Three structurally related sets of hydroisobenzofuran analogs of sclerophytin A were prepared in 3 or 4 steps from (S)-(+)-carvone via an aldol-cycloaldol sequence. The most potent members of each set of analogs exhibited IC50’s of 1–3 µM in growth inhibitory assays against KB3 cells. The NCI 60 cell line 5-dose assay for analog 6h revealed a GI50=0.148 µM and LC50=9.36 µM for the RPMI-8226 leukemia cell line, and a GI50=0.552 µM and LC50=26.8 µM for the HOP-92 non-small cell lung cancer cell line.
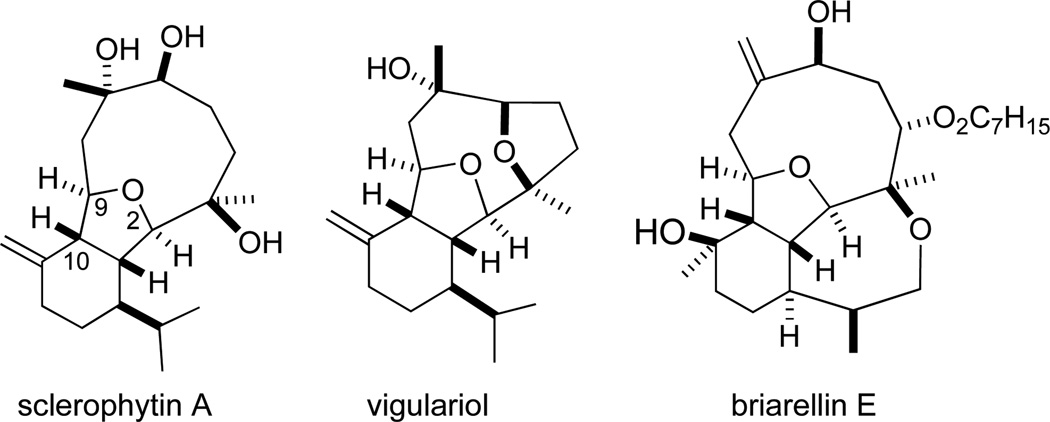
The 2,11-cyclized cembranoids are a class of diterpenoids isolated from a variety of marine sources that exhibit a wide range of biological activities.1,2 Sclerophytin A, for example, was reported to exhibit growth inhibitory activity against the murine L1210 leukemia cell line with an IC50=1.0 ng/mL (Figure 1).3,4
Figure 1.
Representative Hydroisobenzofuran-containing 2,11-Cyclized Cembranoids.
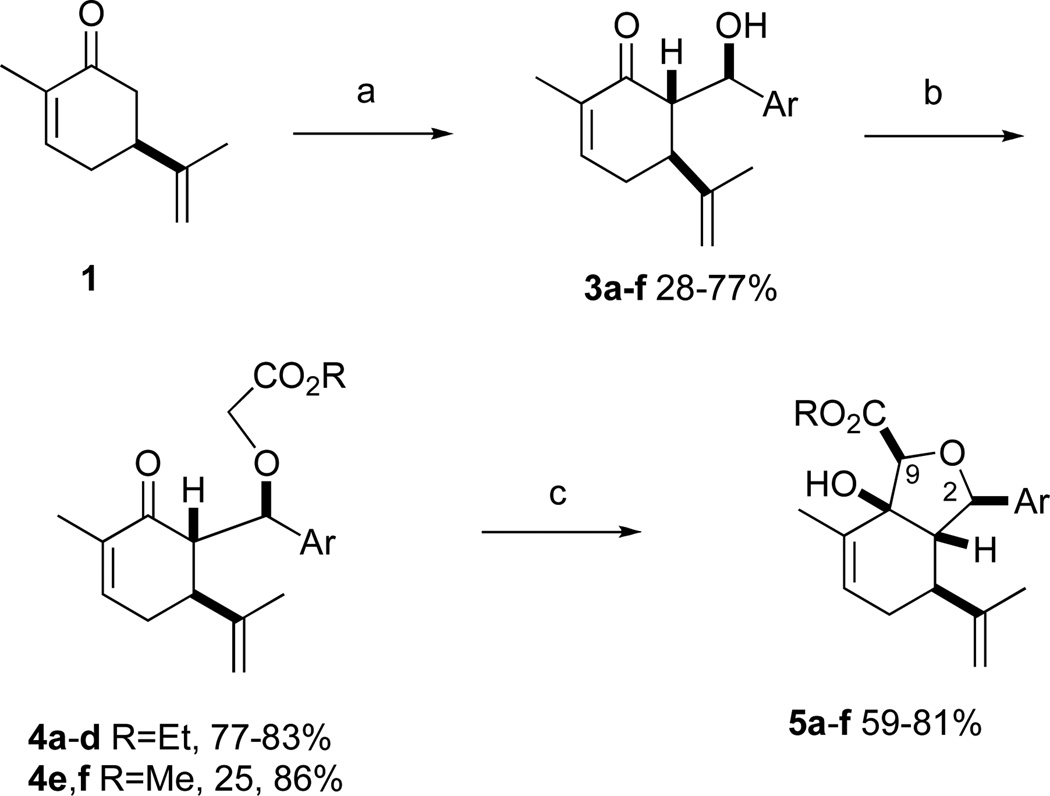
A considerable amount of synthetic effort has been directed toward the synthesis of sclerophytin A and related cembranoids.5,6 Total syntheses of these complex targets typically require in excess of 20 steps from commercially available starting materials. The majority of these diterpenoids possess a cis-fused hydroisobenzofuran core structure.1,2 We hypothesized that seco analogs containing the hydroisobenzofuran core might exhibit some of the same anticancer activities as the parent compounds. We designated sclerophytin A as the nominal target of the analoging study and named the resulting compounds “sclerologs”.7 As part of the design principle, we sought to construct novel scaffolds that would readily lend themselves to diversification. To this end, we chose aryl groups as C2 substituents and an ester as the C9 substituent (Scheme 1, cf. Fig. 1). In order to keep the sclerolog syntheses as short as possible, we elected to retain the isopropenyl alkene. It seems unlikely that the steric and/or electronic differences between the isopropenyl and isopropyl groups would have a significant effect on anticancer activity.
Scheme 1.
Sclerolog Synthesis via an Aldol-Cycloaldol Sequence.
a. 1. LDA, THF, −78 °C. 2. 2a–f. 3. HOAc. b. Ag2O, BrCH2CO2R, DMF, 2,6-lutidine, rt. c. 1. KHMDS, THF, −78 °C. 2. HOAc.
a 2a–d: 2-bromo-, 3-bromo-, 2,3-dichloro-, 2,4-dichlorobenzaldehyde, respectively; 2e: 1-naphthaldehyde; 2f : pyridine-2-carboxaldehyde.
In our own studies toward the total synthesis of selected 2,11-cyclized cembranoids, we developed a 3-step protocol for assembling the hydroisobenzofuran core structure from (S)-(+)-carvone.8 Following the same procedure, intermolecular aldol reaction of carvone and aryl aldehydes 2a–f gave anti-aldol adducts 3a–f with varying levels of diastereoselectivity (1:1 to 10:1 anti:syn) (Scheme 1). Etherification of alcohols 3a–f generally proceeded in good yields to give glycolate esters 4a–f. Cycloaldolization under the influence of KHMDS afforded hydroisobenzofurans 5a–f as single diastereomers based on 1H NMR analysis. The stereochemical assignments were made by analogy to earlier examples whose structure was determined by X-ray crystallographic analysis.8a
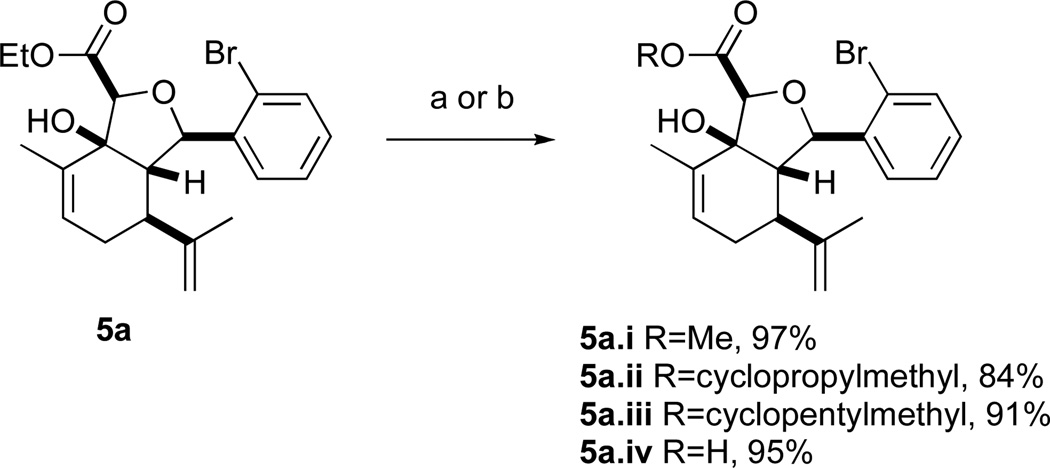
A series of acyl derivatives of ester 5a were prepared to evaluate the effect of the carboxyl substituent on activity (Scheme 2). Esters 5a.i–iii were prepared by transesterification of ethyl ester 5a in the presence of excess alcohol and Bu3SnOAc catalyst, while carboxylic acid 5a.iv was prepared by saponification.
Scheme 2.
Variation of the C9 Ester Moiety
a. Bu3SnOAc, ROH. b. LiOH, THF/MeOH/H2O
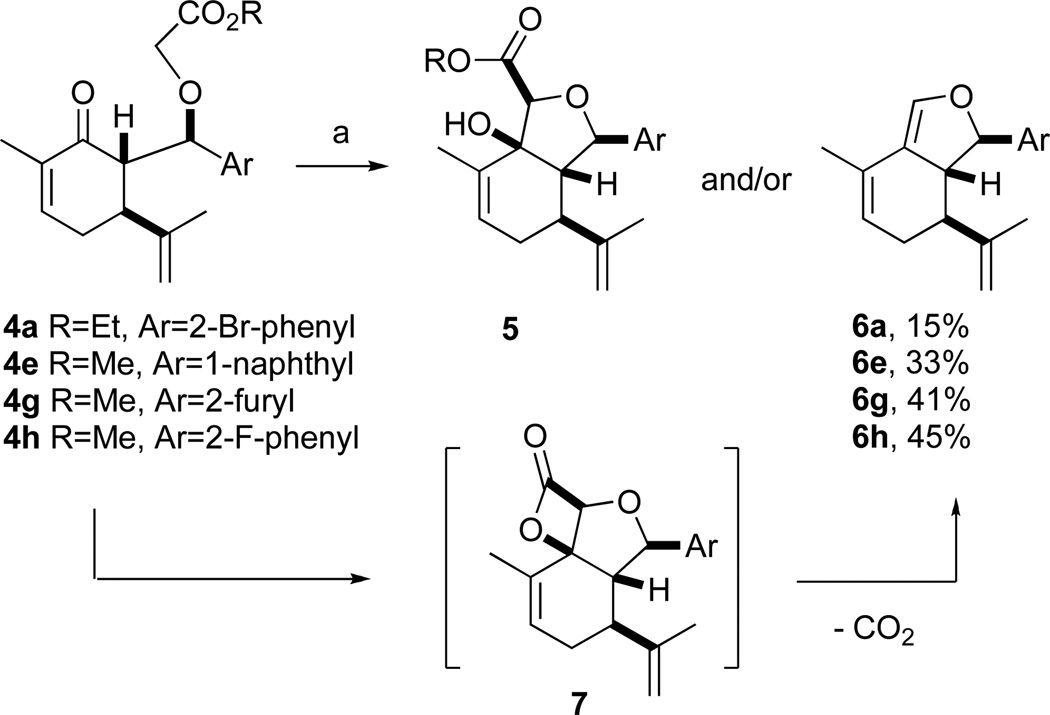
In the course of optimizing the cycloaldol reaction of glycolate 4a to ester 5a, we found that diene 6a was formed in significant amounts if the the reaction mixture was not rapidly quenched with HOAc after addition of KHMDS (Scheme 3). For methyl glycolates 4g and 4h, the diene was the only cyclized product isolated from the reaction mixture.
Scheme 3.
Proposed Sequence for Formation of Dienes 6.
a. 1. KHMDS, THF, −78 °C. b. HOAc
The dienes were presumably formed by in situ lactonization of the aldol intermediates to form β-lactones 7, which underwent unusually facile loss of CO2 to give the dienes (Scheme 3).9 Since the dienes also constituted novel scaffolds, we included several in the subsequent assays (vide infra).
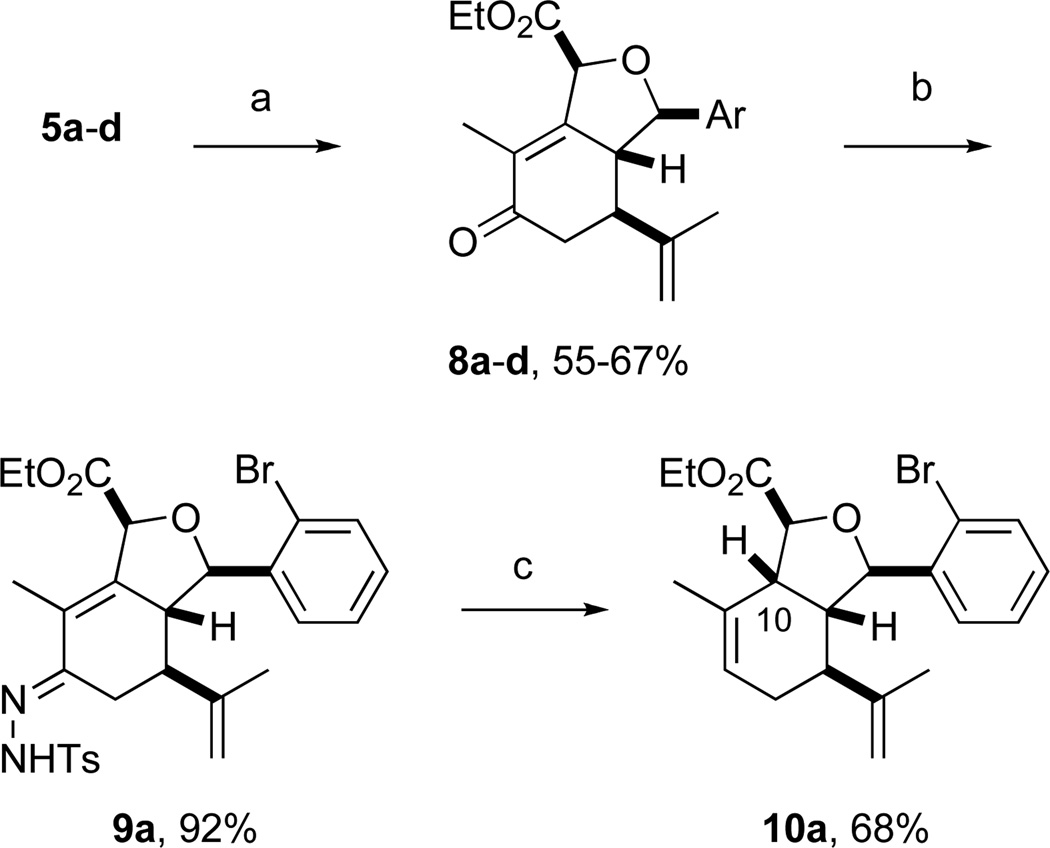
Sclerophytin A and all other hydroisobenzofuran-containing 2,11-cyclized cembranoids possess a C10 stereocenter in the alkane, rather than alcohol, oxidation state (Scheme 3, cf. Figure 1).1,2 We therefore prepared analog 10a to more closely mimic the natural structures. We have previously reported the reduction of alcohols similar to 5a–d in a 3-step oxidative/reductive rearrangement process.8a,10 Enones 8a–d were prepared by oxidative rearrangement of the corresponding 3° alcohols.11 Conversion of enone 6a to tosyl hydrazone 9a was followed by reductive transposition to afford cis-fused hydroisobenzofuran 10a. As the enones also constituted a novel structural class, we included them in the biological assays.
The human KB-3 carcinoma cell line was used to perform the MTT colorimetric assays.12,13,14 Cells were treated with increasing concentrations of compounds to assess their growth inhibitory properties. IC50 values (concentration of the drug required to reduce cell viability by 50%) ranged from 1 to >100 µM (Table).
Table 1.
Inhibition of KB-3 Cell Survival Assessed by MTT Viability Assay.*
| Entry | Compound | IC50 (µM) |
|---|---|---|
| 1 | 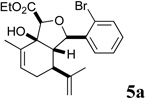 |
20 |
| 2 | 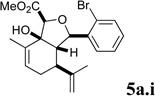 |
5 |
| 3 | 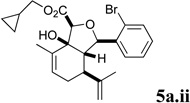 |
50 |
| 4 | 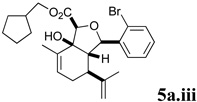 |
> 100 |
| 5 | 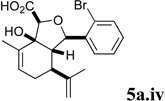 |
> 100 |
| 6 | 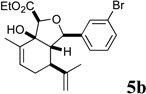 |
3 |
| 7 | 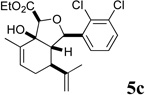 |
5 |
| 8 | 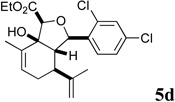 |
3 |
| 9 | 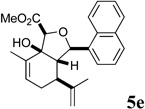 |
10 |
| 10 | 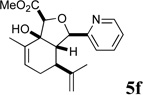 |
100 |
| 11 | 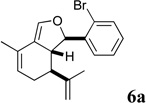 |
4 |
| 12 | 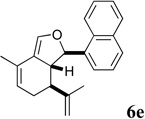 |
> 100 |
| 13 | 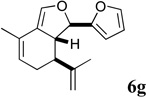 |
2 |
| 14 | 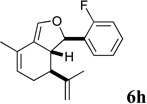 |
1 |
| 15 | 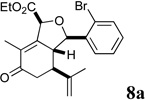 |
70 |
| 16 | 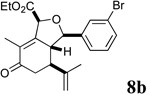 |
30 |
| 17 | 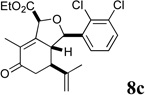 |
5 |
| 18 | 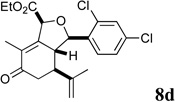 |
3 |
| 19 | 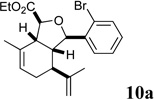 |
> 100 |
Cells were treated with increasing concentration of compounds and MTT viability assays were performed after 96 h. Values are the means of triplicate assays and are expressed as mean relative to untreated controls (see Supplementary data for details and representative concentration curves).
Several notable features become apparent upon examination of the assay data. Firstly, for the 2-Br esters 5a and 5a.i–iii (entries 1–4), the smaller the alcohol moiety of the ester, the lower the IC50, i.e. Me < Et < cyclopropylmethyl < cyclopentylmethyl. Secondly, two or more members of each class (alcohols 5, dienes 6 and enones 8) possess single digit micromolar activity. Reduction of C10 from the alcohol to the alkane oxidation state significantly diminished activity (5a vs. 10a)
The essentially equal potency of the alcohols and dienes is intriguing and initially tempted us to speculate that the esters might undergo lactonization and decarboxylation to the dienes under the assay conditions (cf. Scheme 3). This notion is supported by the substantial difference in activity between C10-OH ester 5a, which can undergo lactonization, and the corresponding C10 reduction product 10a, which cannot. However, the 1-napthyl methyl ester 5e is at least 10-fold more active than the corresponding diene 6e. Furthermore, enones 8a–d exhibit essentially equal potency to the C10-OH methyl and ethyl esters, and also cannot undergo β-lactone formation. Further studies will be needed to determine whether the alcohols, dienes and enones are inhibiting growth by the same or different mechanisms of action.
The most active representatives of the compound classes (alcohol 5d, diene 6h and enone 8d) were submitted to NCI’s Developmental Therapeutics program for 60-cell line screening (see Supplementary data for complete results). Single dose assays revealed no significant activity for alcohol 5d. Enone 8d exhibited significant differential activity against the RPMI-8226 leukemia and the PC-3 prostate cancer cell lines. Diene 6h was the most active, possessing significant differential activity against the entire leukemia panel and the NCI-H522 non-small cell lung cancer cell line. Subsequent 5-dose testing of 6h revealed a GI50=0.148 µM and LC50=9.36 µM for the RPMI-8226 leukemia cell line, and a GI50=0.552 µM and LC50=26.8 µM for the HOP-92 non-small cell lung cancer line.
The results described herein suggest that the three novel hydroisobenzofuran-containing scaffolds 5, 6 and 8 exhibit promising anticancer activity. Further SAR studies and investigations into their mechanism of action are pending.
Supplementary Material
Scheme 4.
Oxidative-Reductive Rearrangement Sequence.
a. PCC, silica gel, CH2Cl2. b. TsNHNH2, EtOH, HOAc. c.1. catecholborane, CHCl3, 0 °C. 2. NaOAc-3H2O, reflux.
Acknowledgements
We thank the NIH (CA75577, RR15569, CA125602, CA109821) and the Arkansas Biosciences Institute for support of this work; the NCI Developmental Therapeutics Program (http://dtp.cancer.gov) for 60-cell line testing; and John Beutler, NCI, for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:
References and notes
- 1.Wahlberg I, Eklund A-M. Prog. Chem. Org. Nat. Prod. 1992;60:1. [Google Scholar]; Bernardelli P, Paquette LA. Heterocycles. 1998;49:531. [Google Scholar]
- 2.(a) Wu S-L, Su J-H, Wen Z-H, Hsu C-H, Chen B-W, Dai C-F, Kuo Y-H, Sheu J-H. J. Nat. Prod. 2009;72:994. doi: 10.1021/np900064a. [DOI] [PubMed] [Google Scholar]; (b) Ospina CA, Rodriguez AD. J. Nat. Prod. 2006;69:1721. doi: 10.1021/np060317y. [DOI] [PubMed] [Google Scholar]; (c) Kyeremeh K, Baddeley TC, Stein BS, Jasparsa M. Tetrahedron. 2006;62:8770. [Google Scholar]; (d) Ahmed AF, Wu M-H, Wang G-H, Wu Y-C, Shue J-H. J. Nat. Prod. 2005;68:1051. doi: 10.1021/np0500732. [DOI] [PubMed] [Google Scholar]; (e) Chill L, Berrer N, Benayahu Y, Kashman Y. J. Nat. Prod. 2005;68:19. doi: 10.1021/np049772p. [DOI] [PubMed] [Google Scholar]; Ata A, Ackerman J, Bayoud A, Radhika P. Helv. Chim. Acta. 2004;87:592. and references cited therein. [Google Scholar]
- 3.Sharma P, Alam M. J. Chem. Soc. Perkin Trans I. 1988:2537. [Google Scholar]
- 4.Structure revision: Friedrich D, Doskotch RW, Paquette LA. Org. Lett. 2000;2:1879. doi: 10.1021/ol000084g. Friedrich D, Paquette LA. J. Nat. Prod. 2002;65:126. doi: 10.1021/np0103822.
- 5.For a comprehensive review, see: Ellis JM, Crimmins MT. Chem. Rev. 2008;108:5278. doi: 10.1021/cr078117u.
- 6.Corminboeuf O, Overman LE, Pennington DL. J. Org. Chem. 2009;74:5458. doi: 10.1021/jo9010156. [DOI] [PMC free article] [PubMed] [Google Scholar]; Campbell MJ, Johnson JS. J. Am. Chem. Soc. 2009;131:10370. doi: 10.1021/ja904136q. [DOI] [PubMed] [Google Scholar]
- 7.There have been very few biological studies of analogs of isobenzofuran-containing 2,11-cyclized cembranoids: Jung ME, Pontillo J. J. Org. Chem. 2002;67:6848. doi: 10.1021/jo016246j. Davidson JEP, Gilmour R, Ducki S, Davies JE, Green R, Burton JW, Holmes AB. Synlett. 2004:1434.
- 8.(a) Chai Y, Vicic DA, McIntosh MC. Org. Lett. 2003;7:1039–1042. doi: 10.1021/ol034052f. [DOI] [PubMed] [Google Scholar]; (b) Chai Y, McIntosh MC. Tetrahedron Lett. 2004;45:3269. doi: 10.1016/j.tetlet.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam W, Arias Encarnacion LA. Synthesis. 1979:388. [Google Scholar]
- 10.Hutchison JM, Lindsay HA, Dormi SS, Jones GD, Vicic DA, McIntosh MC. Org. Lett. 2006;8:3663. doi: 10.1021/ol061072j. [DOI] [PubMed] [Google Scholar]
- 11.Dauben WG, Michno DM. J. Org. Chem. 1977;42:682. [Google Scholar]
- 12.Mosmann T. J. Immunol. Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Fan M, Du L, Stone AA, Gilbert KM, Chambers TC. Cancer Res. 2000;60:6403. [PubMed] [Google Scholar]
- 14.Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Abbott BJ, Mayo JG, Shoemaker RH, Boyd MR. Cancer Res. 1988;48:589. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.