Abstract
Animal studies show that increasing large bowel butyrate concentration through ingestion of butyrylated or resistant starches opposes carcinogen-induced tumorigenesis, which is consistent with population data linking greater fiber consumption with lowered colorectal cancer (CRC) risk. Butyrate has been shown to regulate the apoptotic response to DNA damage. This study examined the impact of increasing large bowel butyrate concentration by dietary butyrylated starch on the colonic epithelium of rats treated with the genotoxic carcinogen azoxymethane (AOM). Four groups of 10 male rats were fed AIN-93G based-diets containing either low amylose maize starch (LAMS), LAMS with 3% tributyrin, 10% high amylose maize starch (HAMS) or 10% butyrylated HAMS (HAMSB). HAMS and HAMSB starches were cooked by heating in water. After 4 weeks, rats were injected once with AOM and killed 6 h later. Rates of apoptosis and proliferation were measured in colonic epithelium. Short-chain fatty acid concentrations in large bowel digesta and hepatic portal venous plasma were higher in HAMSB than all other groups. Apoptotic rates in the distal colon were increased by HAMSB and correlated with luminal butyrate concentrations but cellular proliferation rates were unaffected by diet. The increase in apoptosis was most marked in the base and proliferative zone of the crypt. Regulation of luminal butyrate using HAMSB increases the rates of apoptotic deletion of DNA-damaged colonocytes. We propose this pro-apoptotic function of butyrate plays a major role reducing tumour formation in the AOM-treated rat and that these data support a potential protective role of butyrate in CRC.
Introduction
Colorectal cancer (CRC) is a major cause of death and its incidence is increasing throughout the world (1). The majority of case-controlled epidemiological studies have shown a dose-dependent protective effect against CRC of diets high in fiber, although prospective studies have shown less effect (see (2)). These apparent inconsistencies may reflect the different quantities and relative contributions of the major components of the predominant dietary fiber sources consumed in the various studies. Based on early definitions of dietary fiber (3), it was thought that non-starch polysaccharides (NSPs) were the most important contributors to the health benefits of fiber while the contribution of resistant starch (RS) was discounted. RS is that fraction of ingested starch that escapes small intestinal digestion and enters the human large bowel and it is now recognized as a dietary fiber component (4). Population studies have identified RS consumption as protective against CRC (5) and it appears that populations at low risk of CRC consume relatively little NSP but have high intakes of RS (6). In the large bowel, RS together with a fraction of NSP that varies by source undergoes bacterial fermentation with the production of short-chain fatty acids (SCFAs), mainly acetate, propionate and butyrate (7). Butyrate may protect against oncogenesis as it enhances apoptosis of genetically damaged cells, inhibits cell proliferation and promotes differentiation in CRC cells in vitro (7–10).
Apoptosis provides a system for the removal of aged or damaged cells through programmed autolysis. Though first recognized for its role in early development and morphogenesis (11,12) apoptosis also provides a defence against tumorigenesis. Genotoxin-induced cellular anomalies such as DNA double-strand breaks, inappropriate polyploidy, telomere dysfunction and abnormal mitoses can all induce apoptosis (13). Rates of apoptosis are usually low in the colon (14); disruption of this protective process is often involved with tumour initiation and development (15,16).
Increasing large bowel digesta butyrate concentration by the ingestion of RS increases rates of apoptosis in distal colonic epithelium (17,18), as well as reducing large bowel tumour number and incidence in carcinogen-treated rats (19–21). Although fermentation of RS by the microflora can elevate butyrate levels in the large bowel, there may be limitations to the effectiveness of RS as the SCFA profile produced can vary with the source (22). Acylated starches have been shown to deliver specific SCFA to the large bowel and ingestion of butyrylated starch has been shown to deliver butyrate to the colon in animals (23,24) and humans (25,26). Butyrylated high amylose maize starch (HAMSB) has been shown to reduce the incidence and number of tumours in carcinogen-treated rats (27) and to protect from diet-induced genomic damage to colonic epithelium (28).
The aim of this study was to investigate the effects of increasing large bowel butyrate concentration by dietary butyrylated starch on the colonic epithelium of rats treated with the genotoxic carcinogen azoxymethane (AOM). The study examined the effects of RS and butyrylated-RS on the acute apoptotic and proliferative cellular responses of the colonic epithelium to exposure to the pro-carcinogen AOM, which causes the methylation of guanine to the adduct O6-methylguanine in colonocytes of rats. To differentiate between the effects of butyrate delivered to the colon directly (as opposed to systemically through small intestinal absorption), a group of rats were fed a low amylose maize starch diet with added tributyrin (LAMST). In addition, changes in the colonic luminal environment relevant to CRC risk were examined.
Materials and methods
Animals and diets
Forty male Sprague–Dawley rats of 4 weeks of age were purchased from the Animal Resource Centre, Murdoch University, Western Australia. The animals were housed in wire-bottomed cages in a temperature-controlled room (23 ± 1°C) with a 12 h light/dark cycle. After 2 weeks acclimatization, the rats were randomized to 1 of 4 groups (n = 10 per group) with similar mean body weights (215 ± 2 g).
The animals were given free access to food and water throughout the study and were fed 1 of 4 experimental diets. These diets have been described previously (27) and were based on a balanced modification of the AIN-93G diet (29). Briefly, they contained 530g low RS maize starch (low amylose maize starch, LAMS), 200g casein, 70g sunflower seed oil and 50g α-cellulose/kg of diet. Choline, methionine, minerals, vitamins and a permitted anti-oxidant were added as described previously (24). The amount of LAMS starch in the diet was reduced to allow for the addition of high amylose maize starch (HAMS), HAMSB and tributyrin (Sigma Chemical Co., St Louis, MO). The experimental diets were control (LAMS, low RS), low amylose maize starch with 3% tributyrin (LAMST, low RS), 10% HAMS (high RS) and 10% HAMSB (high RS). The latter was manufactured by National Starch Food Innovation (Bridgewater, New Jersey) and had a degree of substitution of 0.25 [i.e. 0.25 of the hydroxyl groups on each starch D-glucopyranosyl unit were derivatized or replaced by the substituent acid (30)]. HAMS and HAMSB were heated with water and spray dried to minimize retrogradation and crystal formation (31). They were added to the diets to mimic the condition of starches consumed in foods by humans as cooking decreases resistance to amylolysis (24). All diets were prepared weekly at CSIRO and stored at 4°C. The rats were fed fresh diet daily.
After 28 days of feeding the experimental diets rats were injected with 15 mg of AOM/kg subcutaneously (Sigma Chemical Co.). Six hours later the animals were anaesthetized with halothane and blood collected from the hepatic portal vein and abdominal aorta. The colon was excised, flushed with chilled saline, the distal and proximal 0.5 cm discarded and 2cm sections taken from both the proximal and the distal ends. The tissues were fixed in 10% buffered formalin for 20 h, then washed and stored in 70% alcohol before being embedded in paraffin. The caecum was removed, weighed and samples of caecal, proximal colonic and distal colonic digesta were collected for pH measures. Digesta samples were mixed with 3 volumes of internal standard (heptanoic acid, 1.68 mmol/l) and stored at −20° C for SCFA analysis.
All procedures involving animals were approved by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) Human Nutrition Animal Ethics Committee and complied with the NHMRC Australian Code of Practice for the Care and Use of Animals for Scientific Purposes 7th ed (32).
All slides assessed for rates of colonic epithelial apoptosis and proliferation were randomly encoded and examined by an independent observer unaware of the dietary treatments.
Short-chain fatty acids
Digesta homogenate samples were processed for SCFA analysis using gas chromatography (GC) as described previously (24). Plasma SCFA were extracted for GC analysis using diethyl ether as described previously (33).
Apoptosis measurement
Cross sections of 4μm thickness were cut for all histological techniques. Rates of apoptosis were determined using morphological assessment (34) of the distal and proximal colon. The rate of apoptosis was also measured in the distal colon using immunohistochemical staining and quantification of caspase-3 to enable comparison with the determination by morphological assessment. For the morphological assessment, tissue sections from the distal and proximal colon were rehydrated through xylene and ethanol, stained with Harris’s hematoxylin (BDH Laboratory Supplies, England) and evaluated under a light microscope (BX41, Olympus, Tokyo, Japan). Twenty intact crypts were used for apoptotic cell identification per animal. Cell shrinkage, presence of condensed chromatin, and sharply delineated cell borders surrounded by a clear halo (34) were used as defining variables for morphological assessment. The number of apoptotic cells in each crypt, the length of the crypt (in cells) and the position of the apoptotic cells within the crypt where position one was at the base were recorded. The apoptotic index was calculated as the mean number of apoptotic cells per crypt column divided by the total number of cells in the column multiplied by 100.
Rates of apoptosis were also quantified using an immunohistochemical staining technique that was a modified version of the method of Marshmann et al. (35). The method used rabbit monoclonal Asp175 caspase-3 primary antibody (Cell Signalling Technology, Beverley, MA) to detect the large (p17) fragment of activated caspase-3; this antibody does not recognize procaspase-3 or other caspases. Following deparaffinisation and rehydration, antigen retrieval was performed in 10 mM sodium citrate/TBST buffer pH 6.0 at 95°C for 20 min. Slides were cooled, washed in TBS and immersed in 3% H2O2 for 10 min to block endogenous peroxidase activity. Powervision+ preantibody blocking solution (Immunovision Technologies, Brisbane, CA) was used for 20 min, followed by incubation with anticleaved caspase-3 diluted 1:200 in blocking solution overnight at 4°C. Next morning, sections were washed and incubated for 20 min in post-antibody blocking solution (Immunovision Technologies) before addition of Poly-HRP anti-mouse/rabbit IgG 2° antibody (Immunovision Technologies) for 30 min. Sections were developed in 3,3′-diaminobenzidine (DAB) for 10 min and counterstained with hematoxylin for 1 min before dehydration and mounting. Twenty intact crypts per animal were scored for caspase-3 and the number of cells per crypt column recorded. The apoptotic index was calculated as the mean number of caspase-3 stained cells per crypt column divided by the total number of cells in the column multiplied by 100. Sections were visualized on an Olympus BX41 light microscope equipped with a Diagnostic Instruments Spot RT camera. Images were taken and assessed using the Image Pro Plus v.4.0.1 software package (media Cybernetics, Silver Spring, MD).
Cell proliferation
Distal colonic epithelial proliferation was measured using immunohistochemical staining with Ki67 monoclonal antibody (DAKO, Glostrup, Denmark). Briefly, sections of the distal colon were placed in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed in a 95°C water bath with 0.1 M citrate buffer for 25 min. Endogenous peroxidase activity was blocked by immersing the slides in 0.3% H2O2 in methanol for 20 min. Slides were incubated in 5% goat serum to block non-specific binding before Ki67 antibody was applied 1:500 in blocking solution for 1 h at room temperature. Following washing and HRP anti-mouse IgG secondary antibody application (DAKO), the antigen–antibody complex was made visible by incubation with DAB before counterstaining with hematoxylin for 1 min. Twenty intact crypts per animal were chosen for analysis and scored for presence of Ki67 staining and the number of cells per crypt column recorded. The proliferative index was calculated as the mean number of Ki67 stained cells per crypt column divided by the total number of cells in the column multiplied by 100.
Statistical analyses
Analyses were performed using GraphPad Prism Version 4.00 (GraphPad Software Inc. San Diego, CA). Data are expressed as mean ± standard error of the mean. The apoptotic indices derived from morphological assessment were transformed using y = log y before a significance test was performed. One-way analysis of variance with Tukey post hoc tests were used to compare data between treatment groups and differences were considered significant when P < 0.05. The correlations involving distal colonic apoptotic index derived from morphological assessment were analyzed using Spearman’s correlation tests as these data were non-parametrically distributed. Pearson’s correlation tests were used to analyse correlations between other parametrically distributed variables.
Results
Animal and digesta measurements
There were no significant differences in the final body weights or digesta weights in the proximal and distal colon (Table I). Rats fed HAMSB had higher caecal tissue and digesta weights (P < 0.001) and lower pH (P < 0.001) in the large bowel compared with rats fed LAMS, LAMST and HAMS. The large bowel digesta pH values of rats fed HAMS were lower than those of rats fed LAMST (P < 0.01).
Table I.
Final measures of rats fed diets for 28 days containing LAMS, LAMS with 3% tributyrin (LAMST), HAMS and HAMSB
| LAMS | LAMST | HAMS | HAMSB | |
| Body weight(g) | 383 ± 11 | 385 ± 9 | 382 ± 9 | 378 ± 6 |
| Caecal tissue weight (g) | 0.66 ± 0.03a | 0.64 ± 0.03a | 0.78 ± 0.02a | 1.11 ± 0.08b |
| Digesta weight (g) | ||||
| Caecum (g) | 1.24 ± 0.10a | 1.30 ± 0.08a | 1.48 ± 0.16a | 2.43 ± 0.31b |
| Proximal colon (g) | 0.22 ± 0.07 | 0.13 ± 0.04 | 0.39 ± 0.12 | 0.37 ± 0.09 |
| Distal colon (g) | 0.55 ± 0.10 | 0.39 ± 0.06 | 0.48 ± 0.08 | 0.74 ± 0.14 |
| Digesta pH | ||||
| Caecum | 7.19 ± 0.07ac | 7.37 ± 0.07a | 7.00 ± 0.06c | 6.45 ± 0.10b |
| Proximal colon | 7.28 ± 0.08ac | 7.30 ± 0.05a | 6.94 ± 0.09c | 6.57 ± 0.06b |
| Distal colon | 6.93 ± 0.07ac | 7.03 ± 0.06a | 6.74 ± 0.06c | 6.38 ± 0.04b |
Values are mean ± standard error of the mean (n = 9–10). Means with a different superscript within each row are significantly different (P < 0.05).
Apoptosis and cell proliferation in the colon
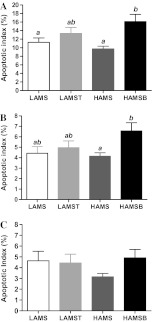
The apoptotic index (as measured by morphological assessment of distal colonic epithelium) was higher in rats fed HAMSB compared with rats fed LAMS (P < 0.05) and HAMS (P < 0.01, Figure 1A) whereas no difference was observed in the proximal colon (Figure 1C). Apoptotic indices were higher in the distal than in the proximal colon in all groups (P < 0.001). HAMSB increased the rate of apoptosis compared with LAMS in cells in the distal colon in cellular positions 7–12, 14–15 and 17; compared with LAMST in cellular positions 11–12 and compared with HAMS in cellular positions 1–2, 11–12, 14–15 and 17 (Figure 2).
Fig. 1.
Apoptotic index of the colonic epithelium of rats fed LAMS, LAMS with 3% tributyrin (LAMST), HAMS and HAMSB 6 h after AOM injection as determined by (A) morphological assessment of the distal colon, (B) caspase-3 immunohistochemistry of distal colon and (C) morphological assessment of the proximal colon. Data are mean ± standard error of the mean (n = 9–10). Means with a different superscript are significantly different (P < 0.05).
Fig. 2.
Crypt cell position of AOM-induced apoptosis measured by morphological assessment of distal colonic epithelium. Data are mean ± standard error of the mean (n = 10). aLAMS significantly different to HAMSB; bLAMST significantly different to HAMSB; cHAMS significantly different to HAMSB (P < 0.05).
Rats fed HAMSB showed significantly higher active caspase-3 in the distal colon crypts compared with those fed HAMS (P < 0.05, Figure 1B). The distribution of the increase in active caspase-3 staining was similar in cellular position to that described for apoptosis measured by morphological assessment (data not shown).
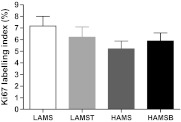
The Ki67 labelling index in the distal colon was not affected by diet (Figure 3).
Fig. 3.
(A) Cell proliferation rates in the distal colon as measured by Ki67 immunohistochemistry 6 h after AOM injection in rats fed diets containing LAMS, LAMS with tributyrin (LAMST), HAMS and HAMSB. Data are mean ± standard error of the mean (n = 10). Means with a different superscript are significantly different (P < 0.05).
Digesta and plasma SCFA concentrations
The effects of diet on caecal digesta SCFA concentrations are shown in Table II. Propionate (P < 0.05) and butyrate concentrations (P < 0.001) were significantly higher in rats fed HAMSB compared with all other diets. Total caecal digesta SCFA concentrations were also higher for rats fed HAMSB compared with those fed LAMST (P < 0.05).
Table II.
Digesta (μmol/g) and hepatic portal (μM) SCFA concentrations of rats fed diets containing LAMS, LAMS with 3% tributyrin (LAMST), HAMS and HAMSB
| LAMS | LAMST | HAMS | HAMSB | |
| Caecum | ||||
| Acetate | 56.0 ± 3.3 | 44.6 ± 5.9 | 51.1 ± 4.4 | 47.3 ± 5.7 |
| Propionate | 16.8 ± 0.9a | 15.5 ± 1.8a | 19.4 ± 2.0a | 28.2 ± 3.1b |
| Butyrate | 14.0 ± 1.3a | 11.0 ± 1.4a | 13.3 ± 1.4a | 32.1 ± 3.7b |
| Total | 91.1 ± 5.1ab | 75.6 ± 9.3a | 88.2 ± 7.3ab | 111.0 ± 11.5b |
| Proximal colon | ||||
| Acetate | 48.2 ± 3.2 | 29.5 ± 4.3 | 41.8 ± 5.0 | 39.3 ± 5.7 |
| Propionate | 12.9 ± 0.7a | 9.1 ± 1.3a | 16.2 ± 2.0ab | 22.3 ± 2.7b |
| Butyrate | 12.5 ± 1.8a | 7.3 ± 2.1a | 11.9 ± 2.1a | 28.4 ± 5.0b |
| Total | 77.2 ± 5.3ab | 48.6 ± 7.7a | 73.8 ± 8.8ab | 93.1 ± 13.0b |
| Distal colon | ||||
| Acetate | 46.6 ± 5.1 | 31.2 ± 4.6 | 43.2 ± 4.0 | 38.1 ± 3 |
| Propionate | 9.8 ± 1.5ab | 8.6 ± 1.5b | 15.4 ± 1.8a | 24.1 ± 2.0c |
| Butyrate | 12.4 ± 2.2a | 9.3 ± 2.1a | 12.0 ± 2.2a | 29.8 ± 3.0b |
| Total | 71.6 ± 8.8a | 2.2 ± 8.4a | 74.6 ± 7.2ab | 95.4 ± 8.0b |
| Hepatic portal venous plasma | ||||
| Acetate | 631 ± 60 | 466 ± 49 | 558 ± 39 | 681 ± 75 |
| Propionate | 123 ± 33a | 59 ± 8a | 107 ± 10a | 251 ± 38b |
| Butyrate | 50 ± 9a | 102 ± 27ab | 51 ± 8a | 201 ± 47b |
Values are mean ± standard error of the mean (n = 9–10, except proximal colon n = 6–8). Means with a different superscript within each row are significantly different (P < 0.05).
Rats fed HAMSB had higher propionate concentrations in their proximal colons compared with those consuming LAMST (P < 0.001) and LAMS (P < 0.05). Proximal butyrate concentrations were elevated in HAMSB fed rats compared with LAMST (P < 0.001), LAMS and HAMS (P < 0.01). Total SCFA concentrations were higher in rats fed HAMSB than LAMST (P < 0.05).
Distal colonic propionate concentrations were higher in rats fed HAMSB compared with those of LAMS, LAMST (both P < 0.001) and HAMS (P < 0.05). HAMSB more than doubled the distal colon digesta butyrate concentrations compared with all other dietary treatments (P < 0.001). Total SCFA concentrations were higher for rats fed HAMSB than LAMST (P < 0.01).
Ingestion of HAMSB increased hepatic portal venous propionate concentrations compared with HAMS and LAMS (P < 0.01) and LAMST (P < 0.001). It also increased butyrate concentrations compared with HAMS and LAMS (P < 0.01). The hepatic portal butyrate levels of LAMST rats were intermediate between HAMSB, and LAMS and HAMS.
Correlations between large bowel variables and epithelial response
Distal colon apoptotic index as measured by morphological assessment was positively correlated with distal colonic propionate and butyrate concentrations (r = 0.34, P < 0.05; r = 0.41, P < 0.02, respectively) and distal colonic butyrate pools (r = 0.36, P < 0.05) and caecal acetate pool (r = 0.32, P < 0.05). There was no correlation between SCFA concentrations and cellular proliferation.
Discussion
This trial showed that increased large bowel digesta butyrate through the feeding of HAMSB induced higher rates of apoptotic deletion of cells following acute exposure to a genotoxic agent in rats. In the gastrointestinal tract, apoptosis is an important protective process eliminating cells with DNA damage that may otherwise progress to malignancy. Enhancing rates of apoptosis may be one of the mechanisms by which butyrate protects against cancer induction (36,37). This SCFA has been shown to promote apoptosis in genetically damaged cells (8) through involvement in both the extrinsic pathway activated via death receptors and intrinsic apoptotic pathways involving mitochondria and the release of caspase-activating proteins (38). Butyrate also promotes apoptosis by regulating gene expression, particularly those inhibiting histone deacetylase (HDAC) (39).
The ability of butyrate to cause apoptosis in cancer cells may be linked with specific tumour suppressors that are silenced during the oncogenic process. These include GPR109A, a receptor in the lumen-facing apical membrane of colonic epithelial cells that binds butyrate with low affinity. The expression of GPR109A is silenced in colon cancer by DNA methylation whereas its re-expression by butyrate exposure in cancer cells results in apoptosis without the involvement of HDAC (40). GPR109A and butyrate also block NF-κB activation (40,41) which is a pathway also linked to inflammatory diseases of the bowel (42). SLC5A8 (SMCT1) is another transporter that actively moves butyrate from the colonic lumen into epithelial cells leading to HDAC inhibition and subsequent tumour suppression. SMCT1 is expressed in normal colonocytes but is silenced when the cells are transformed (41). Re-expression of SMCT1 in the presence of butyrate results in cellular apoptosis (43). These studies suggest that butyrate plays an important role in regulating apoptosis using both extra and intracellular mechanisms.
In the colon stem, cells proliferate at the base of the crypt producing both pluripotent stem cells and daughter cells; the latter divide for up to six generations as they migrate up the crypt column (44). The present study demonstrated that increased digesta butyrate promoted apoptosis in the base and mid sections of the colonic crypts. These sections represent the stem cell and proliferative functional zones, and the data suggest butyrate may promote the deletion of damaged stem cells and daughter cells. Deletion of damaged stems cells by butyrate delivered by acylated starch could explain the reduced incidence and number of colonic tumours in rats fed butyrylated starch (27).
Ingestion of cooked HAMS did not increase rates of apoptosis in the distal colon of rats in this study and reduced the incidence but not numbers of tumours in AOM-treated rats in a previous experiment (27). Despite the different experimental protocols, the digesta and hepatic portal butyrate concentrations in the two groups of HAMS rats were similar but substantially lower than those in the HAMSB-fed rats. This suggests that there may be mechanisms of cancer protection associated with RS fermentation other than the effects of butyrate and could include reduced digesta pH and increased digesta bulking which may minimize exposure to procarcinogens and minimize damage or activate repair (45).
In this study, HAMSB was effective at increasing butyrate concentration throughout the entire large bowel, including the distal colon, which was associated with higher rates of apoptosis. Based on previous data, it had been expected that the dietary intervention with HAMS would also lead to higher digesta butyrate and increased rates of apoptosis (46). This did not occur in this study in which the high RS starches were cooked and was most likely due to the subsequent partial gelatinisation of the starches that would reduce the RS content of HAMS (47). In contrast, the RS content of HAMSB is more stable on heating and the esterified butyrate is retained (24) which is reflected in the higher butyrate concentrations in all sections of the large bowel of rats fed cooked HAMSB relative to cooked HAMS.
Tumours occur more frequently in the distal large bowel in the rat AOM model (48), which is also the primary gastrointestinal cancer site in humans in the developed world (49). Increasing the supply of butyrate and hence optimizing the rate of apoptosis in the distal colon may have public health benefits. Rates of cellular proliferation in the distal colon were not affected by increased digesta butyrate, an experimental finding that may be important in the context of predicting human CRC risk. It has been suggested that greater fiber fermentation could lead to crypt hyperproliferation and increased risk of carcinogenesis (50). This suggestion was supported by the finding that fermentable fiber carbohydrate stimulated cell proliferation in the Apc(Min/+) mouse model of intestinal cancer in association with enhanced polyp development (51). However, this effect was seen largely in the small intestine that is neither the site of CRC nor fiber fermentation in the human intestine. The current data together with previous studies from this laboratory (e.g. (52)) suggest that greater SCFA availability in the large bowel acts to promote colonocyte genetic stability.
The hepatic portal venous butyrate concentration was intermediate in rats fed LAMST and large bowel digesta butyrate concentrations were significantly lower than in rats fed HAMSB diet. The rate of distal colonic apoptosis was intermediate in the LAMST animals suggesting that tributyrin may have had some protective effect on the colonic epithelium. Oral tributyrin (glycerol tributyrate) is absorbed in the small intestine and at high doses increases free butyrate concentration in peripheral plasma for up to 4 h. However, the hepatic uptake of intestinal butyrate is known to be almost complete (53), suggesting that systemic delivery of butyrate to the colon would be limited.
To our knowledge, this is the first study to compare the measurement of apoptosis by caspase-3 activation and morphological assessment in the colonic epithelium of rats treated acutely with AOM. Caspase-3 activation was up-regulated by butyrylated starch, however the increase was not as large as that seen with morphological assessment. The effector caspases (caspases 3, 6 and 7), responsible for intrinsic mitochondrial apoptotic pathways are thought to cause all the morphological apoptotic features assessed using the hematoxylin staining method (15). Caspase-3 immunostaining has been considered a suitable alternative for apoptotic quantification in other models (35), but the present study suggests other caspases also need to be considered when quantifying colonic epithelial apoptosis in this model.
In conclusion, we have demonstrated that elevated luminal butyrate as delivered by HAMSB increased the rate of apoptosis but not colonocyte proliferation in the distal colon of rats in acute response to AOM-induced genotoxicity. This effect provides further evidence supporting a possible protective role for butyrate against CRC.
Funding
Commonwealth Scientific and Industrial Research Organisation Preventative Health National Research Flagship (PO Box 10041, Adelaide BC, Adelaide, South Australia 5000); CSIRO Flagship Collaboration Fund Fellowship (to G.P.Y.).
Acknowledgments
The authors would like to thank National Starch Food Innovation (Bridgewater, New Jersey) for providing the HAMSB, Ms Julie Dallimore for her technical assistance and Dr Shusuke Toden for his contribution to statistical analyses. In addition, the statistical advice of Dr Ian Saunders of CSIRO Mathematical and Information Sciences (Urrbrae, South Australia) was greatly appreciated. G.P.Y., J.M.C., L.C., D.L.T. and A.R.B. designed the study; J.M.C., B.L.S. and J.G.W. conducted the research; J.M.C., G.P.Y., L.C., D.L.T., A.R.B., B.L.S. and J.G.W. analyzed the data; J.M.C., T.J.L., D.L.T., G.P.Y., A.R.B., B.L.S. and J.G.W. prepared the manuscript and J.M.C. had primary responsibility for the final content. All authors have read and revised the drafts and approved the final manuscript.
Conflict of Interest Statement: D.L.T. and A.R.B. are inventors on patents WO 01/02016 A1 and WO 02/02102 A1 as part of their employment by CSIRO and have no financial interest in them. J.M.C., G.P.Y., L.C., B.S., J.G.W., and T.J.L. declare no conflicts of interest.
Glossary
Abbreviations
- AOM
azoxymethane
- CRC
colorectal cancer
- HAMS
high amylose maize starch
- HAMSB
butyrylated high amylose maize starch
- LAMS
low amylose maize
- LAMST
low amylose maize starch with tributyrin; RS
- resistant starch
-
SCFA,
short-chain fatty acids
References
- 1.Garcia M, et al. Global Cancer Facts & Figures 2007. American Cancer Society, Atlanta, Georgia; 2007. [Google Scholar]
- 2.Young GP, et al. Dietary fibre and colorectal cancer: a model for environment–gene interactions. Mol. Nutr. Food Res. 2005;49:571–584. doi: 10.1002/mnfr.200500026. [DOI] [PubMed] [Google Scholar]
- 3.Trowell H. Definition of dietary fiber and hypotheses that it is a protective factor in certain diseases. Am. J. Clin. Nutr. 1976;29:417–427. doi: 10.1093/ajcn/29.4.417. [DOI] [PubMed] [Google Scholar]
- 4. Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commission, FAO., World Health Organisation Programme Mixte FAO/OMS Sur Les Normes Alimentaires, OAA, & Organisation Mondiale de la Santé. (2009) Report of the 30th session of the Codex Committee on Nutrition and Foods for Special Dietary Uses: Cape Town, South Africa, 3-7 November 2008. Rome, Italy: World Health Organization WHO / Food and Agriculture Organization of the United Nations FAO. paras 27--54 and Appendix II, p. 46. [Google Scholar]
- 5.Cassidy A, et al. Starch intake and colorectal cancer risk: an international comparison. Br. J. Cancer. 1994;69:937–942. doi: 10.1038/bjc.1994.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topping DL, et al. Resistant starch and human health. In: Van der Kamp JW, Jones J, McCleary BV, Topping DL, editors. Dietary Fiber: New Frontiers in Food and Health. Wageningen, The Netherlands: Wageningen Academic Publishers; 2010. pp. 311–321. [Google Scholar]
- 7.Topping DL, et al. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 8.Hague A, et al. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p.53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int. J. Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 9.Singh B, et al. Butyrate can act as a stimulator of growth or inducer of apoptosis in human colonic epithelial cell lines depending on the presence of alternative energy sources. Carcinogenesis. 1997;18:1265–1270. doi: 10.1093/carcin/18.6.1265. [DOI] [PubMed] [Google Scholar]
- 10.Avivi-Green C, et al. Different molecular events account for butyrate-induced apoptosis in two human colon cancer cell lines. J. Nutr. 2002;132:1812–1818. doi: 10.1093/jn/132.7.1812. [DOI] [PubMed] [Google Scholar]
- 11.Glucksmann A. Cell death in normal development. Arch. Biol. (Liege) 1965;76:419–437. [PubMed] [Google Scholar]
- 12.Chen Y, et al. Shaping limbs by apoptosis. J. Exp. Zool. 1998;282:691–702. [PubMed] [Google Scholar]
- 13.Zhivotovsky B, et al. Apoptosis and genomic instability. Nat. Rev. Mol. Cell Biol. 2004;5:752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
- 14.Chang WC, et al. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 15.Watson AJM. Apoptosis and colorectal cancer. Gut. 2004;53:1701–1709. doi: 10.1136/gut.2004.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong MY, et al. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol. Biomarkers Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 17.Le Leu RK, et al. Effect of resistant starch on genotoxin-induced apoptosis, colonic epithelium, and lumenal contents in rats. Carcinogenesis. 2003;24:1347–1352. doi: 10.1093/carcin/bgg098. [DOI] [PubMed] [Google Scholar]
- 18.Le Leu RK, et al. Effects of resistant starch and nonstarch polysaccharides on colonic luminal environment and genotoxin-induced apoptosis in the rat. Carcinogenesis. 2002;23:713–719. doi: 10.1093/carcin/23.5.713. [DOI] [PubMed] [Google Scholar]
- 19.Caderni G, et al. Slow-release pellets of sodium butyrate increase apoptosis in the colon of rats treated with azoxymethane, without affecting aberrant crypt foci and colonic proliferation. Nutr. Cancer. 1998;30:175–181. doi: 10.1080/01635589809514660. [DOI] [PubMed] [Google Scholar]
- 20.Avivi-Green C, et al. Apoptosis cascade proteins are regulated in vivo by high intracolonic butyrate concentration: correlation with colon cancer inhibition. Oncol. Res. 2000;12:83–95. doi: 10.3727/096504001108747558. [DOI] [PubMed] [Google Scholar]
- 21.McIntyre A, et al. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993;34:386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen PB, et al. Fermentation to short-chain fatty acids and lactate in human faecal batch cultures. Intra- and inter-individual variations versus variations caused by changes in fermented saccharides. Scand. J. Gastroenterol. 1991;26:1285–1294. doi: 10.3109/00365529108998626. [DOI] [PubMed] [Google Scholar]
- 23.Annison G, et al. Acetylated, propionylated or butyrylated starches raise large bowel short-chain fatty acids preferentially when fed to rats. J. Nutr. 2003;133:3523–3528. doi: 10.1093/jn/133.11.3523. [DOI] [PubMed] [Google Scholar]
- 24.Bajka BH, et al. Butyrylated starch is less susceptible to enzymic hydrolysis and increases large-bowel butyrate more than high-amylose maize starch in the rat. Br. J. Nutr. 2006;96:276–282. doi: 10.1079/bjn20061807. [DOI] [PubMed] [Google Scholar]
- 25.Clarke JM, et al. Excretion of starch and esterified short chain fatty acids by ileostomists after the ingestion of acylated starches. Am. J. Clin. Nutr. 2007;86:1146–1151. doi: 10.1093/ajcn/86.4.1146. [DOI] [PubMed] [Google Scholar]
- 26.Clarke JM, et al. Butyrate esterified to starch is released in the human gastrointestinal tract. Am. J. Clin. Nutr. 2011 doi: 10.3945/ajcn.111.017228. doi: 10.3945/ajcn.111.017228. [DOI] [PubMed] [Google Scholar]
- 27.Clarke JM, et al. Effects of high amylose maize starch and butyrylated high amylose maize starch on azoxymethane-induced intestinal cancer in rats. Carcinogenesis. 2008;29:2190–2194. doi: 10.1093/carcin/bgn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajka BH, et al. Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis. 2008;29:2169–2174. doi: 10.1093/carcin/bgn173. [DOI] [PubMed] [Google Scholar]
- 29.Reeves PG, et al. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 30.Thomas DJ, et al. Starches: Practical Guides for the Food Industry. St. Paul, MN: Eagan Press; 1999. [Google Scholar]
- 31.Brown MA, et al. Cooking attenuates the ability of high-amylose meals to reduce plasma insulin concentrations in rats. Br. J. Nutr. 2003;90:823–827. doi: 10.1079/bjn2003958. [DOI] [PubMed] [Google Scholar]
- 32.National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. 2004. 7th edn. http://www.nhmrc.gov.au/publications/synopses/ea16syn.htm. (3 June 2005, date last accessed) [Google Scholar]
- 33.Murase M, et al. Determination of portal short-chain fatty acids in rats fed various dietary fibers by capillary gas chromatography. J. Chromatogr. B. 1995;664:415–420. doi: 10.1016/0378-4347(94)00491-m. [DOI] [PubMed] [Google Scholar]
- 34.Potten CS, et al. A possible explanation for the differential cancer incidence in the intestine, based on distribution of the cytotoxic effects of carcinogens in the murine large bowel. Carcinogenesis. 1992;13:2305–2312. doi: 10.1093/carcin/13.12.2305. [DOI] [PubMed] [Google Scholar]
- 35.Marshman E, et al. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J. Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- 36.Hague A, et al. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int. J. Cancer. 1995;60:400–406. doi: 10.1002/ijc.2910600322. [DOI] [PubMed] [Google Scholar]
- 37.Heerdt BG, et al. Short-chain fatty acid-initiated cell cycle arrest and apoptosis of colonic epithelial cells is linked to mitochondrial function. Cell Growth Differ. 1997;8:523–532. [PubMed] [Google Scholar]
- 38.Pajak B, et al. Sodium butyrate sensitizes human colon adenocarcinoma COLO 205 cells to both intrinsic and TNF-alpha-dependent extrinsic apoptosis. Apoptosis. 2009;14:203–217. doi: 10.1007/s10495-008-0291-9. [DOI] [PubMed] [Google Scholar]
- 39.Myzak MC, et al. Dietary agents as histone deacetylase inhibitors. Mol. Carcinog. 2006;45:443–446. doi: 10.1002/mc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thangaraju M, et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganapathy V, et al. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol. Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Andoh A, et al. Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. Design. 2003;9:347–358. doi: 10.2174/1381612033391973. [DOI] [PubMed] [Google Scholar]
- 43.Thangaraju M, et al. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J. Gastrointest. Surg. 2008;12:1773–1781. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- 44.Marshman E, et al. The intestinal epithelial stem cell. BioEssays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 45.Young GP, et al. Resistant starch and colorectal neoplasia. J. AOAC Int. 2004;87:775–786. [PubMed] [Google Scholar]
- 46.Le Leu RK, et al. Effect of high amylose maize starches on colonic fermentation and apoptotic response to DNA-damage in the colon of rats. Nutr. Metab. (Lond) 2009;6:11. doi: 10.1186/1743-7075-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symonds EL, et al. A combined 13CO2/H2 breath test can be used to assess starch digestion and fermentation in humans. J. Nutr. 2004;134:1193–1196. doi: 10.1093/jn/134.5.1193. [DOI] [PubMed] [Google Scholar]
- 48.Holt PR, et al. Regional distribution of carcinogen-induced colonic neoplasia in the rat. Nutr. Cancer. 1996;25:129–135. doi: 10.1080/01635589609514435. [DOI] [PubMed] [Google Scholar]
- 49.Cats A, et al. Regional differences of physiological functions and cancer susceptibility in the human large intestine. Int. J. Oncol. 1996;9:1055–1069. doi: 10.3892/ijo.9.5.1055. [DOI] [PubMed] [Google Scholar]
- 50.Goodlad RA, et al. Redefining dietary fibre: potentially a recipe for disaster. Lancet. 2001;358:1833–1834. doi: 10.1016/S0140-6736(01)06882-9. [DOI] [PubMed] [Google Scholar]
- 51.Mandir N, et al. Resistant carbohydrates stimulate cell proliferation and crypt fission in wild-type mice and in the Apc (Min+) mouse model of intestinal cancer, association with enhanced polyp development. Br. J. Nutr. 2008;100:711–721. doi: 10.1017/S0007114508901276. [DOI] [PubMed] [Google Scholar]
- 52.Toden S, et al. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer Biol. Ther. 2007;6:253–258. doi: 10.4161/cbt.6.2.3627. [DOI] [PubMed] [Google Scholar]
- 53.Bloemen JG, et al. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin. Nutr. 2009;28:657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]