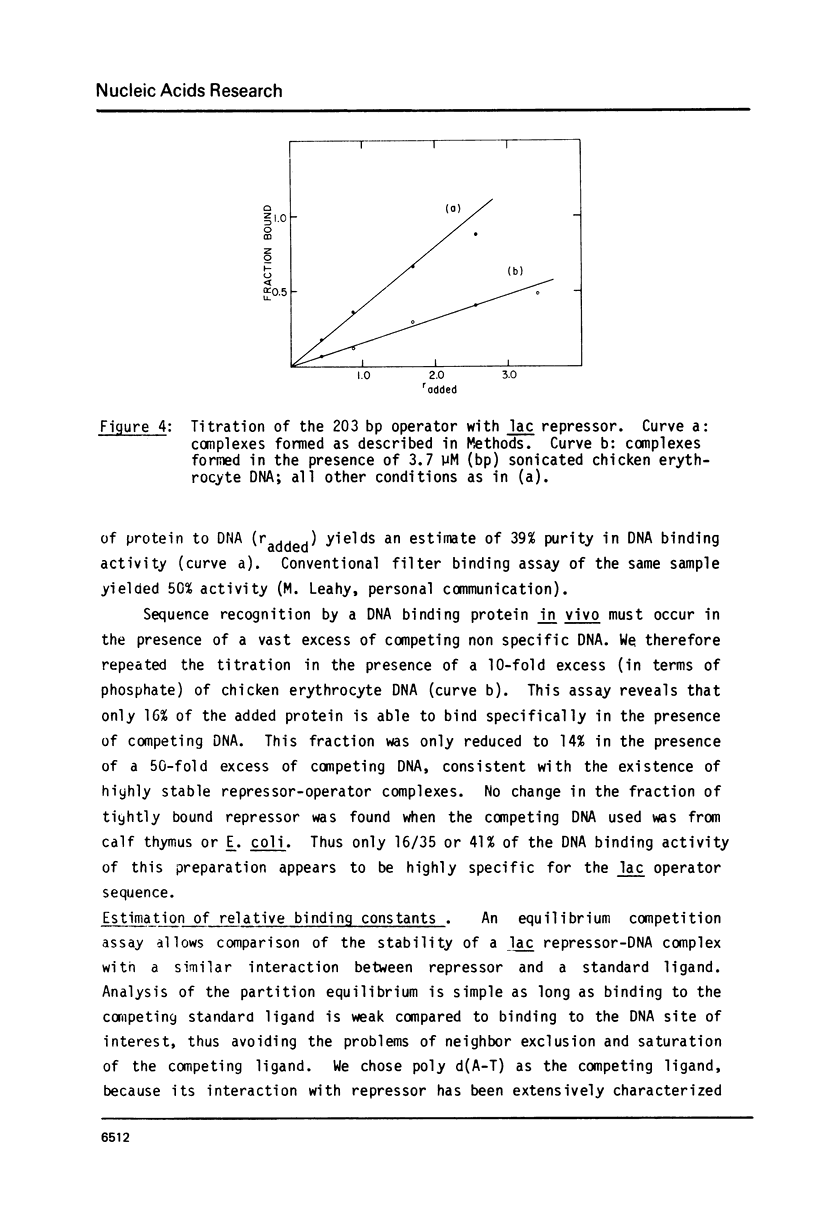
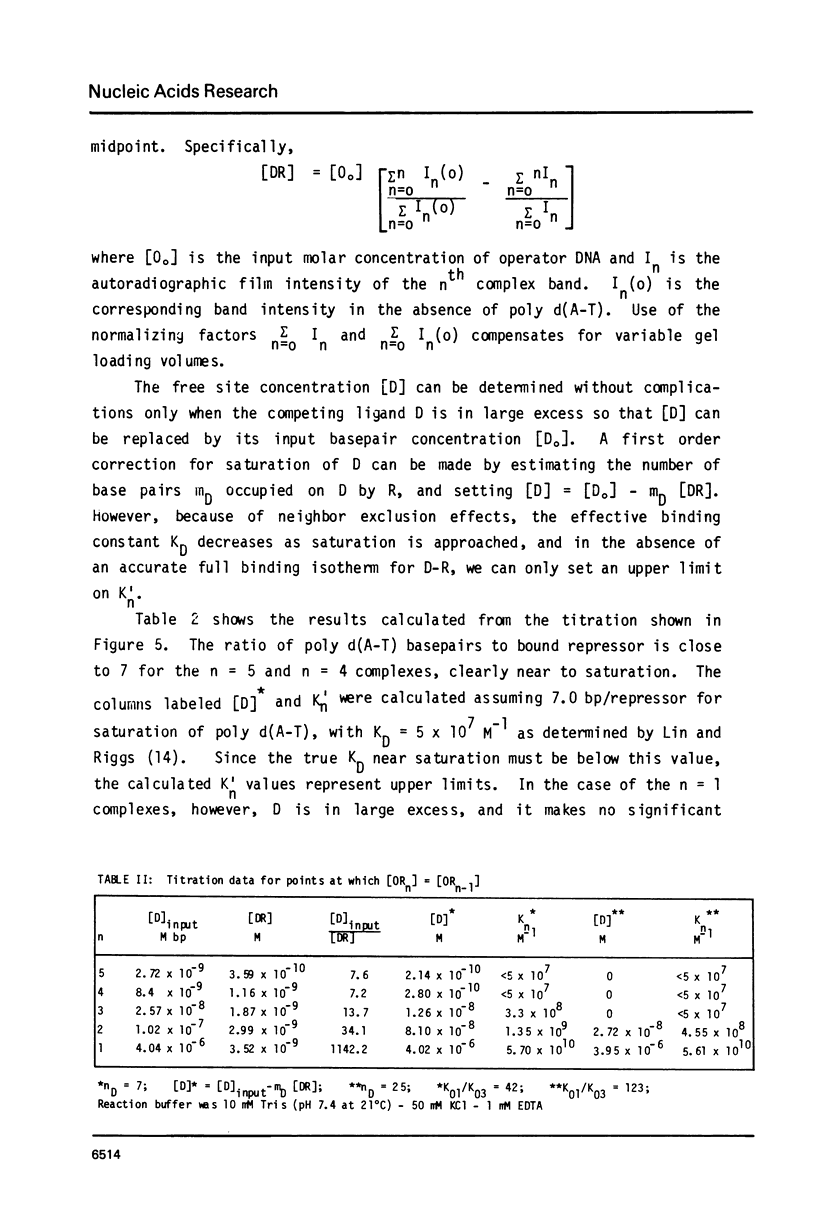
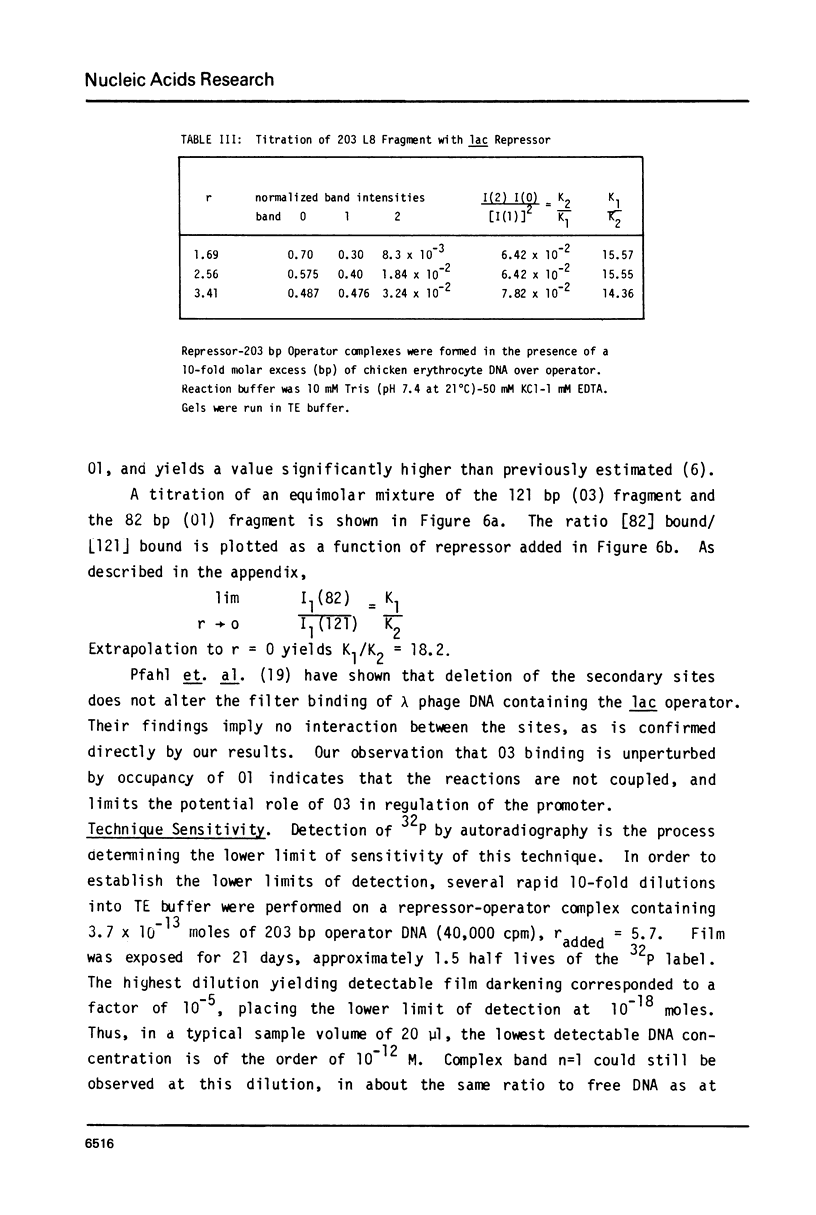
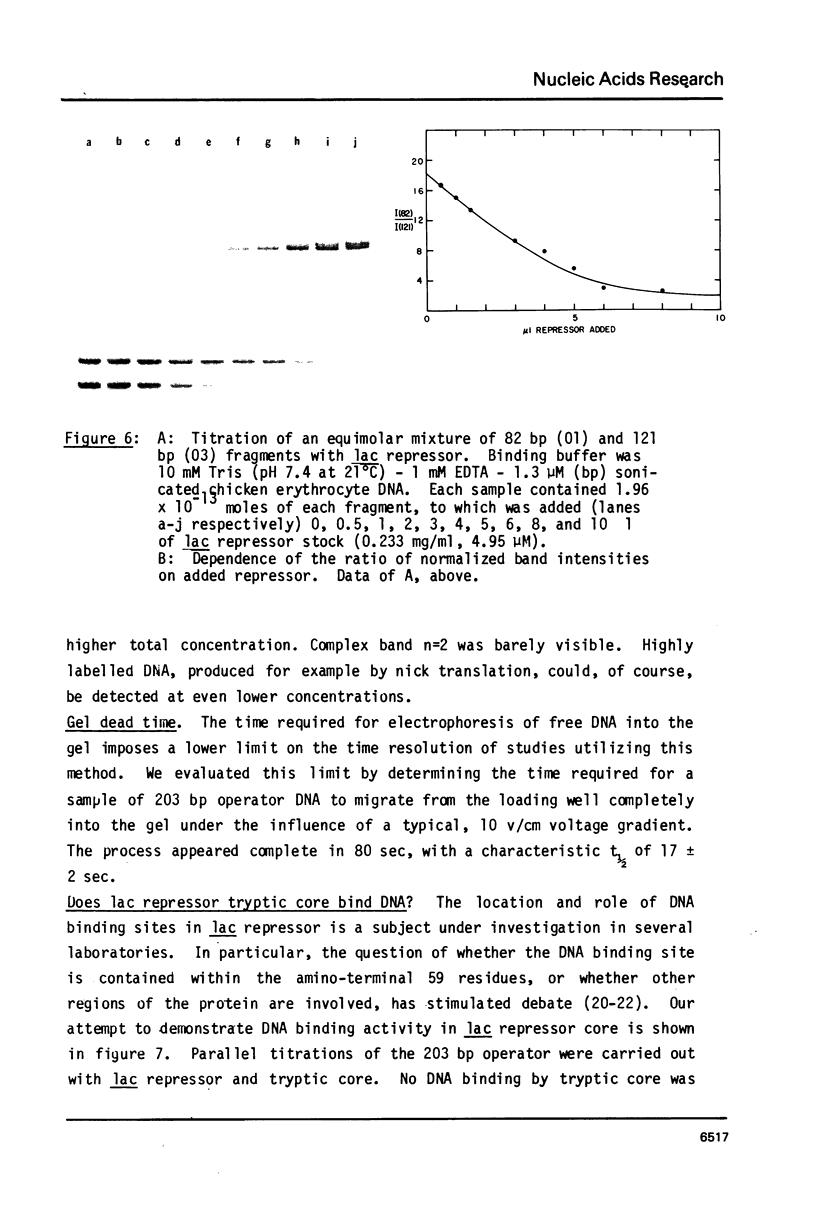
Abstract
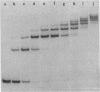
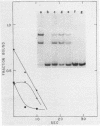
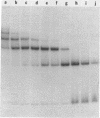
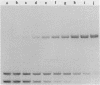
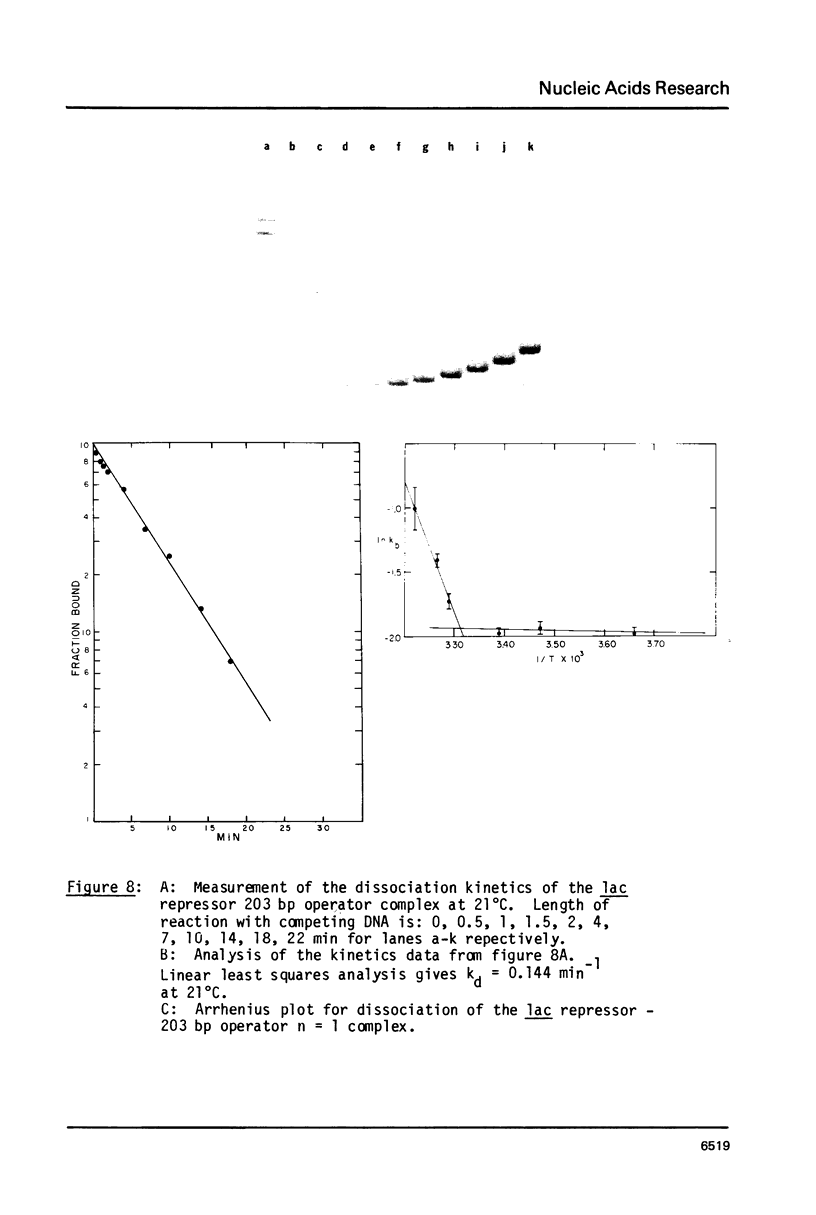
We describe the use of gel electrophoresis in studies of equilibrium binding, site distribution, and kinetics of protein-DNA interactions. The method, which we call protein distribution analysis, is simple, sensitive and yields thermodynamically rigorous results. It is particularly well suited to studies of simultaneous binding of several proteins to a single nucleic acid. In studies of the lac repressor-operator interaction, we found that binding to the so-called third operator site (03) is 15-18 fold weaker than operator binding, and that the binding reactions with the first and third operators are uncoupled, implying that there is no communication between the sites. Pseudo-first order dissociation kinetics of the repressor-203 bp operator complex were found to be temperature sensitive, with delta E of 80 kcal mol-1 above 29 degrees C and 26 kcal mol-1 below. The half life of the complex (5 min at 21 degrees C) is shorter than that reported for very high molecular weight operator-containing DNAs, but longer than values reported for much shorter fragments. The binding of lac repressor core to DNA could not be detected by this technique: the maximum binding constant consistent with this finding is 10(5) M-1.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bresloff J. L., Crothers D. M. DNA-ethidium reaction kinetics: demonstration of direct ligand transfer between DNA binding sites. J Mol Biol. 1975 Jun 15;95(1):103–123. doi: 10.1016/0022-2836(75)90339-3. [DOI] [PubMed] [Google Scholar]
- Clement R., Daune M. P. Binding of lactose repressor to poly d(A-T) : OD AND CD melting of the complex. Nucleic Acids Res. 1975 Mar;2(3):303–318. doi: 10.1093/nar/2.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M. Calculation of binding isotherms for heterogenous polymers. Biopolymers. 1968 Apr;6(4):575–584. doi: 10.1002/bip.1968.360060411. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of a set of hybrid lac repressors made in vitro between normal lac repressor and its homogeneous tryptic core. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3103–3106. doi: 10.1073/pnas.73.9.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of amino-terminal fragment of lactose repressor necessary for DNA binding. Biochemistry. 1977 Mar 8;16(5):938–943. doi: 10.1021/bi00624a020. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yansura D. G., Caruthers M. H. Binding of synthetic lactose operator DNAs to lactose represessors. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3292–3296. doi: 10.1073/pnas.74.8.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M. H. Non-linear Arrhenius plots in temperature-dependent kinetic studies of enzyme reactions. I. Single transition processes. J Theor Biol. 1972 Jun;35(3):543–568. doi: 10.1016/0022-5193(72)90150-6. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to non-operator DNA: detailed studies and a comparison of eequilibrium and rate competition methods. J Mol Biol. 1972 Dec 30;72(3):671–690. doi: 10.1016/0022-2836(72)90184-2. [DOI] [PubMed] [Google Scholar]
- Lin S., Riggs A. D. A comparison of lac repressor binding to operator and to nonoperator DNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):704–710. doi: 10.1016/0006-291x(75)90456-8. [DOI] [PubMed] [Google Scholar]
- Matthews K. S. Tryptic core protein of lactose repressor binds operator DNA. J Biol Chem. 1979 May 10;254(9):3348–3353. [PubMed] [Google Scholar]
- Matthews K. S. Tryptic core protein of lactose repressor binds operator DNA. J Biol Chem. 1979 May 10;254(9):3348–3353. [PubMed] [Google Scholar]
- Maurizot J. C., Charlier M., Hélène C. Lac repressor binding to poly (d(A-T)). Conformational changes. Biochem Biophys Res Commun. 1974 Oct 8;60(3):951–957. doi: 10.1016/0006-291x(74)90406-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfahl M., Gulde V., Bourgeois S. "Second" and "third operator" of the lac operon: an investigation of their role in the regulatory mechanism. J Mol Biol. 1979 Jan 25;127(3):339–344. doi: 10.1016/0022-2836(79)90333-4. [DOI] [PubMed] [Google Scholar]
- Platt T., Files J. G., Weber K. Lac repressor. Specific proteolytic destruction of the NH 2 -terminal region and loss of the deoxyribonucleic acid-binding activity. J Biol Chem. 1973 Jan 10;248(1):110–121. [PubMed] [Google Scholar]
- Revzin A., von Hippel P. H. Direct measurement of association constants for the binding of Escherichia coli lac repressor to non-operator DNA. Biochemistry. 1977 Nov 1;16(22):4769–4776. doi: 10.1021/bi00641a002. [DOI] [PubMed] [Google Scholar]
- Richter P. H., Eigen M. Diffusion controlled reaction rates in spheroidal geometry. Application to repressor--operator association and membrane bound enzymes. Biophys Chem. 1974 Oct;2(3):255–263. doi: 10.1016/0301-4622(74)80050-5. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Bourgeois S., Cohn M. The lac repressor-operator interaction. 3. Kinetic studies. J Mol Biol. 1970 Nov 14;53(3):401–417. doi: 10.1016/0022-2836(70)90074-4. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]