neoplastic transformation, functional annotation of the lists of genes modulated in cells at each PD was performed using David (http://david.abcc.ncifcrf.gov/). This analysis gave 1 BP5 of the GO overrepresented (Benjamini adjusted enrichment p-value <0.01) at PD 37, 1 at PD 97, 19 at PD 167, 15 at PD 618, and 22 at PD 1034. A total of 30 BP5 were overrepresented in at least one PD (Fig. 2).
FIG. 2.
Heatmap of the union of biological processes (level 5 of the GO) in which at least one PD specific list of differentially expressed genes was enriched. The enrichment score is given by the negative log base 10 of the Benjamini adjusted enrichment p-value. A yellow-to-red gradient was used to indicate, for each process, the level of significance.
Among the overrepresented processes, we found three terms associated with cell movement: regulation of cell migration, positive regulation of cell motion, and cell migration. Analyzing all the genes falling in the three classes and modulated in cen3tel cells at at least one PD (92 in total), we found that the number of modulated genes increased with PDs being 14, 24, 41, 44, and 72 in cells at PD 37, 97, 167, 618, and 1034, respectively (Fig. 3 and SupplementaryTable S1).
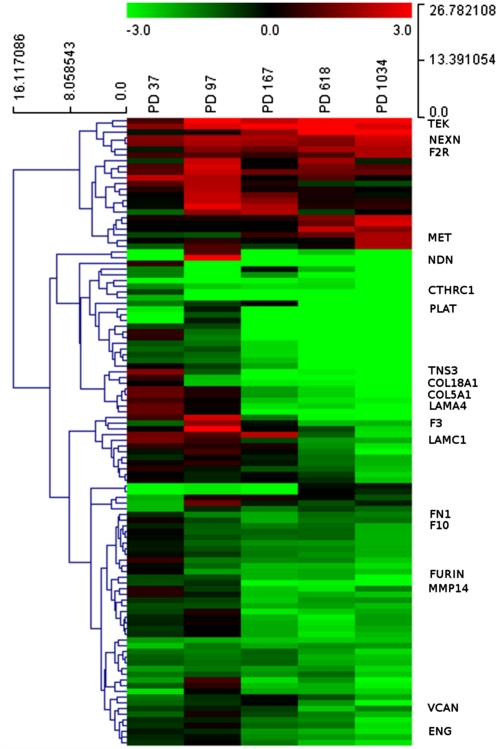
FIG. 3.
Hierarchical clustering applied to the expression matrix of genes involved in cell migration in cen3tel cells at each PD, using Euclidean distance as similarity metrics and complete linkage as linkage method. A red-to-green gradient was used to indicate, for each gene, levels of up- or downregulation in cells at each PD. Genes discussed in the text are indicated. The logFC values of the entire matrix used for hierarchical clustering are provided as Supplementary Table 1.
Mainly starting from PD 167, we found the downregulation of several genes involved in extracellular matrix formation (FN1, VCAN, LAMA4, COL18A1, COL5A1, LAMC1, CTHRC1) or degradation (MMP14, FURIN, PLAT). PLAT is also involved in the coagulation cascade, which is frequently impaired in cancer patients (Kvolik et al., 2010). Three additional genes belonging to this pathway (F10, F2R, F3) showed an early expression alteration in cen3tel cells.
Other interesting downregulated genes were those encoding for the proteins tensin 3 (TENS3) and endoglin (ENG), which act as metastasis suppressors in kidney and prostate cancer, respectively (Lakshman et al., 2011; Martuszewska et al., 2009). Necdin (NDN) was highly downregulated from PD 97 (logFC <−4.7); this gene has tumor suppressive functions and was found to be downregulated also in telomerase immortalized urothelial cells (Chapman and Knowles, 2009).
Finally, among the few upregulated genes, we found nexilin (NEXN, since PD 97), a gene coding for an actin binding protein, which promotes cell migration (Wang et al., 2005); TEK (since PD 97), encoding a tyrosin kinase receptor that binds angiopoietin 1 and promotes angiogenesis and breast cancer metastasis (Min et al., 2010); MET, which was overexpressed in phase III tumorigenic cells only, encodes for the hepatocyte growth factor receptor and is involved in many biological processes, among which metastasis formation (Trusolino et al., 2010).
To have a complete view of the genes coding for ECM proteins and ECM degrading enzymes during neoplastic transformation of cen3tel cells, we analyzed the expression levels of all the genes associated with “proteinaceous extracellular matrix” GO term at different PDs. We found that 304 out of the 313 gene products associated with this term were present on the Agilent platform and more than 100 were downregulated in tumorigenic cells (40 with logFC <−1 and 72 with logFC <−2 in at least one PD), with a tendency to become more downregulated at higher PDs. Only five genes showed a progressive upregulation during transformation (Supplementary Table S2). Similarly, out of about 350 products associated with ECM remodeling, 32 genes were present in our matrix and were downregulated (12 with logFC <−2, 17 with logFC <−1), in particular in tumorigenic cells. On the other hand, three genes were upregulated (MMP16, ADAM15, and ADAM9) (Supplementary Table S3).
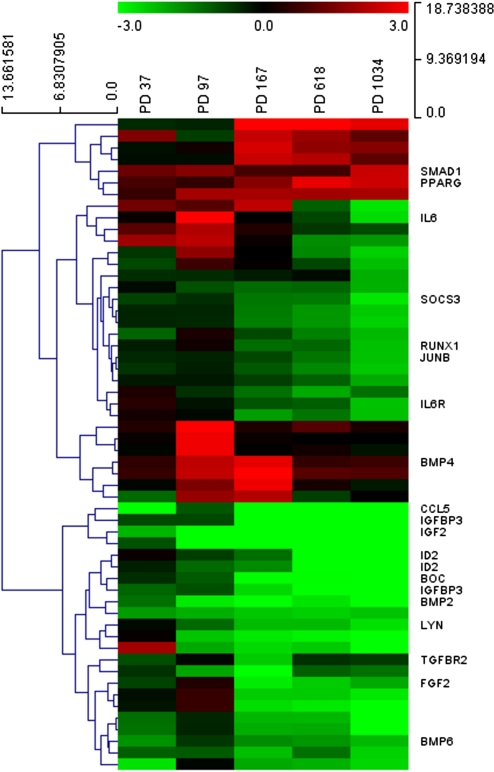
Functional enrichment analysis showed many GO terms referring to organ development and morphogenesis, suggesting that alterations in developmental and differentiation pathways occurred during cen3tel cell transformation. Focusing our attention on the more general term “positive regulation of cell differentiation,” which was found strongly overrepresented in phase I tumorigenic cells (PD 167), we found that several markers of cell differentiation started to be repressed in cells at PD 167, maintaining this downregulation at later stages of transformation, even if at those stages the enrichment of this process was not significant (Fig. 4 and Supplementary Table S4). More in details, we identified 49 cell differentiation promoting genes that were differentially expressed in cells at at least one PD (3, 12, 24, 19, and 36 in cells at PD 37, 97, 167, 618, and 1034, respectively). About 37% of these genes were involved in mesenchymal cell differentiation, in particular, in osteoblast/osteoclast differentiation, a differentiation process that can be undertaken by human skin fibroblasts (Alt et al., 2011). The products of these genes included extracellular signalling molecules involved in bone formation such as BMP2 and BMP6 (BMP2 and BMP6 expression modulation has been confirmed by real-time RT-PCR) (Supplementary Table S5), SMAD1, which is one of the immediate downstream molecules of BMP receptors (Shi et al., 1999), ID2, an early BMP-responsive gene also regulated by transforming growth factor-beta (TGF-β) itself and by other TGF-β family members (Cao et al., 2009), and RUNX1, another integral component of the signaling cascade mediated by bone morphogenetic proteins (Ito and Miyazono, 2003). All these genes showed a decreased expression during the transformation process, except SMAD1, which was upregulated in cells at PD 1034 only and whose main function is, however, carried out by the phosphorylated form of its protein. We also found a peculiar pattern of expression of BMP4, which was overexpressed in cells at PD 97 and 167 (logFC ∼2), but was not modulated in cells at later stages. It is important to mention that BMP proteins belong to the TGF-β superfamily and a role for BMP signaling as suppressor of intestinal tumorigenesis has been described both in humans and in mice (Hardwick et al., 2008).
FIG. 4.
Hierarchical clustering applied to the expression matrix of genes involved in positive regulation of cell differentiation in cen3tel cells at each PD, using Euclidean distance as similarity metrics and complete linkage as linkage method. A red-to-green gradient was used to indicate, for each gene, levels of up- or downregulation in cells at each PD. Genes discussed in the text are indicated. The logFC values of the entire matrix used for hierarchical clustering are provided as Supplementary Table 4.
Other modulated genes were related to osteoclast differentiation (IGFBP3, TGFBR2, SOCS3, PPARG, IL6R) and, more in general, to ossification and bone remodeling processes (JUNB, LYN, CCL5, BOC, IL6, IGF2) (Roux and Orcel, 2000). Also in this case, expression of these transcripts decreased at various steps of transformation, with the only exception of PPARG.
An orchestrated reduction in expression of several differentiation markers of the nervous/immune system, of keratinocytes and adipocytes was also noticeable.
These results highlight a dedifferentiation program in cen3tel cells that started at phase I tumorigenic stage (PD 167) and persisted or got stronger at later steps of transformation.
Interestingly, processes concerning cell cycle (including: mitosis, M phase, negative regulation of cell proliferation, positive regulation of cell proliferation, positive regulation of cell cycle, spindle organization) became overrepresented at PDs 167 and 618, with a stronger enrichment at PD 1034, when many cell cycle-related genes started to be deregulated (Fig. 5, blue bars, and Supplementary Table S6). A total of 215 genes belonged to these processes and, in particular, 15 were differentially expressed in cells at PD 37, 32 in cells at PD 97, 86 in cells at PD 167, 106 in cells at PD 618, and 191 in cells at PD 1034.
FIG. 5.
Hierarchical clustering applied to the expression matrix of genes involved in cell cycle control in cen3tel cells at each PD, using Euclidean distance as similarity metrics and complete linkage as linkage method. A red-to-green gradient was used to indicate, for each gene, levels of up- or downregulation in cells at each PD. The logFC values of the entire matrix used for hierarchical clustering are provided as Supplementary Table 5. Red bars indicate positive regulators of cell proliferation; green bars indicate negative regulators of cell proliferation; blue bars indicate genes that are exclusively modulated at PD 1034.
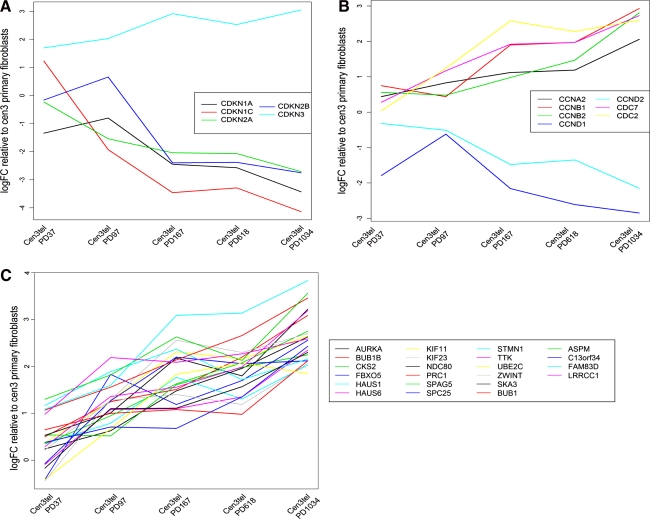
Among the genes belonging to these processes, four cyclin-dependent kinase inhibitors (CDKN1A, CDKN2A, CDKN1C, CDKN2B) were found to be progressively downregulated during transformation, reaching very low expression values in cells at PD 1034 (Fig. 6A). CDKN1C showed the greatest level of downregulation and was downregulated from PD 97, as well as CDKN2A, whose reduced expression was also confirmed by Western blotting (Zongaro et al., 2005). For CDKN1A and CDKN2B, downregulation was more pronounced in tumorigenic cells. CDKN3, which prevents CDK2 kinase activation, was the only cyclin-dependent kinase inhibitor whose expression gradually increased along with culture propagation (Fig. 6A). However, CDKN3 was found to be overexpressed in breast and prostate cancer, suggesting that it can positively contribute to malignant transformation (Lee et al., 2000).
FIG. 6.
Expression of some genes involved in cell cycle control, spindle organization, and check points in cen3tel cells at different PDs. LogFC values are reported. (A) cyclin inhibitors genes, (B) cyclins and cell cycle genes, and (C) genes involved in spindle organization and check point.
Cyclins of the A and B families (CCNA2, CCNB1, CCNB2), whose modulation was also confirmed by Western blotting (Donà et al., in press), showed an increasing expression trend, which culminated in high expression levels in cells at PD 1034 (Fig. 6B). Two cyclins of the D family (CCND1, confirmed by Western blotting, CCND2) showed decreasing expression trends (Fig. 6B); however, because the pRB/CCND1/cdk4/p16INK4a pathway is compromised in these cells because of CDKN2A downregulation, cyclins D reduced expression does not probably negatively influence cen3tel proliferation. Cell cycle kinases, such as the cyclin-dependent kinases CDC7 and CDC2, were overexpressed at late passage cen3tel cells (Fig. 6B). The expression patterns of these genes support the experimental observation of a higher, and increasing with increasing PDs, proliferation rate observed in tumorigenic cells compared to non-tumorigenic ones (Donà et al., in press).
We also found the overrepresentation of mitotic spindle organization and check point genes, whose expression drastically increased, or started to be positively regulated at PD 1034 (Fig. 6C), together with genes involved in chromosome condensation, movement, alignment, and segregation during cell division (AURKB, CENPV, CLASP2, KIF15, KIF18A, KIF20B, KIF2C, KPNA2, NCAPD2, NCAPG, NCAPH, NEK2, NUF2, NUP37, PLK1, PLK3, SMC4), all of which showed increasing expression trends.
Furthermore, we found 28 upregulated genes that are positive regulators of cell proliferation (Fig. 5, red bars) and 44 downregulated genes that are negative regulators of cell proliferation (Fig. 5, green bars).
Besides cell cycle genes, several transcripts belonging to the cancer testis antigen family were upregulated. Cancer testis antigens are characterized by a peculiar pattern of expression, being highly expressed in normal human germ lines and cancer cells, and almost undetectable in normal somatic cells (Caballero and Chen, 2009). Out of 250 genes included in this category (Ludwig Institute list at http://www.cta.lncc.br/modelo.php), 43 were upregulated (logFC >1), mainly in phase II and III tumorigenic cells, with higher levels in cells at the highest PD, six also in phase I and mid cen3tel cells, and none in early cells. We found upregulation of six members of the MAGE family, four of the PAGE and SPANX families, and five of the SSX family (Supplementary Table S7).
The negative regulation of programmed cell death was strongly overrepresented at PD 1034. Most of the genes belonging to this process were downregulated (some of these already at early stages), with a small subset induced, among which the apoptosis inhibitor survivin (BIRC5), which showed increasing expression from phase I (logFC 1.2) to phase III tumorigenic cells (logFC 2.6) (Fig. 7 and Supplementary Table S8). The expression modulation of several genes involved in different aspects of apoptosis highlights the complexity of the regulation of this process during neoplastic transformation.
FIG. 7.
Hierarchical clustering applied to the expression matrix of genes involved in negative regulation of programmed cell death in cen3tel cells at each PD, using Euclidean distance as similarity metrics and complete linkage as linkage method. A red-to-green gradient was used to indicate, for each gene, levels of up- or downregulation in cells at each PD. The logFC values of the entire matrix used for hierarchical clustering are provided as Supplementary Table 7.
Finally, running GSEA (Gene Set Enrichment Analysis; see Materials and Methods) on differentially expressed genes in cells at PD 97, we found a statistically significant enrichment (FDR q-value=0.008) in the “HAN_SATB1_TARGETS_DN” geneset, which is formed by genes downregulated in MDA-MB-231 cells (breast cancer) after RNAi knockdown of SATB1. SATB1 is a genome organizer that regulates chromatin structure and gene expression and it has been shown to promote breast tumor growth and metastasis formation (Han et al., 2008). A total of 20 out of 22 genes that fell into this geneset were upregulated in cells at PD 97, and most of them only at this PD (Supplementary Table S9), reflecting a putative participation of the pathway regulated by SATB1 in determining the initiation of the transformation program.
The same analysis run on upregulated genes at PD 1034 showed the enrichment in a geneset formed by genes induced in melanomas developing distant metastases within 4 years (Winnepenninckx et al., 2006). In this case, 23 genes were found to be induced at the latest stage of transformation only (PD 1034), while 18 were upregulated also at previous stages (Supplementary Table S10).
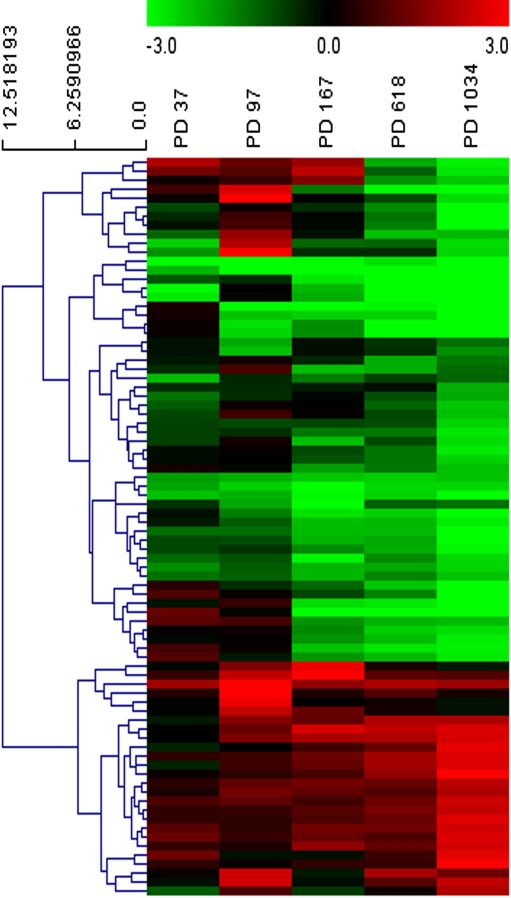
miRNA expression profiling of cen3tel cells at different stages of transformation
miRNA expression profiles were obtained using the Agilent Human miRNA platform containing probes corresponding to 534 unique miRNAs and the same RNA samples used for gene expression analysis (from cells at PDs 97, 167, 618, and 1034), which were analyzed against RNA prepared from cen3 fibroblasts at PD 15. Global miRNA expression profiles divided non-tumorigenic cen3tel cells from tumorigenic ones. Cells at PDs 618 and 1034 were the most similar to one another. Cells at PD 167 were closer to cells at PD 618/1034 than to cells at PD 97, which were in a separated cluster (not shown). A total of 43 miRNAs resulted differentially expressed with an adjusted p-value lower than 0.01 and a logFC greater than +1 or lower than −1 in at least one RNA sample (Table 2). In the majority of cases, miRNA expression appeared progressively modulated with PD progression. To validate the microarray analysis, we analyzed the expression of 4 modulated miRNAs (miR-34a, miR-145, miR-let7b, and miR-20a) in cen3tel cells at different stages of propagation by real-time RT-PCR and, for all of them, we confirmed the same expression trend previously observed (Supplemenentary Table S5).
Table 2.
List of the 43 miRNAs Differentially Expressed in cen3tel Cells at Different PDs Relative to Parental cen3 Fibroblastsa and Number of Negatively Correlated Putative Targets from TargetScan Database
| miRNA name | Cen3tel 97 | Cen3tel 167 | Cen3tel 618 | Cen3tel 1034 | # of negatively correlated targets (Pearson <−0.8) |
|---|---|---|---|---|---|
| hsa-let-7a | −0.73 | −0.90 | −1.59 | −1.49 | 5 |
| hsa-let-7b | −0.95 | −1.32 | −2.70 | −3.06 | 2 |
| hsa-let-7c | −1.21 | −1.35 | −2.36 | −2.30 | 0 |
| hsa-let-7d | −0.55 | −0.50 | −1.49 | −1.62 | 8 |
| hsa-let-7e | −0.71 | −0.75 | −1.42 | −1.36 | 1 |
| hsa-let-7f | −0.72 | −0.85 | −1.67 | −1.67 | 2 |
| hsa-let-7g | −0.54 | −0.68 | −0.84 | −1.03 | 5 |
| hsa-let-7i | −0.50 | −0.82 | −1.13 | −1.20 | 0 |
| hsa-miR-106a | −0.19 | 0.68 | 1.19 | 1.31 | #N/D |
| hsa-miR-10a | −0.39 | −0.66 | −1.53 | −1.32 | 16 |
| hsa-miR-10b | −0.20 | −0.49 | −1.36 | −1.30 | 2 |
| hsa-miR-125a | −0.68 | −0.94 | −1.43 | −1.44 | #N/D |
| hsa-miR-143 | −0.06 | −0.60 | −1.55 | −1.45 | 26 |
| hsa-miR-145 | −0.30 | −0.82 | −2.04 | −1.86 | 22 |
| hsa-miR-148a | −0.82 | −1.11 | −1.51 | −1.53 | 19 |
| hsa-miR-152 | −0.67 | −1.13 | −1.34 | −1.33 | 0 |
| hsa-miR-193b | −0.48 | −0.99 | −1.10 | −1.59 | 26 |
| hsa-miR-195 | −0.44 | −1.07 | −1.04 | −1.16 | 3 |
| hsa-miR-199a* | −1.58 | −2.39 | −2.52 | −2.63 | #N/D |
| hsa-miR-199a-5p | −1.43 | −1.56 | −1.72 | −1.65 | #N/D |
| hsa-miR-199b-5p | −0.97 | −2.36 | −2.56 | −2.56 | #N/D |
| hsa-miR-19a | −0.11 | 0.86 | 1.40 | 1.61 | 50 |
| hsa-miR-19b | −0.29 | 0.56 | 1.19 | 1.20 | 11 |
| hsa-miR-20a | −0.22 | 0.68 | 1.44 | 1.52 | 16 |
| hsa-miR-21 | −1.32 | −1.00 | −0.31 | −0.33 | 46 |
| hsa-miR-214 | −1.75 | −2.42 | −1.81 | −1.95 | 9 |
| hsa-miR-22 | −1.14 | −1.80 | −2.37 | −2.13 | 40 |
| hsa-miR-23a | −0.77 | −0.93 | −1.75 | −1.86 | 72 |
| hsa-miR-23b | −0.95 | −1.81 | −2.44 | −2.56 | 6 |
| hsa-miR-24 | −0.70 | −0.93 | −2.04 | −2.07 | 33 |
| hsa-miR-26a | −0.68 | −1.22 | −1.46 | −1.59 | 26 |
| hsa-miR-27a | −0.59 | −0.54 | −1.32 | −1.40 | 37 |
| hsa-miR-27b | −0.49 | −0.83 | −1.33 | −1.43 | 5 |
| hsa-miR-29a | −0.67 | −2.36 | −1.31 | −1.40 | 5 |
| hsa-miR-30a | −0.53 | −1.02 | −1.51 | −1.79 | 13 |
| hsa-miR-34a | −1.07 | −4.00 | −3.96 | −3.79 | 9 |
| hsa-miR-34b* | −0.87 | −2.12 | −2.11 | −2.13 | 21 |
| hsa-miR-34c-5p | −0.54 | −1.38 | −1.39 | −1.38 | 2 |
| hsa-miR-376a | −0.26 | −1.00 | −1.05 | −1.13 | 22 |
| hsa-miR-377 | 0.00 | −0.86 | −0.91 | −1.03 | 35 |
| hsa-miR-422a | 1.74 | −0.08 | −0.09 | −0.11 | 0 |
| hsa-miR-768-3p | −0.60 | −0.66 | −1.12 | −1.20 | 56 |
| hsa-miR-99a | −1.00 | −0.97 | −1.69 | −1.45 | 0 |
The values are the log2 of the ratio.
Most modulated miRNAs resulted downregulated (39 of 43; 91%). Only four showed increasing levels of expression starting from PD 167 and reached significant overexpression from PD 618 (i.e., miR-106a, miR-19a, miR-19b, and miR-20a). A total of 87% of the differentially expressed miRNAs had at least one negatively correlated predicted target among the genes modulated in at least one PD (Table 2). Gene enrichment analysis on the non-redundant union of the anticorrelated predicted targets (374 in total) showed an involvement into the following GO biological processes (level 2): cell cycle, cell adhesion, development, organelle organization, and chromosome segregation (Benjamini adjusted enrichment p-value <0.01).
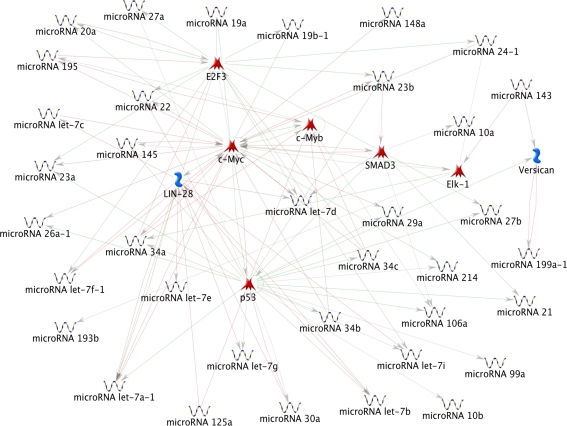
Analyzing the 43 differentially expressed miRNAs with the “shortest path” algorithm implemented in MetaCore, we found that 37 miRNAs out of 43 (86%) resulted connected in a single network (Fig. 8), that also contains the transcription factors p53, c-Myc, c-Myb, Elk-1, Smad3, and E2F3 together with the VCAN and LIN28 gene products. Interestingly, TP53, E2F3, MYC, and SMAD3 gene products resulted connected in the cell cycle pathway (KEGG; K04110) and p53, c-Myc and ELK-1 factors in the highly correlated MAPK signalling pathway (KEGG; k04010), involved in cell proliferation, differentiation and migration. All the eight genes connecting the pathway resulted involved at different levels in neoplastic transformation.
FIG. 8.
Network created with the shortest paths algorithm, which calculates the shortest directed paths between the list of 43 differentially expressed miRNAs.
Based on MetaCore annotation, 21 miRNAs were direct targets of p53: 20 of them showed decreased expression, consistently with p53 inactivation previously described (Zongaro et al., 2005). Among these 21 miRNAs there were the three members of the miRNA-34 family (a, b, and c-5p), demonstrated to be activated by p53 and for which many lines of evidence establish a role as mediators of p53 action in cell-cycle arrest and induction of apoptosis (Chang et al., 2007; He et al., 2007). Putative target genes for the miR-34 family were reported enriched in cell-cycle control and DNA damage response. Among the validated target genes, repressed by miR-34 family members, there were again MYC and E2F3, demonstrating the complex pattern of interactions inside the molecular network controlling neoplastic transformation (Hermeking, 2010). Both at the RNA and protein level, we have found a clear increase in MYC expression in phase I tumorigenic cells followed by a further increase in cells at subsequent phases (Zongaro et al. 2005). Moreover, at least four of the miR-34 predicted target genes appeared to increase their expression in tumorigenic cells, probably because of miR-34 expression inhibition. Interestingly, three of the four genes, E2F5, RUNX2, and FOXG1, encode for transcription factors involved in the TGF-β receptor signalling pathway, impaired in many different tumors; the fourth gene was the cell cycle gene CCNA2.
miR-145 was also shown to act as a tumor suppressor activated by the direct action of p53 on its promoter, and to silence MYC (Sachdeva et al., 2009). Consistently with what reported and similarly to the expression profiles of miR-34 family members, miR-145 was downregulated in tumorigenic cen3tel cells, when p53 became inactivated and c-Myc expression increased (Zongaro et al., 2005), eventually due to the combined action of these miRNAs.
c-Myc transcription factor activation was demonstrated to repress the expression of many different miRNAs, and in particular, through the repression of the LIN28 gene product, of the let-7 miRNA family members (Chang et al., 2009), known to act as tumor suppressors and shown to increase tumor aggressiveness and patient poor prognosis when repressed (Bussing et al., 2008). In our study, eight let-7 miRNAs (a, b, c, d, e, f, g, and i) appeared repressed, in the majority of cases, starting from PD 97 and reaching significance at PD 618.
The proto-oncogene MYC was demonstrated to induce the miR-17-92 cluster (He et al., 2005; O'Donnell et al., 2005), a class of potent oncogenic miRNAs promoting cell proliferation and inhibiting differentiation (Olive et al., 2010). Interestingly, the only four miRNAs that showed increased expression in our dataset, miR-106a, miR-19a, miR-19b, and miR-20a, belong to the miR-17-92 cluster. Among the remaining members of the miR-17-92 cluster, some did not pass the p-value cutoff; the others (i.e., miR-20b, miR17-3p, miR-17-5p, miR-18a; data not shown) showed increasing expression levels without reaching the logFC treshold. Moreover, miR-106a and miR-20a are predicted by miRanda or TargetScan algorithms to bind to the 3′UTR of the RND3 transcripts, whose expression appeared to be significantly anticorrelated (Pearson score <−0.9) and whose role in cell invasion was already established and characterized in the cen3tel cellular system (Belgiovine et al., 2010).
Finally, at least six miRNAs (miR-20a, miR-106a, miR-19a, miR-145, let-7b, miR-34c) could be directly associated with transcriptional modulation of some cell cycle genes (CCND1, CCNB1, CCNA2, CDKN1A) during cen3tel transformation; in fact, their participation in direct regulation of those genes was experimentally validated or predicted in silico (Borgdorff et al., 2010; Bouhallier et al., 2010; Inomata et al., 2009; Jiang et al., 2009; Schultz et al., 2008; Wang et al., 2009).
Discussion
Cen3tel cells gradually underwent spontaneous neoplastic transformation, thus cells at different stages of propagation allowed us to analyze gene expression variations occurring during the transformation process and to determine their temporal onset and accumulation. We determined gene expression profiles of cen3tel cells at different stages of propagation relatively to parental cen3 fibroblasts and found a total of 1,776 genes highly modulated in cells at at least one PD, while micro-RNA expression analysis revealed 43 modulated miRNAs
The majority of the differentially expressed transcripts showed a specific trend of expression (up- or downregulation) during the transformation process. However, we also found transcripts differentially expressed specifically in cells of a single PD. These transcripts represented about 20% of the total expression variations observed in cells of a single PD, except for cen3tel cells at PD 97, in which they corresponded to about 30% of the total changes. In mid-cen3tel cells, which represent the transition stage from non-tumorigenic to tumorigenic cells, we found that most differentially expressed transcripts were upregulated, many of them being upregulated only at this stage, or in some cases also in phase I tumorigenic cells, but not in the subsequent phases. Unexpectedly, many of the genes upregulated in cells at PD 97 overlapped with a group of SATB1-induced genes in breast cancer, which could mediate a SATB1-positive role in tumor growth and metastasis formation, thus in the latest stages of tumor development (Han et al., 2008). Given our observation of their activation in cells at early phases of transformation, we are tempted to speculate that the same genes can act both in the initial and in the late stages of tumorigenesis, probably depending on the genetic background of the cells in which they are overexpressed.
In cen3tel cells at PD 167, the number of genes downregulated greatly increased compared to those upregulated. This tendency, which was maintained in cells at the successive stages, correlated with an increase in DNMT3B expression (logFC 2.5 in cells at PD 167 and ∼1.7 at PD 618 and 1034), a de novo DNA methyl transferase involved in gene silencing and cancer development (Taberlay and Jones, 2011). This observation is in agreement with the known relevant role played by epigenetic phenomena in cancer development (Taberlay and Jones, 2011).
We found 17 genes that were modulated in cen3tel cells at an early stage after hTERT expression and at all the subsequent phases. A total of 15 of these genes showed the same type of modulation at all cen3tel stages; one of them, IGF2, was also found downregulated in urothelial human cells as soon after hTERT expression (Chapman et al., 2008), suggesting that its expression might be regulated by hTERT itself.
The NDN gene was also found highly downregulated both in the hTERT immortalized urothelial cells (Chapman et al., 2008) and in our cellular system from PD 97 and throughout all the subsequent phases (logFC <−4), confirming that this gene plays a role in tumor initiation and progression. NDN is a potential tumor suppressor gene, being downregulated in several types of tumors, and can modulate gene expression interacting with the transcription factor E2F1 (Chapman and Knowles, 2009).
In our cellular system, a decrease in the expression of the cyclin-dependent kinase inhibitors CDKN2A and CDKN1C was an early event during transformation, followed by the accumulation of additional changes in the expression of cell cycle and cell proliferation genes, together with changes in genes involved in chromosome maintenance and segregation. Our results indicate that accumulation of transcriptional variations in genes involved in proliferation and genome instability correlate with increasing aggressiveness and metastasis formation, in agreement with phase III tumorigenic cells' ability to induce subcutaneous tumors in immunocompromised mice with shorter latency compared to previous phase cells, and to form metastases as well. These results are in line and extend to more advanced phases of tumorigenesis those previously reported by Milyavsky et al. (2005), who studied human telomerase immortalized lung fibroblasts just after experimental p53 inactivation. Moreover, it is worth noticing that several genes upregulated at late stages of transformation were previously found to be positively implicated in melanoma metastases (Winnepenninckx et al., 2006), indicating that transcription variations found during transformation progression of cen3tel cells can highlight changes relevant for human tumor progression.
A strong transcriptional signature of phase III tumorigenic cells, and to a less extent of phase II cells, was the overexpression of several cancer-testis antigen genes whose role in tumorigenesis is not completely understood. However, in agreement with our observations, evidence has been reported that their expression correlates with more advanced stages of the disease (Caballero and Chen, 2009; Smith and McNeel, 2010).
Cellular transformation was also associated with impairment in cellular differentiation programs and transcriptional regulation of several genes involved in cell movement. As we already described (Belgiovine et al., 2010), and as we show in more details here, a peculiar characteristic of cen3tel cells was the downregulation of many ECM degrading enzymes, as well as of many ECM proteins. We previously showed that cen3tel cells adopted a protease independent/ROCK dependent mechanism of invasion during transformation, and that this mechanism was strictly linked to RND3 downregulation. Interestingly, miRNA expression analysis revealed that the expression of miR-106a, which has predicted binding sites on the RND3 transcript, was negatively correlated with that of RND3 in cen3tel cells at different PDs, suggesting that RND3 regulation might occur at posttranscriptional levels.
On the whole, miRNA profiles of cen3tel cells at different stages of transformation showed that, as for gene transcripts, most differentially expressed miRNAs were repressed and progressively modulated along PD progression. In particular, we found that members of the miR-34 and let-7 families, the most highly studied miRNAs with a proved tumor suppressor function in cancer (Garzon et al., 2009), drastically decreased their expression in cen3tel cells. On the contrary, several members of the oncogenic miR-17-92 cluster (Garzon et al., 2009) were significantly upregulated starting from PD 618. Moreover, two miRNAs with an antimetastatic (miR-34b/c) and two with a pro-metastatic role (miR-19a/b) (Hurst et al., 2009; Valastyan and Weinberg, 2009) maintained low/high expression levels in cen3tel cells at PD 1034, suggesting their possible contribution in leading cells through metastatic progression.
A relevant finding was that about half of the 43 miRNAs we found as differentially expressed were described to be direct targets of p53; an attractive possibility is that their downregulation was due to p53 inactivation, which we demonstrated in phase I tumorigenic cen3tel cells. In turn, eight of these miRNAs (miR-34 family members, let-7a/c/f, miR-24, and miR-145) putatively silence MYC, another key regulator of cellular transformation, whose expression started to increase in tumorigenic phase I cells also. We also found many other deregulated miRNAs that were described to be repressed by c-Myc. Some of them seem to be involved in a feed-forward loop that operates in the fine tuning of MYC transcriptional regulation (miR-34a, let-7a, and miR-145). c-Myc also seemed to induce members of the miR-17-92 family, which represented the only upregulated miRNAs in our experiments.
Finally, several miRNAs could be associated with the observed impairment in cell cycle control, as suggested by their participation in directly regulating some cell cycle genes that we found to be deregulated in gene expression experiments.
Conclusions
The analysis of the gene and miRNA transcriptional programs in cen3tel cells at different phases of transformation allowed us to highlight alterations in specific biological processes during neoplastic transformation and to follow their progressive deregulation. Moreover, our results revealed a fine regulation circuitry constituted by a tight crosstalk between genes and miRNA signalling pathways, which both contribute to the control of tumorigenesis and malignant transformation.
Supplementary Material
Acknowledgments
This work was supported by Fondazione Cariplo (Grant nos. 2006-0734 and 2010-0253). P.O. was supported by a grant by Lauretana S.P.A.; C.G. and G.C. were supported by a grant from Compagnia di San Paolo.
Author Disclosure Statement
The authors declare that no conflicting financial interests exist.
References
- Alt E. Yan Y. Gehmert S. Song Y.H. Altman A. Vykoukal D., et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103:197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- Belgiovine C. Frapolli R. Bonezzi K. Chiodi I. Favero F. Mello-Grand M., et al. Reduced expression of the ROCK inhibitor Rnd3 is associated with increased invasiveness and metastatic potential in mesenchymal tumor cells. PLoS One. 2010;5:e14154. doi: 10.1371/journal.pone.0014154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y. Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- Borgdorff V. Lleonart M.E. Bishop C.L. Fessart D. Bergin A.H. Overhoff M.G., et al. Multiple microRNAs rescue from Ras-induced senescence by inhibiting p21(Waf1/Cip1) Oncogene. 2010;29:2262–2271. doi: 10.1038/onc.2009.497. [DOI] [PubMed] [Google Scholar]
- Bouhallier F. Allioli N. Lavial F. Chalmel F. Perrard M.H. Durand P., et al. Role of miR-34c microRNA in the late steps of spermatogenesis. RNA. 2010;16:720–731. doi: 10.1261/rna.1963810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussing I. Slack F.J. Grosshans H. let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14:400–409. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Caballero O.L. Chen Y.T. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci. 2009;100:2014–2021. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Liu X. Zhang W. Deng X. Zhang H. Liu Y., et al. TGF-beta repression of Id2 induces apoptosis in gut epithelial cells. Oncogene. 2009;28:1089–1098. doi: 10.1038/onc.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.C. Wentzel E.A. Kent O.A. Ramachandran K. Mullendore M. Lee K.H., et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T.C. Zeitels L.R. Hwang H.W. Chivukula R.R. Wentzel E.A. Dews M., et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci USA. 2009;106:3384–3389. doi: 10.1073/pnas.0808300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J. Kelly G. Knowles M.A. Genes involved in differentiation, stem cell renewal, and tumorigenesis are modulated in telomerase-immortalized human urothelial cells. Mol Cancer Res. 2008;6:1154–1168. doi: 10.1158/1541-7786.MCR-07-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J. Knowles M.A. Necdin: a multi functional protein with potential tumor suppressor role? Mol Carcinog. 2009;48:975–981. doi: 10.1002/mc.20567. [DOI] [PubMed] [Google Scholar]
- Donà F. Chiodi I. Belgiovine C. Raineri T. Ricotti R. Mondello C., et al. Poly(ADP-ribosylation) and neoplastic transformation: effect of PARP inhibitors. Curr Pharmaceut Biotechnol. doi: 10.2174/138920101405131111104642. (in press). [DOI] [PubMed] [Google Scholar]
- Garzon R. Calin G.A. Croce C.M. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- Gentleman R.C. Carey V.J. Bates D.M. Bolstad B. Dettling M. Dudoit S., et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H.J. Russo J. Kohwi Y. Kohwi-Shigematsu T. SATB1 reprogrammes gene expression to promote breast tumour growth and metastasis. Nature. 2008;452:187–193. doi: 10.1038/nature06781. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hardwick J.C. Kodach L.L. Offerhaus G.J. Van den Brink G.R. Bone morphogenetic protein signalling in colorectal cancer. Nat Rev Cancer. 2008;8:806–812. doi: 10.1038/nrc2467. [DOI] [PubMed] [Google Scholar]
- He L. Thomson J.M. Hemann M.T. Hernando-Monge E. Mu D. Goodson S., et al. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. He X. Lim L.P. De Stanchina E. Xuan Z. Liang Y., et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- Hurst D.R. Edmonds M.D. Welch D.R. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69:7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inomata M. Tagawa H. Guo Y.M. Kameoka Y. Takahashi N. Sawada K. MicroRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood. 2009;113:396–402. doi: 10.1182/blood-2008-07-163907. [DOI] [PubMed] [Google Scholar]
- Ito Y. Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43–47. doi: 10.1016/s0959-437x(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Jiang Q. Feng M.G. Mo Y.Y. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer. 2009;9:194. doi: 10.1186/1471-2407-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvolik S. Jukic M. Matijevic M. Marjanovic K. Glavas-Obrovac L. An overview of coagulation disorders in cancer patients. Surg Oncol. 2010;19:e33–46. doi: 10.1016/j.suronc.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Lakshman M. Huang X. Ananthanarayanan V. Jovanovic B. Liu Y. Craft C.S., et al. Endoglin suppresses human prostate cancer metastasis. Clin Exp Metastasis. 2011;28:39–53. doi: 10.1007/s10585-010-9356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W. Reimer C.L. Fang L. Iruela-Arispe M.L. Aaronson S.A. Overexpression of kinase-associated phosphatase (KAP) in breast and prostate cancer and inhibition of the transformed phenotype by antisense KAP expression. Mol Cell Biol. 2000;20:1723–1732. doi: 10.1128/mcb.20.5.1723-1732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B.P. Burge C.B. Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Martuszewska D. Ljungberg B. Johansson M. Landberg G. Oslakovic C. Dahlback B., et al. Tensin3 is a negative regulator of cell migration and all four Tensin family members are downregulated in human kidney cancer. PLoS One. 2009;4:e4350. doi: 10.1371/journal.pone.0004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michor F. Iwasa Y. Lengauer C. Nowak M.A. Dynamics of colorectal cancer. Semin Cancer Biol. 2005;15:484–493. doi: 10.1016/j.semcancer.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Milyavsky M. Tabach Y. Shats I. Erez N. Cohen Y. Tang X., et al. Transcriptional programs following genetic alterations in p53, INK4A, and H-Ras genes along defined stages of malignant transformation. Cancer Res. 2005;65:4530–4543. doi: 10.1158/0008-5472.CAN-04-3880. [DOI] [PubMed] [Google Scholar]
- Min Y. Ren X. Vaught D.B. Chen J. Donnelly E. Lynch C.C., et al. Tie2 signaling regulates osteoclastogenesis and osteolytic bone invasion of breast cancer. Cancer Res. 2010;70:2819–2828. doi: 10.1158/0008-5472.CAN-09-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondello C. Chiesa M. Rebuzzini P. Zongaro S. Verri A. Colombo T., et al. Karyotype instability and anchorage-independent growth in telomerase-immortalized fibroblasts from two centenarian individuals. Biochem Biophys Res Commun. 2003;308:914–921. doi: 10.1016/s0006-291x(03)01484-0. [DOI] [PubMed] [Google Scholar]
- O'Donnell K.A. Wentzel E.A. Zeller K.I. Dang C.V. Mendell J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Olive V. Jiang I. He L. mir-17-92, a cluster of miRNAs in the midst of the cancer network. Int J Biochem Cell Biol. 2010;42:1348–1354. doi: 10.1016/j.biocel.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradervand S. Weber J. Thomas J. Bueno M. Wirapati P. Lefort K., et al. Impact of normalization on miRNA microarray expression profiling. RNA. 2009;15:493–501. doi: 10.1261/rna.1295509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou P. Villalonga P. Ridley A.J. Rnd proteins: multifunctional regulators of the cytoskeleton and cell cycle progression. Bioessays. 2010;32:986–992. doi: 10.1002/bies.201000060. [DOI] [PubMed] [Google Scholar]
- Roux S. Orcel P. Bone loss. Factors that regulate osteoclast differentiation: an update. Arthritis Res. 2000;2:451–456. doi: 10.1186/ar127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B.C. Aoyagi T. Sarkar S. Nomura K. Kanda H. Iwaya K., et al. Pathological characterization of Kank in renal cell carcinoma. Exp Mol Pathol. 2005;78:41–48. doi: 10.1016/j.yexmp.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Sachdeva M. Zhu S. Wu F. Wu H. Walia V. Kumar S., et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci USA. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A.I. Bhagabati N.K. Braisted J.C. Liang W. Sharov V. Howe E.A., et al. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- Schultz J. Lorenz P. Gross G. Ibrahim S. Kunz M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–557. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- Shi X. Yang X. Chen D. Chang Z. Cao X. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem. 1999;274:13711–13717. doi: 10.1074/jbc.274.19.13711. [DOI] [PubMed] [Google Scholar]
- Smith H.A. McNeel D.G. The SSX family of cancer-testis antigens as target proteins for tumor therapy. Clin Dev Immunol. 20102010:150591. doi: 10.1155/2010/150591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Subramanian A. Tamayo P. Mootha V.K. Mukherjee S. Ebert B.L. Gillette M.A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlay P.C. Jones P.A. DNA methylation and cancer. Prog Drug Res. 2011;67:1–23. doi: 10.1007/978-3-7643-8989-5_1. [DOI] [PubMed] [Google Scholar]
- Tauber S. Jais A. Jeitler M. Haider S. Husa J. Lindroos J., et al. Transcriptome analysis of human cancer reveals a functional role of heme oxygenase-1 in tumor cell adhesion. Mol Cancer. 2010;9:200. doi: 10.1186/1476-4598-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusolino L. Bertotti A. Comoglio P.M. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Valastyan S. Weinberg R.A. MicroRNAs: Crucial multi-tasking components in the complex circuitry of tumor metastasis. Cell Cycle. 2009;8:3506–3512. doi: 10.4161/cc.8.21.9802. [DOI] [PubMed] [Google Scholar]
- Vie N. Copois V. Bascoul-Mollevi C. Denis V. Bec N. Robert B., et al. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol Cancer. 2008;7:14. doi: 10.1186/1476-4598-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Tang H. Thayanithy V. Subramanian S. Oberg A.L. Cunningham J.M., et al. Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res. 2009;69:9490–9497. doi: 10.1158/0008-5472.CAN-09-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Zhang W. Han Y. Chen J. Wang Y. Zhang Z., et al. NELIN, a new F-actin associated protein, stimulates HeLa cell migration and adhesion. Biochem Biophys Res Commun. 2005;330:1127–1131. doi: 10.1016/j.bbrc.2005.03.082. [DOI] [PubMed] [Google Scholar]
- Winnepenninckx V. Lazar V. Michiels S. Dessen P. Stas M. Alonso S.R., et al. Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98:472–482. doi: 10.1093/jnci/djj103. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Kakinuma N. Wang Y. Kiyama R. Kank proteins: a new family of ankyrin-repeat domain-containing proteins. Biochim Biophys Acta. 2008;1780:128–133. doi: 10.1016/j.bbagen.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Zongaro S. De Stanchina E. Colombo T. D'Incalci M. Giulotto E. Mondello C. Stepwise neoplastic transformation of a telomerase immortalized fibroblast cell line. Cancer Res. 2005;65:11411–11418. doi: 10.1158/0008-5472.CAN-05-1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.