Abstract
Pancreatic cancer has a poor prognosis and is associated with high levels of psychological stress that may adversely affect clinical outcomes. However, the potential influence of neuropsychological factors on pancreatic cancer has not been investigated to date. Using a mouse model of social stress, we have tested the hypothesis that psychological stress promotes the progression of pancreatic cancer xenografts via neurotransmitter-induced activation of multiple pathways and that the inhibitory neurotransmitter γ-aminobutiric acid (GABA) inhibits these responses. Sytemic and xenograft levels of noradrenalin, adrenalin, GABA, cortisol, vascular endothelial growth factor (VEGF) and cyclic adenosine 3′, 5′-monophosphate (cAMP) were measured by immunoassays. Xenograft expression of nicotinic acetylcholine receptors (nAChRs) α3, α4, α5, α6 and α7 and β-adrenergic receptors 1 and 2 were assessed by real-time PCR and western blots. Expression of glutamate decarboxylases GAD65 and GAD67 and phosphorylated and unphosphorylated signaling proteins of relevance to pancreatic cancer were determined in tumor tissue by western blots. Psychological stress significantly promoted xenograft growth and increased systemic and tumor levels of noradrenalin, adrenalin, cortisol, VEGF and cAMP while GABA and GAD were suppressed. Stress upregulated nAChR proteins but not RNAs and induced phosphorylated ERK, CREB, Src and AKT in xenografts. Reduction of cAMP by treatment with GABA prevented tumor progression and activation of signaling proteins. Our findings suggest that neurotransmitter responses to psychological stress negatively impact clinical outcomes of pancreatic cancer via the activation of multiple pathways and that replacement of the suppressed inhibitory neurotransmitter GABA prevents these effects.
Introduction
Pancreatic cancer is the fourth leading cause of cancer deaths in developed countries with a 5 year survival rate <5% (1,2). The introduction of targeted agents that block cellular regulatory pathways has failed to add significant survival benefits despite very promising preclinical data (1). Novel strategies for the improvement of pancreatic cancer intervention are therefore urgently needed.
One neglected area of cancer research is the influence of neuropsychological factors on disease progression and cancer intervention outcomes. High levels of psychological stress have been associated with increased cancer mortality (3) but the underlying mechanisms are poorly understood. Pancreatic cancer patients demonstrated the highest levels of psychological stress among 9000 investigated cancer patients (4). In accord with these findings, the histochemical analysis of 30 pancreatic cancer cases showed overexpression of the stress neurotransmitter noradrenalin in all tumor tissues (5), suggesting a potential influence on tumor regulation.
Stress responses are governed by the nicotinic acetylcholine receptor (nAChR) regulated release of the stress neurotransmitters noradrenalin and adrenalin and of the stress hormone cortisol (6). The effects of noradrenalin and adrenalin are mediated by adrenergic receptors (7). Human pancreatic cancer cell lines express β1- and β2-adrenergic receptors, with β2 predominating (8). Treatment of the cells with selective β-adrenergic ligands revealed that agonists stimulated while antagonists inhibited DNA synthesis and cell migration (5,8). These responses were cyclic adenosine 3′,5′-monophosphate (cAMP) dependent and involved phosphorylation of CREB and transactivation of the epidermal growth factor receptor pathway (9). The inhibitory neurotransmitter γ-aminobutyric acid (GABA) reversed these effects (5). Gene knockdown of the Gαi-coupled GABA-B receptor blocked the effects of GABA, suggesting Gαi-mediated inhibition of adenylyl cyclase activation as the underlying mechanism. These findings suggest that neurotransmitter responses to psychological stress may significantly promote the progression of pancreatic cancer and that inhibition of the resulting hyperactive cAMP signaling by GABA may reverse this effect. Using an established mouse model of chronic social stress (10), we have tested this hypothesis in nude mice carrying xenografts from two human pancreatic cancer cell lines.
Materials and methods
Cell lines
The human pancreatic cancer cell lines Panc-1 and BXPC-3 (American Type Culture Collection, Rockville, MD) were cultured as suggested by the vendor. Both the cell lines were authenticated by Research Animal Diagnostic Laboratory (RADIL, Columbia, MO) immediately prior to the start of the current experiments.
Animal experiment
The animal experiment was approved by the Institutional Animal Care and Use Committee. Male, 6-week-old athymic nude mice (Harlan Sprague Dawley, Indianapolis, IN) were housed under standard laboratory conditions with free access to autoclaved food (Purina Rodent Chow) and water. The mice were provided with numbered ear tags and were randomly assigned five mice per cage to treatment groups (n = 20). Mice in two groups were exposed to social stress for 4 weeks according to published procedure (10) by changing the group composition of each cage twice per week. One group of these animals was subcutaneously inoculated in the flank with Panc-1 or BXPC-3 cells (3 × 106 in 0.2 ml of phosphate-buffered saline, viability >95%). Social stress was continued in both the groups for another 30 days. Two additional groups of mice that were not exposed to stress were inoculated with identical numbers of cancer cells from each cell lines. Two additional groups of mice (one group with and one group without social stress) inoculated with the more aggressively growing BXPC-3 cells were treated by intraperitoneal injections of GABA (Sigma, St Louis, MO; 10 mg/kg 5 days/week for 30 days). The dose of GABA is within the recommended daily human dose (10–32 mg/kg). Two perpendicular diameters (length and width) of each xenograft were measured weekly and tumor volumes were calculated (length/2) × (width2). The animals were euthanized by CO2 inhalation 30 days after tumor cell inoculation. Blood samples and tumors were collected and snap frozen in liquid nitrogen.
Immunoassays for the detection of noradrenalin, adrenalin, cortisol, GABA, VEGF and cAMP
Systemic and xenograft levels of noradrenalin, adrenalin, GABA, cortisol, vascular endothelial growth factor (VEGF) and cAMP were measured by immunoassays according to the manufacturer's instruction (adrenalin and noradrenalin: 2-CAT ELISA, GABA: GABA Elisa, Rocky Mountain Diagnostic, Colorado Spring, CO; cortisol: EIA kit: Assay Designs, Ann Arbor, MI; VEGF: Enzo Life Sciences International, Plymouth Meeting, PA; cAMP: direct cyclic AMP enzyme immunoassay, Assay Designs). Absorbance was read with an ELISA reader at 450 nm for the neurotransmitters, cortisol and VEGF and at 405 nm for cAMP.
Protein analyses by semiquantitative western blotting
Western blots were performed as described (11) with the following primary antibodies: nAChR subunits α3, α5, α7 (Abcam, Cambridge, MA), α4 and α6 (Millipore, Billerica, MA); β1- and β2-adrenergic receptor, GAD 65 and GAD 67 (Abcam), total CREB (Millipore), p-CREB, p-ERK1/2, ERK1/2, non-phospho-Src, phospho-Src, pan AKT and phospho-AKT (Cell Signaling, Danvers, MA); β-actin (Sigma). Densitometry (NIH Image Gel) was conducted on three independent western blots. Following background subtraction, mean densities of four rectangular areas of standard size per band were determined (n = 12 for three Westerns). Values were adjusted for actin (nAChRs, β-adrenergic receptors and GADs) or for unphosphorylated proteins (CREB, ERK, Src, AKT).
Real-time PCR
RNA isolation and quantitative analysis of messenger RNAs (mRNAs) were done by real-time PCR as described (12). The primers for α5 subunit mRNA were forward 5′-aaggcggaggagaccctatc-3′ and reverse 5′-gctggcaggcaatctaaattcatc-3′ (GenBank accession no. NM_000745), and the internal TaqMan probe was 6-FAM-ttaatcgtaggcaggtgtttaatgcca-BHQ1 (Biosearch Technologies, Novato, CA). The real-time PCR conditions for α5 were 95°C for 120 s, followed by 45 cycles of 95°C, 15 s; 56°C, 10 s and 72°C, 15 s. QuantiTect Primer assays (Qiagen, Valencia, CA) were used along with the QuantiFast SYBR Green PCR kit for mRNAs of nAChR subunits α3, α4, α6, α7 and the β1- and β2-adrenergic receptors. 18S rRNA detection reagents (Eurogentec, San Diego, CA) served for normalization. Real-time PCR data were analyzed using the 2-ΔΔCT method (13).
Statistical analysis of data
Statistical analysis was performed using Graphpad Instat software. Statistical analysis of tumor volumes of the two treatment groups (n = 20) with Panc-1 xenografts was by non-parametric Mann–Whitney test. Statistical analysis of tumor volumes of the four treatment groups (n = 20) with BXPC-3 xenografts was by non-parametric analysis of variance (Kruskal–Wallis) followed by Dunn's multiple comparison test.
Statistical evaluation of noradrenalin (n = 5), adrenalin (n = 5), GABA (n = 5), cortisol (n = 5), VEGF (n = 5) and cAMP (n = 5) in blood and xenograft tissues was assessed by Mann–Whitney test.
Statistical analysis of four densitometric readings per protein band from three independent western blots prepared from three randomly selected xenografts per treatment group (n = 12) was by Mann–Whitney test.
Statistical evaluation of real-time PCR data (n = 5) was by unpaired two-tailed t-test.
Results
Xenograft growth
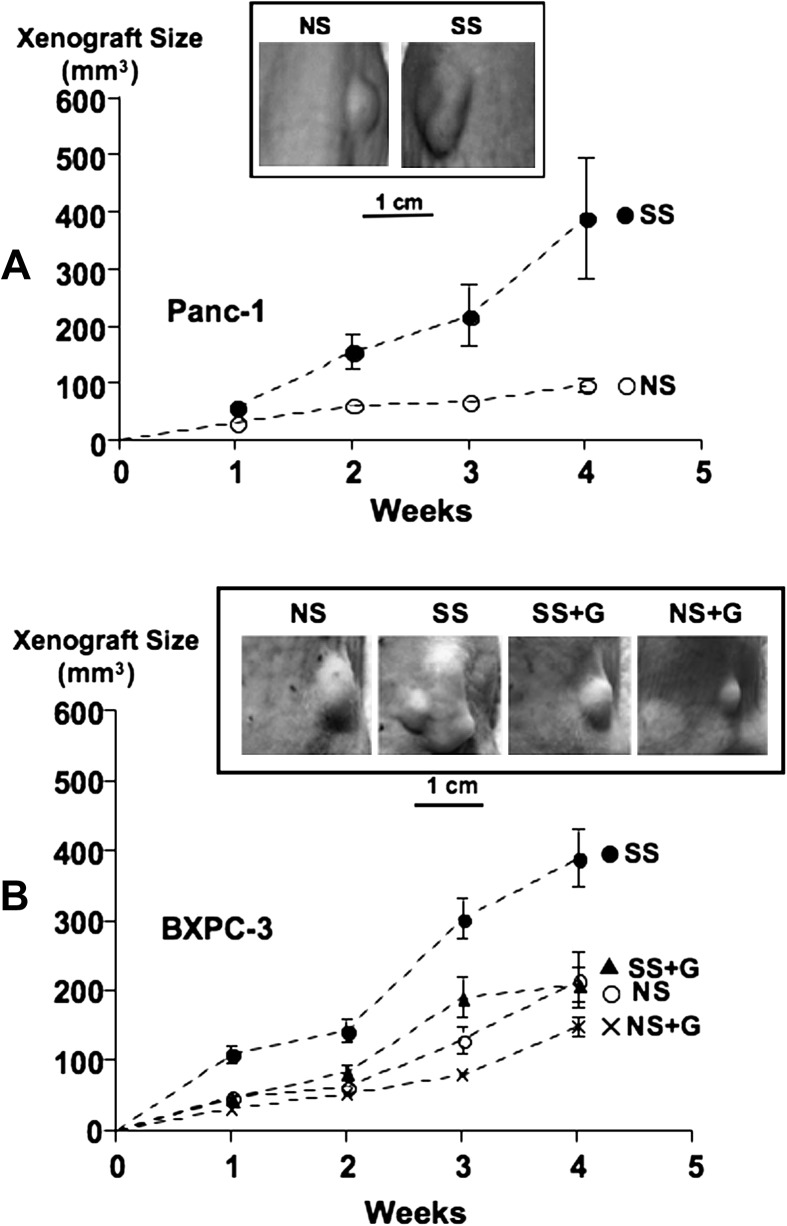
The growth of xenografts from both pancreatic cancer cell lines was promoted by psychological stress as indicated by increased tumor sizes (Figure 1). Tumor sizes from the four treatment groups with BXPC-3 xenografts showed highly significant differences (P < 0.001 weeks 1 through 4, analysis of variance). Dunn's multiple comparison test additionally showed significant increases in tumor size in stress-exposed versus unstressed mice (week 1: P < 0.01, week 2: P < 0.001, weeks 3 and 4: P < 0.01). Sizes of the slower growing Panc-1 xenografts were not significantly larger in stress-exposed mice in the first week. However, social stress significantly increased the size of xenografts from this cell line in all subsequent weeks (week 2: P = 0.0293, week 3: P = 0.0387, week 4: P = 0.0056). GABA treatment completely reversed the stress-induced increases in tumor sizes in BXPC-3 xenografts (week 1: P < 0.01, week 2: P < 0.05, weeks 3 and 4: P < 0.05). GABA also reduced tumor sizes in mice not exposed to stress (Figure 1), but these reductions were not significant.
Fig. 1.
Xenograft sizes over time from cell line Panc-1 (A) or BXPC-3 (B) in groups of mice exposed to social stress (SS) or not exposed to stress (NS) in the presence and absence of GABA treatment (G). Each group consisted of 20 mice. The photographs show representative examples of xenografts for each treatment group.
Neurotransmitter and hormonal stress responses
Psychological stress significantly (P = 0.0079) increased the serum and xenograft levels of noradrenalin, adrenalin and cortisol (Table I). In contrast, GABA was significantly (P = 0.0079) reduced (Table I).
Table I.
Social stress-induced changes in systemic and xenograft levels of neurotransmitters and cortisol
| Stress response | Fold change |
|
| Mean | Standard deviation | |
| Panc-1 | ||
| Serum noradrenalin | 2.2 | 0.005 |
| Xenograft noradrenalin | 2.7 | 0.009 |
| Serum adrenalin | 1.7 | 0.007 |
| Xenograft adrenalin | 2.0 | 0.004 |
| Serum cortisol | 2.5 | 0.120 |
| Xenograft cortisol | 2.1 | 0.100 |
| Serum GABA | 0.67 | 0.006 |
| Xenograft GABA | 0.74 | 0.084 |
| BXPC-3 | ||
| Serum noradrenalin | 2.46 | 0.100 |
| Xenograft noradrenalin | 3.13 | 0.090 |
| Serum adrenalin | 2.20 | 0.140 |
| Xenograft adrenalin | 2.87 | 0.090 |
| Serum cortisol | 2.63 | 0.150 |
| Xenograft cortisol | 2.34 | 0.130 |
| Serum GABA | 0.65 | 0.030 |
| Xenograft GABA | 0.69 | 0.03 |
Data are mean values and standard deviations of five samples per treatment group expressed as fold change over controls. All stress-induced changes in neurotransmitter and cortisol levels were significant (P = 0.0079, by Mann–Whitney test).
Effects of stress on neurotransmitter receptors
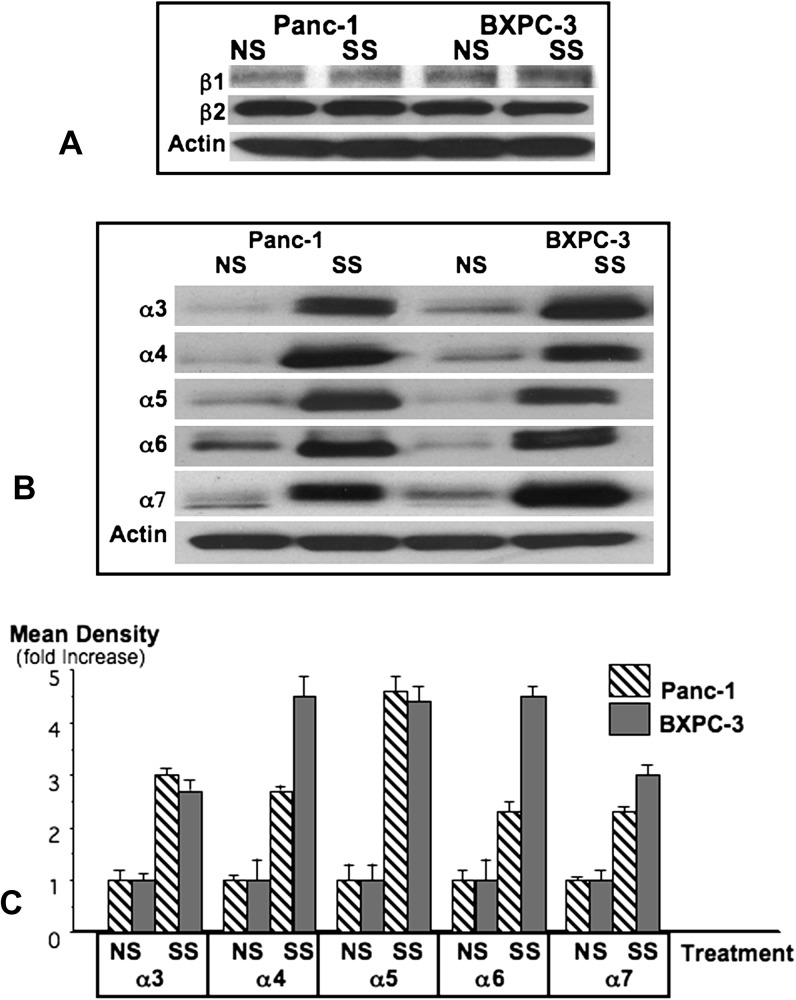
Noradrenalin and adrenalin are agonists for β1- and β2-adrenergic receptors that regulate pancreatic cancer cells in vitro (5,8,9). As reported in vitro (8), the β2-adrenergic receptor predominated over β1 in xenografts from both cell lines (Figure 2). Psychological stress did not significantly change the protein expression of either receptor in xenograft tissues (Figure 2). However, the RNA expression of both receptors was significantly downregulated in Panc-1 xenografts of stress-exposed mice (β1: P = 0.0151, β2: P = 0.0150, Table II), whereas β1 RNA remained unchanged and β2 was downregulated in BXPC-3 xenografts (P = 0.0458, Table II).
Fig. 2.
The western blot (A) showing protein expression of β1-and β2-adrenergic receptors is representative for three independent blots prepared from three randomly selected xenografts per treatment group. The western blot (B) exemplifies the protein expression of nAChR subunits α3–α7 in xenograft tissues of unstressed (NS) and stress-exposed (SS) mice. Columns in the graph (C) are mean values and standard deviations of four densitometric readings per band adjusted for actin in independent blots prepared from three randomly selected xenografts per treatment group.
Table II.
Social stress-induced changes in the expression levels of nAChR subunits and β-adrenergic receptors by real-time PCR
| Xenograft type | Gene | Fold change (mean ±SE) | P value |
| Panc-1 | α3 nAChR | 0.52 ± 0.06 | 0.0370* |
| α4 nAChR | 0.33 ± 0.04 | 0.0065* | |
| α5 nAChR | 0.48 ± 0.09 | 0.0148* | |
| α6 nAChR | 0.53 ± 0.05 | 0.0277* | |
| α7 nAChR | 0.35 ±0.09 | 0.0001* | |
| β1-AR | 0.58 ± 0.08 | 0.0151* | |
| β2-AR | 0.50 ± 0.04 | 0.0150* | |
| BXPC-3 | α3 nAChR | 1.62 ± 0.52 | 0.5557 |
| α4 nAChR | 0.40 ± 0.08 | 0.0191* | |
| α5 nAChR | 0.68 ± 0.07 | 0.0137* | |
| α6 nAChR | 0.26 ± 0.07 | 0.0500* | |
| α7 nAChR | 0.54 ± 0.04 | 0.0067* | |
| β1-AR | 0.70 ± 0.12 | 0.0883 | |
| β2-AR | 0.61 ± 0.13 | 0.0458* |
Data are mean values and standard errors of fold changes (n = 5). Significant differences (by unpaired two-tailed t-test) are identified by asterix.
nAChRs containing subunits α3, α4, α5, α6 and α7 are expressed in numerous cancers, regulating cell proliferation, migration, angiogenesis and apoptosis (14). In our study, receptor protein of all investigated alpha subunits was significantly (P < 0.0001) increased in xenografts from both cell lines by stress (Figure 2). In contrast, psychological stress downregulated the mRNA expression of these nAChR subunits in Panc-1 xenografts while in BXPC-3 xenografts α4, α5, α6 and α7 were downregulated and α3 was not significantly changed (Table II).
Effects of psychological stress on GABA synthesizing enzymes
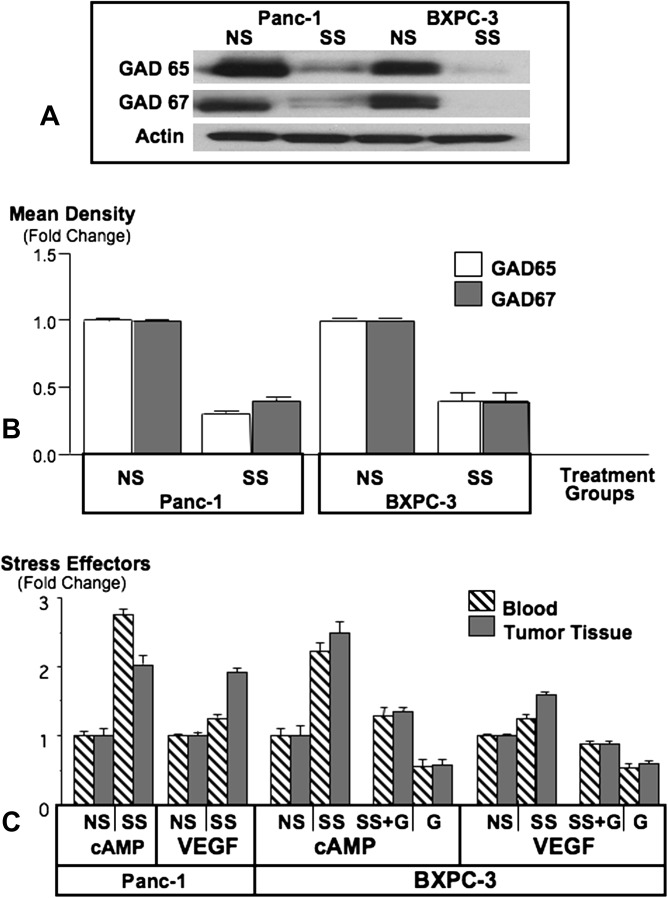
Western blots showed protein expression of both GABA synthesizing enzymes, GAD 65 and GAD 67, in pancreatic cancer xenografts from either cell line (Figure 3), suggesting the ability of the cancer cells to produce GABA. In accord with the stress-induced reduction in systemic and tumor GABA levels, the protein expression of GAD 65 and GAD 67 was significantly reduced in xenografts by stress (P = 0.0079, Figure 3).
Fig. 3.
Western blot (A) showing suppression of GAD65 and GAD67 by social stress (SS) in xenografts from cell lines Panc-1 and BXPC-3. Columns in the graph (B) are mean values and standard deviations of four densitometric readings per band adjusted for actin in independent blots prepared from three randomly selected xenografts per treatment group. Graph (C) illustrates levels of intracellular cAMP in the cellular fraction of blood and in xenograft tissues and levels of VEGF in serum and xenograft tissues. Columns are mean values and standard deviations of five randomly selected samples per treatment group.
Effects of psychological stress on signal transduction and reversal by GABA treatment
Binding of an agonist to the Gαs-coupled β-adrenergic receptors activates adenylyl cyclase, increasing intracellular cAMP (15). Stress significantly (P = 0.0079) increased the levels of cAMP in blood cells and tumors, a response significantly (P = 0.0079) reduced by GABA treatment (Figure 3). In contrast, GABA treatment significantly (P = 0.0079) reduced systemic and xenograft levels of cAMP in mice not exposed to stress (Figure 3).
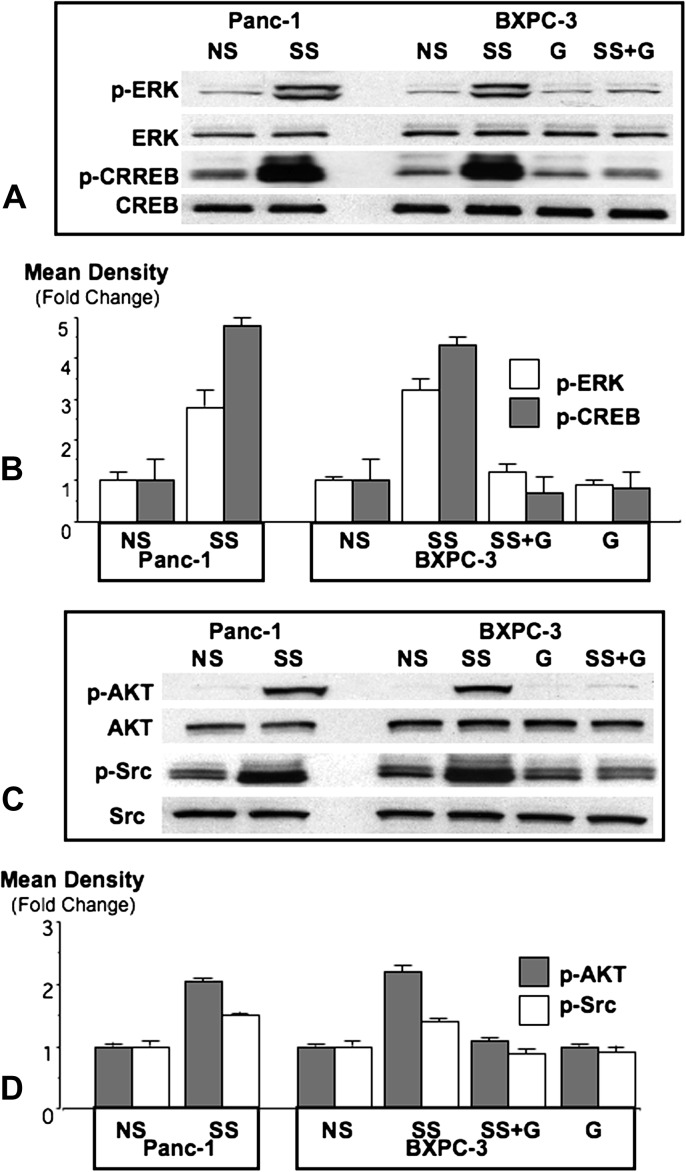
The phosphorylated forms of ERK1/2 are frequently overexpressed in pancreatic cancer (1). In the current study, stress significantly (P < 0.0001) induced p-ERK and p-CREB in xenograft tissues from both cell lines (Figure 4), a response completely inhibited by treatment with GABA (P < 0.0001, Figure 4).
Fig. 4.
Western blots showing induction of p-ERK and p-CREB (A) or p-AKT and p-Src (C) by social stress (SS) in Panc-1 and BXPC-3 xenografts and inhibition of these responses by GABA (G) in BXPC-3 xenografts. Columns in the graphs (B and D) are mean values and standard deviations of four densitometric readings per band adjusted for the unphosphorylated proteins in independent blots prepared from three randomly selected xenografts per treatment group.
The phosphorylated forms of Src tyrosine kinases are frequently overexpressed in pancreatic cancer (16). Stress significantly (P < 0.0001) induced of p-Src in xenografts from both cell lines, a response completely abrogated (P < 0.0001) by treatment with GABA (Figure 4).
The serine/threonine protein kinase B, AKT, is frequently activated in pancreatic cancer and thought to contribute to the resistance of this malignancy to cancer therapy (17). Social stress significantly (P < 0.0001) induced p-AKT (Ser 473) in xenografts from both cell lines, a response completely reversed (P < 0.0001) by treatment with GABA (Figure 4).
Psychological stress induces and GABA reverses VEGF
VEGF is a mediator of cancer angiogenesis. Social stress significantly (P = 0.0079) increased VEGF levels in both serum and xenografts of mice carrying xenografts from either cell line. GABA treatment completely blocked this response and additionally reduced VEGF below base levels (P = 0.0079) in unstressed mice (Figure 3).
Discussion
Our data show, for the first time, that neurotransmitter responses to psychological stress significantly induced multiple signaling pathways that regulate the proliferation, migration, angiogenesis and apoptosis of pancreatic cancer, resulting in a significant promotion of tumor growth in mouse xenografts. Since most stressful stimuli in people are social in nature (10), these findings suggest psychological stress as an important factor that stimulates the progression of pancreatic cancer and negatively impacts intervention outcomes. The translational relevance of our findings is underlined by the reported excessively high levels of psychological stress in pancreatic cancer patients (4). Moreover, our findings imply that the disappointing clinical performance of anticancer agents that have shown great promise in preclinical studies may, at least in part, be caused by the absence of psychological stress in preclinical test systems. The frequent overexpression in pancreatic cancer of phosphorylated signaling proteins significantly induced by stress (16–19) identifies neuropsychological stress responses as important regulators of this malignancy that should be targeted for cancer intervention.
GABA treatment completely prevented stress-induced tumor growth and inhibited the associated activation of multiple signaling proteins while also reducing VEGF. In conjunction with the reduction in systemic and tumor cAMP below base levels by GABA, these findings emphasize an important role of cAMP downstream of β-adrenergic receptors in the observed tumor promoting effects of stress. A major problem of current pancreatic cancer therapy is the fact that this cancer expresses so many different signaling pathways. The inhibition of ERK, Src and AKT activity and angiogenic regulators are thus current targets of pancreatic cancer therapy that require treatment with multiple agents (17,20). In contrast, treatment of the mice with a single agent, GABA, inhibited all of these targets in the current study. The observed effects of GABA are in accord with its inhibitory function on excitatory neurotransmitters in the brain via inhibition of adenylyl cyclase by binding to the Gαi-coupled GABA-B receptor (21). GABA inhibited cell proliferation, migration and phosphorylation of CREB and ERK induced by the β-adrenergic agonist isoproterenol in Panc-1 cells in vitro, effects blocked by gene knockdown of the GABA-B receptor (5). The effects of psychological stress bear strong resemblance to nicotine-induced promotion of Panc-1 xenograft progression that was also reversed by GABA (22). Our findings also indicate that the stress-induced phosphorylations of CREB, ERK, Src and AKT and the increase in VEGF were cAMP dependent. The stress-induced suppression of GABA in our study is in accord with reported impairment of the GABA system in the brain by stress (23).
The stress-induced increase in nAChR protein without accompanying upregulation in mRNA in xenografts represents a typical reaction of these receptors to chronic agonist (in this case acetylcholine that initiates stress responses by binding to nAChRs) and is achieved by posttranscriptional and posttranslational mechanisms (24). However, the function of nAChRs in pancreatic cancer cells is poorly understood. Nicotine induced osteopontin-dependent migration and angiogenesis in pancreatic cancer cells, responses inhibited by the general nAChR antagonist mecamylamine (25,26). But the subunit composition of the involved nAChR(s) was not investigated. It has been shown that the α7nAChR regulates the synthesis and release of noradrenalin and adrenalin in colon cancer (27) and small airway epithelial cells (11), thereby stimulating the proliferation of these cells via β-adrenergic signaling. In contrast, analogous to its function in the brain, the α4nAChR regulates the synthesis and release of GABA in small airway epithelial cells (11). Pancreatic cancers induced in hamsters by the nicotine-derived nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), a high affinity nAChR agonist (28), overexpressed protein of both receptors and had increased levels of stress neurotransmitters and a decrease in GABA (29). It hence appears that some epithelial cells and the cancers derived from them express the regulatory nAChRs and cellular machinery to produce their own stimulatory and inhibitory neurotransmitters. These nAChRs undergo changes in expression and function analogous to the nicotine-addicted brain (14) upon chronic exposure to agonist. Receptors containing α3 and α5 subunits can assemble together or with subunits α4 or α6 and regulate stress neurotransmitters in the adrenal gland (6,30,31). Their function in pancreatic cancer cells is unknown. It has been shown that α3α5β2 nAChRs stimulate wound repair in airway epithelium (32). Additional studies are needed to delineate the function of these receptors in pancreatic cancer cells.
β-Adrenergic cAMP-dependent signaling has been identified as an important regulatory pathway for adenocarcinoma of the lungs (33–37), pancreas (5,8,9,22,29), stomach (38), colon (27,39), prostate (40), breast (41–43) and ovary (44). Neuropsychological stress responses may therefore significantly promote the progression of all of these cancers and diminish intervention outcomes. A recent study has shown that women receiving β-blocker therapy that inhibits binding of stress neurotransmitters to β-adrenergic receptors significantly improved clinical outcomes (43). β-blocker usage has also been reported to reduce the risk for prostate cancer (45). On the other hand, a recent retrospective cohort study has reported poorer survival of patients with pancreatic and prostate cancer who regularly received primarily β1-adrenoreceptor blocker therapy (46). These apparent discrepancies reflect the ability of some β-blockers to sensitize particularly β2-adrenergic receptors (47), which predominate in pancreatic and prostate cancer cells, while others have intrinsic agonist activity (48). Utilization of GABA for the inhibition of cAMP-dependent signaling offers a valuable alternative to beta blockers. This can be achieved by a nutritional approach as GABA has been safely used as a nutritional supplement for many years. However, it has been shown that pancreatic cancers that overexpress the pi-subunit of the GABA-A receptor react with increased proliferation to GABA in vitro (49). The pathological overexpression of this receptor subunit may reduce binding of GABA to the cAMP-inhibiting GABA-B receptor, thus flipping the function of GABA from inhibitory to excitatory. On the other hand, diabetes and pancreatitis are associated with reduced pancreatic GABA levels (50,51), a factor that may contribute to the increased pancreatic cancer risk of patients with these diseases.
Experiments with tumor xenografts are relatively short compared with the development and progression of cancer in humans and additional studies in human cancer patients are needed to assess the effects of psychological stress on pancreatic cancer. However, immunohistochemistry of 30 pancreatic cancers has shown overexpression of noradrenalin in all cases (5), suggesting that the cancer-stimulating activities of stress identified in the current study are present in numerous human pancreatic cancers. Collectively, these findings suggest that neurotransmitter responses to psychological stress promote pancreatic cancer progression via the activation of multiple cAMP-dependent pathways and simultaneous suppression of endogenous GABA and identify the interruption of these stress responses as a promising target for novel cancer intervention strategies.
Funding
National Cancer Institute (RC1CA144640).
Acknowledgments
H.M.S.: scientific concept, experimental design, quantitative densitometry of Westerns, manuscript writing, statistics of all data except PCR. H.A.-W.: animal experiment, all immunoassays, all western blots, in vitro maintenance of cell lines, manuscript writing. M.F.U.: assistance with animal experiment. H.K.P: PCR analyses, including statistics. Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- GABA
γ-aminobutiric acid
- cAMP
cyclic adenosine 3′,5′-monophosphate
- mRNAs
messenger RNA
- nAChR
nicotinic acetylcholine receptor
- VEGF
vascular endothelial growth factor
References
- 1.Almhanna K, et al. Defining new paradigms for the treatment of pancreatic cancer. Curr. Treat. Options Oncol. 2011;12:111–125. doi: 10.1007/s11864-011-0150-8. [DOI] [PubMed] [Google Scholar]
- 2.Nieto J, et al. Metastatic pancreatic cancer 2008: is the glass less empty? Oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 3.Hamer M, et al. Psychological distress and cancer mortality. J. Psychosom. Res. 2009;66:255–258. doi: 10.1016/j.jpsychores.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Zabora J, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Schuller HM, et al. GABA B receptor is a novel drug target for pancreatic cancer. Cancer. 2008;112:767–778. doi: 10.1002/cncr.23231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 7.Hieble JP, et al. Alpha- and beta-adrenoceptors: from the gene to the clinic. 1. Molecular biology and adrenoceptor subclassification. J. Med. Chem. 1995;38:3415–3444. doi: 10.1021/jm00018a001. [DOI] [PubMed] [Google Scholar]
- 8.Weddle DL, et al. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473–479. doi: 10.1093/carcin/22.3.473. [DOI] [PubMed] [Google Scholar]
- 9.Askari MD, et al. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J. Cancer Res. Clin. Oncol. 2005;131:639–648. doi: 10.1007/s00432-005-0002-7. [DOI] [PubMed] [Google Scholar]
- 10.Sterlemann V, et al. Long-term behavioral and neuroendocrine alterations following chronic social stress in mice: implications for stress-related disorders. Horm. Behav. 2008;53:386–394. doi: 10.1016/j.yhbeh.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Al-Wadei HA, et al. Chronic exposure to estrogen and the tobacco carcinogen NNK cooperatively modulates nicotinic receptors in small airway epithelial cells. Lung Cancer. 2009;69:33–39. doi: 10.1016/j.lungcan.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Plummer HK, III, et al. Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer. 2005;5:104. doi: 10.1186/1471-2407-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, et al. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer. 2009;9:195–205. doi: 10.1038/nrc2590. [DOI] [PubMed] [Google Scholar]
- 15.Lefkowitz RJ. The superfamily of heptahelical receptors. Nat. Cell Biol. 2000;2:E133–E136. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- 16.Shields DJ, et al. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene. 2011;30:2123–2134. doi: 10.1038/onc.2010.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons CM, et al. The role of Akt activation in the response to chemotherapy in pancreatic cancer. Anticancer Res. 2010;30:3279–3289. [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S, et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2004;10:2846–2850. doi: 10.1158/1078-0432.ccr-02-1441. [DOI] [PubMed] [Google Scholar]
- 19.Yokoi K, et al. Identification and validation of SRC and phospho-SRC family proteins in circulating mononuclear cells as novel biomarkers for pancreatic cancer. Transl Oncol. 2011;4:83–91. doi: 10.1593/tlo.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo R, et al. Pancreatic cancer: from molecular signature to target therapy. Crit. Rev. Oncol. Hematol. 2008;68:197–211. doi: 10.1016/j.critrevonc.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M, et al. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 22.Al-Wadei HA, et al. Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid. Carcinogenesis. 2009;30:506–511. doi: 10.1093/carcin/bgp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu W, et al. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Govind AP, et al. Nicotine-induced upregulation of nicotinic receptors: underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chipitsyna G, et al. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. Int. J. Cancer. 2009;125:276–285. doi: 10.1002/ijc.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazar M, et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J. Gastrointest. Surg. 2010;14:1566–1577. doi: 10.1007/s11605-010-1338-0. [DOI] [PubMed] [Google Scholar]
- 27.Wong HP, et al. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol. Appl. Pharmacol. 2007;221:261–267. doi: 10.1016/j.taap.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Schuller HM, et al. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem. Pharmacol. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 29.Al-Wadei HA, et al. Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas. J. Pathol. 2009;218:437–445. doi: 10.1002/path.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Angelantonio S, et al. Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur. J. Neurosci. 2003;17:2313–2322. doi: 10.1046/j.1460-9568.2003.02669.x. [DOI] [PubMed] [Google Scholar]
- 31.Yokotani K, et al. Characterization of functional nicotinic acetylcholine receptors involved in catecholamine release from isolated rat adrenal gland. Eur. J. Pharmacol. 2002;446:83–87. doi: 10.1016/s0014-2999(02)01819-8. [DOI] [PubMed] [Google Scholar]
- 32.Tournier J-M, et al. α3α5β2-nicotinic acetylcholine receptor contributes to the wound repair of the respiratory epithelium by modulating intracellular calcium in migrating cells. Am. J. Pathol. 2006;168:55–68. doi: 10.2353/ajpath.2006.050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuller HM, et al. Regulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell line. Carcinogenesis. 1989;10:1753–1755. doi: 10.1093/carcin/10.9.1753. [DOI] [PubMed] [Google Scholar]
- 34.Park PG, et al. Beta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: implications for individuals with preexisting chronic lung disease. Cancer Res. 1995;55:3504–3508. [PubMed] [Google Scholar]
- 35.Schuller HM, et al. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999;59:4510–4515. [PubMed] [Google Scholar]
- 36.Majidi M, et al. Nongenomic beta estrogen receptors enhance beta1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cells. Cancer Res. 2007;67:6863–6871. doi: 10.1158/0008-5472.CAN-07-0483. [DOI] [PubMed] [Google Scholar]
- 37.Schuller HM, et al. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29:1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin VY, et al. Functional role of beta-adrenergic receptors in the mitogenic action of nicotine on gastric cancer cells. Toxicol. Sci. 2007;96:21–29. doi: 10.1093/toxsci/kfl118. [DOI] [PubMed] [Google Scholar]
- 39.Masur K, et al. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–2869. [PubMed] [Google Scholar]
- 40.Palm D, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int. J. Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- 41.Cakir Y, et al. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 42.Drell TL, IV, et al. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res. Treat. 2003;80:63–70. doi: 10.1023/A:1024491219366. [DOI] [PubMed] [Google Scholar]
- 43.Powe DG, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sood AK, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin. Cancer Res. 2006;12:369–375. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perron L, et al. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 46.Shah SM, et al. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horinouchi T, et al. Different changes of plasma membrane beta-adrenoceptors in rat heart after chronic administration of propranolol, atenolol and bevantolol. Life Sci. 2007;81:399–404. doi: 10.1016/j.lfs.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Taira CA, et al. Measurement of inverse agonism in beta-adrenoceptors. Methods Enzymol. 485:37–60. doi: 10.1016/B978-0-12-381296-4.00003-8. [DOI] [PubMed] [Google Scholar]
- 49.Takehara A, et al. Gamma-aminobutyric acid (GABA) stimulates pancreatic cancer growth through overexpressing GABAA receptor pi subunit. Cancer Res. 2007;67:9704–9712. doi: 10.1158/0008-5472.CAN-07-2099. [DOI] [PubMed] [Google Scholar]
- 50.Al-Salam S, et al. Diabetes mellitus decreases the expression of calcitonin-gene related peptide, gamma-amino butyric acid and glutamic acid decarboxylase in human pancreatic islet cells. Neuro Endocrinol. Lett. 2009;30:506–510. [PubMed] [Google Scholar]
- 51.Schrader H, et al. Amino acid malnutrition in patients with chronic pancreatitis and pancreatic carcinoma. Pancreas. 2009;38:416–421. doi: 10.1097/MPA.0b013e318194fc7a. [DOI] [PubMed] [Google Scholar]