Abstract
Untreated BERKO mice demonstrate few abnormalities in bone phenotype and recent ovariectomy has few effects on various bone characteristics in these mice. Long-term studies on the bone phenotype of intact and ovariectomized mice are unavailable. Using quantitative computed tomography (qCT), we determined various parameters of the metaphysis of the tibia in sham-ovariectomized (intact) and ovariectomized BERKO and wildtype mice. Body weight and estrogen-regulated fat were also measured. Mice underwent surgery (ovariectomy or sham) at 3 mo of age, and qCT analysis was performed every 2 to 4 mo until mice were 12 mo old. Ovariectomized wildtype mice gained body weight and their fat depot increased in size within 2 mo after ovariectomy. Obesity developed later in ovariectomized BERKO mice, which became significantly heavier than their wildtype counterparts. Ovariectomized wildtype mice lost trabecular density more rapidly than did ovariectomized BERKO mice, which did not show similar loss in trabecular density until at least 7 mo after ovariectomy. At the latest studied time point (9 mo after surgery), cortical area was significantly larger in ovariectomized BERKO mice than ovariectomized wildtype mice. The absence of ERβ in ovariectomized BERKO mice during the first 3 to 5 mo after ovariectomy had protective effects against obesity and trabecular rarification; this protective effect disappeared at later time points.
Abbreviations: BERKO, estrogen receptor β knockout; ER, estrogen receptor; qCT, qualitative computer-assisted tomography
Overwhelming evidence indicates that estrogens are necessary for maintenance of normal bone homeostasis. In many species, including humans and rats, depletion of estrogen—either spontaneously after menopause or surgically induced by ovariectomy—often results in severe osteoporosis, which largely can be prevented by estrogen replacement.18,30 These effects of estrogens are receptor-mediated. Two estrogen receptors (ERα and ERβ) have been cloned.5,18 The 2 most important cell types for bone homeostasis are the bone-resorbing osteoclasts and the bone-forming osteoblasts. Both cell types express both ER, with a higher degree of ERα expression.1-3,22 Studies with ERα and ERβ knockout (ERKO and BERKO) mice and with specific ERα and ERβ ligands suggest that the predominant ER mediating the antiosteoporotic effects of estrogens in bone cells is ERα.8,12,16,24,31 Treatment of ovariectomized rats with an ERβ agonist has little effect on various characteristics of bone, indicating a relatively unimportant role of ERβ in this tissue.8,9,12 BERKO mice show minor changes in bone characteristics,18 including trabecular bone structures, and the growth of the cortex of long bones and their mineralization are increased.33 These effects begin to be apparent at the peak of bone mass development, which occurs at 2 to 3 mo of age in mice.14,15 Development of bone in BERKO and wildtype mice has been studied, and decreased trabecular bone mineral density is present in BERKO mice at 60 d of age.18 At 140 d of age, trabecular density was greater in BERKO than wildtype animals,18 and this age-related effect became even more marked as mice aged.15 Therefore, increased growth and mineralization of bone occurs later in BERKO than wildtype mice; the exact time course of these effects has not yet been studied in depth.
Deletion of ERβ does not prevent the development of osteoporosis after ovariectomy.20 In view of the proposed antagonistic effects of ERβ on ERα,19,32 ovariectomy of BERKO mice might be expected to prevent or retard osteoporosis. In addition, some evidence suggests that bone demineralization is decreased in ovariectomized BERKO mice compared with intact BERKO mice.20
Estrogens also regulate adipocytes, and human and rodent adipocytes express both ER.7,13,21,25 The removal of estrogenic signaling by ovariectomy causes obesity.4,6,21,27,28,31 This effect on obesity is ERα-mediated because ERKO but not BERKO mice are obese,16,24 and obesity of ovariectomized rats can be prevented by ERα agonists but not ERβ agonists.9 The role of ERβ in adipocytes remains unknown.
Various bone parameters, such as cancellous and cortical bone densities and surface areas, can be measured by quantitative computer-assisted tomography (qCT);10 the proximal metaphysis of the tibia often is studied because it is extremely sensitive to deficient estrogenic signaling.27 We noted a small fat depot in rats that was lateral to the metaphysis of the tibia (the paratibial fat depot); this fat depot increases in size after gonadectomy.27,28 In rats, the size of the paratibial fat depot correlates very well with general obesity, and because it lies within the qCT plane of the metaphysis, its size can be determined from qCT scans of the metaphysis of the tibia.26,27
Because little is known about the bone phenotype of BERKO mice and the development of osteoporosis in these mice after ovariectomy, we determined the densities and areas of the trabecular and cortical structures of the metaphysis of the tibia. In addition, we monitored the size of the paratibial fat depot as a measure of obesity. Intact and ovariectomized wildtype and BERKO mice underwent qCT of the metaphysis of the tibia at various ages from before puberty until they were 1 y old.
Materials and Methods
Animals.
All experiments were performed after consent by the governmental authorities was obtained (LAVES; AZ: 33.11.42502-04-01 to 30.05).
Male heterozygous ERβ+/− mice were mated with female heterozygous ERβ+/− mice, resulting in ERβ−/−, ERβ+/−, and ERβ+/+ (wildtype) offspring on a mixed C57BL/6J × 129 background.17 These mice were raised and genotyped from DNA (NucleoSpin Tissue kit, Macherey-Nagel, Düren, Germany) at our institution. After weaning, mice were kept on soy-free food (V1354 R-Z, Ssniff, Soest, Germany) containing an equicaloric amount of potato proteins. This food was chosen because most animal chows include soy proteins, which contain estrogenic isoflavones that might have confounded the results. Subgroups (n = 10 to 12) of isoflurane-anesthetized wildtype and BERKO mice underwent qCT of the left proximal metaphysis of the tibia at 3 mo of age. Isoflurane anesthesia was provided through an inhalation apparatus (Penlon Sigma Delta, Penlon Abingdon, Oxon, UK) at a flow rate of 0.45 L/min with oxygen at the same flow rate. Isoflurane-anesthetized mice were ovariectomized or sham-ovariectomized, resulting in 4 groups: wildtype, sham-ovariectomized; wildtype ovariectomized; BERKO sham-ovariectomized; and BERKO ovariectomized.
All mice underwent quantitative qCT at 2, 3, 5, 7, and 9 mo after surgery (5, 6, 8, 10, and 12 mo of age); sham-operated mice served as controls. No animal died during the course of the experiments. Mice were weighed at each imaging session. At the end of the investigative period, all mice were euthanized, and blood and other organs were collected for analysis.
Measurement of bone parameters.
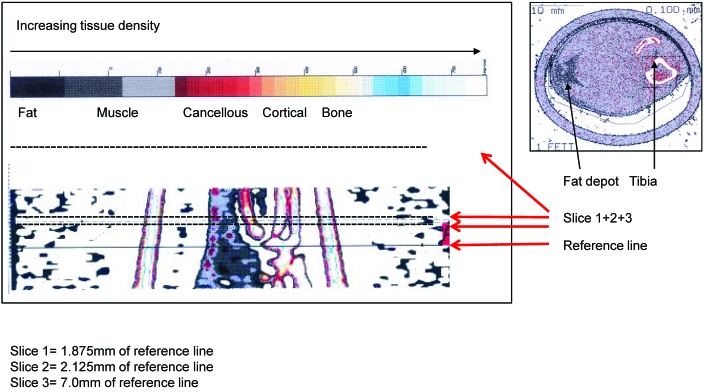
The mineral content of the trabeculae in the proximal metaphysis of the tibia, total endosteal area, cortical mineral density, and cortical area were determined by using qCT (XCT 5.40, Stratec, Pforzheim, Germany) at a resolution of 100 µm. The coefficient of variance as a measure of repeatability of 10 measurements of the same animal was 0.43. The scanner was positioned at the upper end of the left epiphysis of the tibia, and a coronal computer radiograph (scout view) in distal direction was obtained. The scout view was used to position the scanner at the sites of measurement needed to obtain qCT slices 1.86 and 2.11 mm distal of the scout view (Figure 1). Due to the large variations of the cortical thickness at the level of the metaphysis of the tibia, cortical parameters were determined 7.5 mm distal of the reference line, that is, in the diaphyseal part of the tibia. Each mouse was placed in a plastic funnel-like object, such that the left hindleg was inside the funnel with the foot extending from the opening. The foot was clamped to the funnel outlet, an action that ensured consistently repeatable positioning of the investigated structure.
Figure 1.
Scout view and location of the 2 qCT pla nes for quantification of bone parameters (left; slices 1 and 2; 1.875 and 2.125 mm from the reference line, respectively) and of the paratibial fat depot at the level of the metaphysis of the tibia (right; slice 3; a plane through the diaphysis). Tissue densities are color-coded (bar), with a representative qCT image at the right.
Image acquisition, data processing, and calculation of the results were performed by using the software package supplied with the scanner (Strat. Trabecular density and endosteal area in the 2 metaphyseal cross-sections were calculated by acquiring data within the default threshold of 280 to 710 mg/cm3. Tissue with a density exceeding 710 mg/cm3 was considered to be cortical bone, and cortical area and density were calculated from the slice obtained 7.5 mm distal of the reference line.
Determination of fat tissue in lower hind leg.
At the level of the 2 qCT slices within the metaphysis of the tibia, a small fat depot (the paratibial fat pad) is visible. The size of this fat depot can be quantified perimetrically (Figure 1) and is reported as the percentage of the total lower-limb cross-section at the level of the 2 metaphyseal qCT planes.
Statistical analysis.
Data were evaluated by one-way ANOVA followed by the Dunnett post test (Graph Pad Prism, San Diego, CA). A P value of less than 0.05 was considered to be statistically significant. Data are presented as means and SEM (error bars).
Results
None of the measured parameters differed significantly between wildtype and BERKO mice before ovariectomy or sham surgery at 3 mo of age.
Body weights.
Body weight (Figure 2 A) increased steadily in sham-ovariectomized wildtype and BERKO mice until they were 10 mo of age and remained at that level for the rest of the investigative period. Body weight increased significantly (P < 0.05) after ovariectomy in both wildtype and BERKO mice.
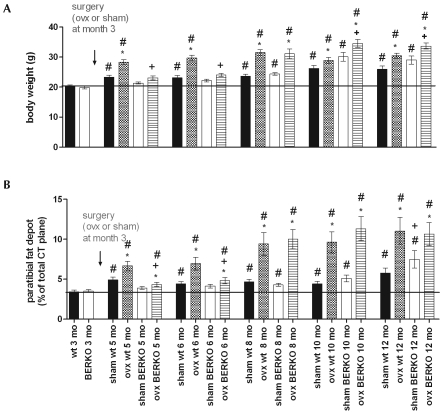
Figure 2.
(A) Body weight and (B) size of the paratibial fat depots in intact (sham) and ovariectomized (ovx) BERKO (−/−) and wildtype (wt) mice after surgery at 3 mo of age. *, P < 0.05 compared with value for intact mice of the same genotype; +, P < 0.05 compared with value for respective wild-type mice; #, P < 0.05 compared with value at 3 mo for mice of the same genotype. Black bars represent the mean value of WT at 3 mo of age (also shown as bar furthest to the left).
At 7 and 9 mo after surgery (that is, at 10 and 12 mo of age), the body weight of ovariectomized BERKO mice was greater (P < 0.05) than that of ovariectomized wildtype animals. In sham-ovariectomized wildtype and BERKO mice, the paratibial fat depot showed only small increases in its size, which were significant (P < 0.05) only at 12 mo of age (Figure 2 B). In contrast, the size of the fat depot increased dramatically (P < 0.05) in ovariectomized wildtype mice within 2 mo after surgery and continued at 3 mo. Interestingly, the paratibial fat had not increased at 2 and 3 mo after ovariectomy of the BERKO mice, but at ages 8, 10, and 12 mo (that is, 5, 7, and 9 mo after ovariectomy), the size of the fat depot in ovariectomized BERKO mice was similar to that in the ovariectomized wildtype mice (Figure 2 B). Therefore, fat accumulation after ovariectomy occurred at a later time point but at a higher magnitude in BERKO mice than in wildtype animals, such that no difference existed at 8, 10, and 12 mo of age (that is, 5, 7, and 9 mo after surgery).
Density and surface area of cancellous bone.
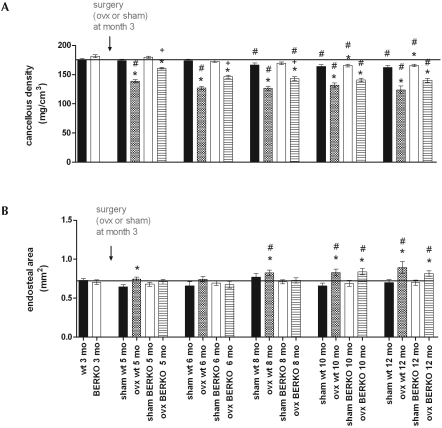
The highest densities in cancellous bone were measured in intact mice at the age of 3 to 6 mo, with no differences between BERKO and wildtype mice (Figure 3 A). Cancellous densities in both groups of sham-ovariectomized mice fell later in life to reach slightly but significantly (P < 0.05) lower levels at 10 mo of age. In ovariectomized wildtype and BERKO mice, cancellous densities were significantly (P < 0.05) reduced within 2 mo after ovariectomy. At 2, 3, and 5 mo after surgery, however, the reduction in density was significantly (P < 0.05) smaller in ovariectomized BERKO than ovariectomized wildtype mice, but these differences were no longer significant at 7 and 9 mo after ovariectomy (Figure 3 A). The areas of endosteal (cancellous) bone in the metaphyses of the tibiae were identical between sham-ovariectomized BERKO and wildtype mice (Figure 3 B) at all investigated time points; the area of cancellous bone increased in both ovariectomized wildtype and BERKO mice, becoming significant (P < 0.05) at 7 and 9 mo after ovariectomy (Figure 3 B).
Figure 3.
(A) Cancellous densities and (B) endosteal areas of the metaphyses of the tibiae of wildtype (wt) and BERKO (−/−) mice after ovariectomy (ovx) or sham surgery. Note the rapid loss of cancellous density at 2, 3, and 5 mo after ovariectomy in wildtype mice; this loss was much slower in BERKO mice. However, these differences had disappeared by 7 and 9 mo after surgery. *, P < 0.05 compared with value for intact mice of the same genotype; +, P < 0.05 compared with value for respective wildtype mice; #, P < 0.05 compared with value at 3 mo for mice of the same genotype. Black bars represent the mean value of WT at 3 mo of age (also shown as bar furthest to the left).
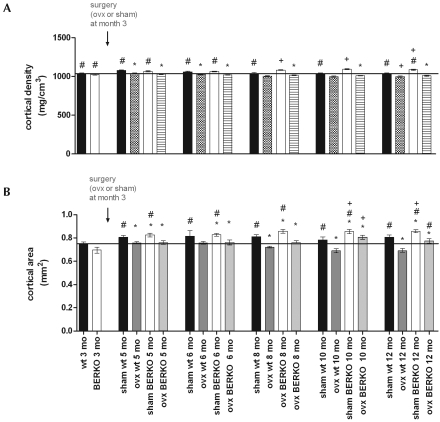
Cortical densities in sham-ovariectomized wildtype and BERKO mice increased significantly throughout the investigation period and were slightly but significantly higher in sham-ovariectomized BERKO mice than in sham-ovariectomized wildtype mice at the ages of 8 mo and later. Wildtype and BERKO mice both had significantly (P < 0.05) lower cortical densities within 4 wk after ovariectomy than did age-matched sham-ovariectomized mice; these differences were retained at all time points thereafter (Figure 4 A). Cortical area increased steadily in both sham-ovariectomized groups and did not differ between wildtype and BERKO mice (Figure 4 B). At all time points after surgery, cortical area was consistently lower in ovariectomized wildtype and BERKO mice than in sham-surgery controls. When mice were 12 mo old (that is, 9 mo after surgery), cortical areas of BERKO mice were at values between ovariectomized intact and ovariectomized wildtype animals. The cortical surface area of ovariectomized BERKO mice was significantly (P < 0.05) greater than that in ovariectomized wildtype mice.
Figure 4.
Cortical (A) densities and (B) areas in wildtype (wt) and BERKO (−/−) mice after ovariectomy (ovx) or sham surgery at the age of 3 mo. The reduction in cortical density after ovariectomy did not differ significantly between wild-type and BERKO mice; however, cortical area was significantly smaller in wild-type than BERKO mice. *, P < 0.05 compared with value for intact mice of the same genotype; +, P < 0.05 compared with value for respective wildtype mice; #, P < 0.05 compared with value at 3 mo for mice of the same genotype. Black bars represent the mean value of WT at 3 mo of age (also shown as bar furthest to the left).
Discussion
In the present study, we confirm previous results indicating that disruption of ERβ does not result in deregulation of body weight or fat tissue19 in sham-ovariectomized mice until month 10 of life. At this and the 12-mo time point, average body weights and paratibial fat depots were significantly (P < 0.05) larger in sham-ovariectomized BERKO mice than in sham-ovariectomized wildtype mice. In addition, ERβ plays a role in the regulation of body weight and fat accumulation after ovariectomy. As reported previously,23 withdrawal of estrogens in ovariectomized wildtype mice resulted in rapid accumulation of body weight and fat depots, but similar increases in body weight and fat accumulation did not occur 1 to 2 mo after ovariectomy in ovariectomized BERKO mice, again confirming previous results.33 However, 5 mo after ovariectomy, body weight and fat accumulation no longer differed between ovariectomized mice, and at 7 and 9 mo after surgery, ovariectomized BERKO mice were significantly heavier than were ovariectomized wildtype mice, and at 7 mo the paratibial fat depot was larger. This late increase in body weight in sham-ovariectomized and ovariectomized BERKO animals had not been described earlier and suggests that ERβ plays an important lipolytic or antilipotrophic role in mice. If this finding is applicable to humans, ERβ agonists may prevent or reduce fat accumulation in women, particularly when administered postmenopausally.
In rodents, the proximal metaphysis of the tibia is known to react most sensitively34 to withdrawal of metabotrophic hormones such as estradiol. In agreement with an earlier study in which bone mineral densities of the proximal tibia were followed for 4 wk after ovariectomy,20 BERKO mice were not protected against ovariectomy-induced loss of bone mineral density, because mineral densities of the trabecular apparatus in the metaphysis and of the cortical areas in the diaphysis of the tibia were affected similarly to those in wildtype mice. Estrogen replacement of ovariectomized BERKO mice prevented development of osteoporosis,20 and the authors concluded that ERα mediates most of the response to estradiol in wildtype mice but that a positive cooperation may exist between the 2 receptors. The overall conclusion of that 4-wk experiment was that the activated ERβ is not efficient in reducing bone resorption.20 In the present experiments, ovariectomy of wildtype mice similarly led to rapid loss of both trabecular and cortical bone mineral density, whereas osteoporosis was attenuated during the first few months after surgery in ovariectomized BERKO mice. However after prolonged estrogen withdrawal, the differences between wildtype and BERKO mice became negligible. These results indicate that initially after estrogen withdrawal, the presence of ERβ enhances the development of osteoporosis. For unknown reasons, the absence of ERβ in the face of prolonged estrogen withdrawal did not affect bone mineral density and metaphyseal structures compared with those in ovariectomized wildtype mice.
The delayed development of maximal osteoporosis in ovariectomized BERKO mice parallels their delayed development of obesity and increase in the paratibial fat depot. In rats, this paratibial fat depot is a highly sensitive marker for estrogenic actions,28 and this appears to be the case also in wildtype and BERKO mice. Therefore, during the first 2 to 4 mo after ovariectomy, mechanisms active in the bones and fat tissue of BERKO mice delay the development of obesity and osteoporosis to the same degree as in ovariectomized wildtype mice. This mechanism, however, seems to disappear at later time points after estrogen withdrawal. Clearly more research is necessary to resolve the molecular mechanisms of this age dependent phenomenon.
In ovariectomized mice, trabecular area (that is, the total surface of trabeculae within the endosteal area at the level of the metaphysis of the tibia) increased slowly in size, similar to effects seen in rats.27 However, cortical area increases in rats after ovariectomy, whereas cortical area in ovariectomized wildtype and BERKO mice was significantly smaller than that in sham-ovariectomized mice. This decrease in cortical area was less pronounced in ovariectomized BERKO than wildtype mice and was significant 2, 7, and 9 mo after ovariectomy. The basic mechanisms underlying this phenomenon need to be explored in the future.
As reported previously,19 neither trabecular nor cortical mineral density nor trabecular nor cortical area differed between ovariectomized wildtype and BERKO mice 4 wk after ovariectomy. However, at 2, 3, and 5 mo after surgery, ovariectomized BERKO mice had lost significantly less trabecular mineral than did ovariectomized wildtype mice. In contrast, trabecular area began to increase after 5 mo but did not differ between these groups at this or any later time point.
At 10 and 12 mo of age (that is, 7 and 9 mo after ovariectomy), both groups of ovariectomized mice had significantly larger endosteal area than did their respective controls. This is a well-known effect in mammals and is believed to compensate for the loss of trabecular structures.11,29 These data indicate that, compared with wildtype mice, ovariectomized BERKO mice are less prone to develop osteoporosis during the first few months after ovariectomy. Therefore, the presence of ERβ in ovariectomized wildtype mice initially causes greater osteoporosis and obesity in the absence of estrogens, confirming the idea that ERβ initially inhibits the activity of ERα. However, because this effect vanishes after several months, treatment with specific ERβ agonists may not have favorable effects in bone and fat.
Acknowledgments
This work was in part funded by the EU Network of Excellence CASCADE (Food-CT-2004-506319). We thank Professor JA Gustafsson (Karolinska Institute, Stockholm, Sweden) for the gift of several pairs of heterozygous ERβ± mice for use as breeding stock.
The authors have no conflict of interest that could be perceived as prejudicing the impartiality of research reported.
References
- 1.Ankrom MA, Patterson JA, d'Avis PY, Vetter UK, Blackman MR, Sponseller PD, Tayback M, Robey PG, Shapiro JR, Fedarko NS. 1998. Age-related changes in human oestrogen receptor α function and levels in osteoblasts. Biochem J 333:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arts J, Kuiper GG, Janssen JM, Gustafsson JA, Lowik CW, Pols HA, van Leeuwen JP. 1997. Differential expression of estrogen receptors α and β mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology 138:5067–5070 [DOI] [PubMed] [Google Scholar]
- 3.Bodine PV, Henderson RA, Green J, Aronow M, Owen T, Stein GS, Lian JB, Komm BS. 1998. Estrogen receptor α is developmentally regulated during osteoblast differentiation and contributes to selective responsiveness of gene expression. Endocrinology 139:2048–2057 [DOI] [PubMed] [Google Scholar]
- 4.Christoffel J, Rimoldi G, Wuttke W. 2006. Effects of 8-prenylnaringenin on the hypothalamo–pituitary–uterine axis in rats after 3-month treatment. J Endocrinol 188:397–405 [DOI] [PubMed] [Google Scholar]
- 5.Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS. 1997. Tissue distribution and quantitative analysis of estrogen receptor α (ERα) and estrogen receptor β (ERβ) mRNA in the wild-type and ERα-knockout mouse. Endocrinology 138:4613–4621 [DOI] [PubMed] [Google Scholar]
- 6.Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW. 2002. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405 [DOI] [PubMed] [Google Scholar]
- 7.Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. 2004. Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 286:C655–C661 [DOI] [PubMed] [Google Scholar]
- 8.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr 2003. Evaluation of an estrogen receptor β agonist in animal models of human disease. Endocrinology 144:4241–4249 [DOI] [PubMed] [Google Scholar]
- 9.Harris HA, Katzenellenbogen JA, Katzenellenbogen BS. 2002. Characterization of the biological roles of the estrogen receptors, ERα and ERβ, in estrogen target tissues in vivo through the use of an ERα-selective ligand. Endocrinology 143:4172–4177 [DOI] [PubMed] [Google Scholar]
- 10.Helterbrand JD, Higgs RE, Jr, Iversen PW, Tysarczyk-Niemeyer G, Sato M. 1997. Application of automatic image segmentation to tibiae and vertebrae from ovariectomized rats. Bone 21:401–409 [DOI] [PubMed] [Google Scholar]
- 11.Higdon K, Scott A, Tucci M, Benghuzzi H, Tsao A, Puckett A, Cason Z, Hughes J. 2001. The use of estrogen, DHEA, and diosgenin in a sustained delivery setting as a novel treatment approach for osteoporosis in the ovariectomized adult rat model. Biomed Sci Instrum 37:281–286 [PubMed] [Google Scholar]
- 12.Hillisch A, Peters O, Kosemund D, Muller G, Walter A, Schneider B, Reddersen G, Elger W, Fritzemeier KH. 2004. Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol Endocrinol 18:1599–1609 [DOI] [PubMed] [Google Scholar]
- 13.Joyner JM, Hutley LJ, Cameron DP. 2001. Estrogen receptors in human preadipocytes. Endocrine 15:225–230 [DOI] [PubMed] [Google Scholar]
- 14.Ke HZ. 2005. In vivo characterization of skeletal phenotype of genetically modified mice. J Bone Miner Metab 23 Suppl:84–89 [DOI] [PubMed] [Google Scholar]
- 15.Ke HZ, Brown TA, Qi H, Crawford DT, Simmons HA, Petersen DN, Allen MR, McNeish JD, Thompson DD. 2002. The role of estrogen receptor β in the early age-related bone gain and later age-related bone loss in female mice. J Musculoskelet Neuronal Interact 2:479–488 [PubMed] [Google Scholar]
- 16. Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP. 1996. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51:159 –186. [PubMed]
- 17.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. 1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson JA, Ohlsson C. 2001. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol 171:229–236 [DOI] [PubMed] [Google Scholar]
- 20.Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O, Erlandsson M, Windahl S, Andersson G, Lubahn DB, Carlsten H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. 2002. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol 174:167–178 [DOI] [PubMed] [Google Scholar]
- 21.Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. 2002. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) α or β. Endocrinology 143:2349–2356 [DOI] [PubMed] [Google Scholar]
- 22.Onoe Y, Miyaura C, Ohta H, Nozawa S, Suda T. 1997. Expression of estrogen receptor β in rat bone. Endocrinology 138:4509–4512 [DOI] [PubMed] [Google Scholar]
- 23.Ornoy A, Giron S, Aner R, Goldstein M, Boyan BD, Schwartz Z. 1994. Gender-dependent effects of testosterone and 17β-estradiol on bone growth and modelling in young mice. Bone Miner 24:43–58 [DOI] [PubMed] [Google Scholar]
- 24.Parikka V, Peng Z, Hentunen T, Risteli J, Elo T, Vaananen HK, Harkonen P. 2005. Estrogen responsiveness of bone formation in vitro and altered bone phenotype in aged estrogen-receptor-α-deficient male and female mice. Eur J Endocrinol 152:301–314 [DOI] [PubMed] [Google Scholar]
- 25.Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. 2001. Demonstration of estrogen receptor subtypes α and β in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol 182:27–37 [DOI] [PubMed] [Google Scholar]
- 26.Rachon D, Vortherms T, Seidlova-Wuttke D, Wuttke W. 2008. Effects of black cohosh extract on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague–Dawley rats. Maturitas 60:209–215 [DOI] [PubMed] [Google Scholar]
- 27.Seidlova-Wuttke D, Hesse O, Jarry H, Christoffel V, Spengler B, Becker T, Wuttke W. 2003. Evidence for selective estrogen receptor modulator activity in a black cohosh (Cimicifuga racemosa) extract: comparison with estradiol 17β. Eur J Endocrinol 149:351–362 [DOI] [PubMed] [Google Scholar]
- 28.Seidlova-Wuttke D, Jarry H, Becker T, Christoffel V, Wuttke W. 2003. Pharmacology of Cimicifuga racemosa extract BNO 1055 in rats: bone, fat, and uterus. Maturitas 44 Suppl 1:S39–S50 [DOI] [PubMed] [Google Scholar]
- 29.Sevil F, Kara ME. 2010. The effects of ovariectomy on bone mineral density, geometrical, and biomechanical characteristics in the rabbit femur. Vet Comp Orthop Traumatol 23:31–36 [DOI] [PubMed] [Google Scholar]
- 30.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331:1056–1061 [DOI] [PubMed] [Google Scholar]
- 31.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. 2000. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA 97:5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. 2000. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc Natl Acad Sci USA 97:5936–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. 1999. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ(−/−) mice. J Clin Invest 104:895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA. 1985. Skeletal alterations in ovariectomized rats. Calcif Tissue Int 37:324–328 [DOI] [PubMed] [Google Scholar]