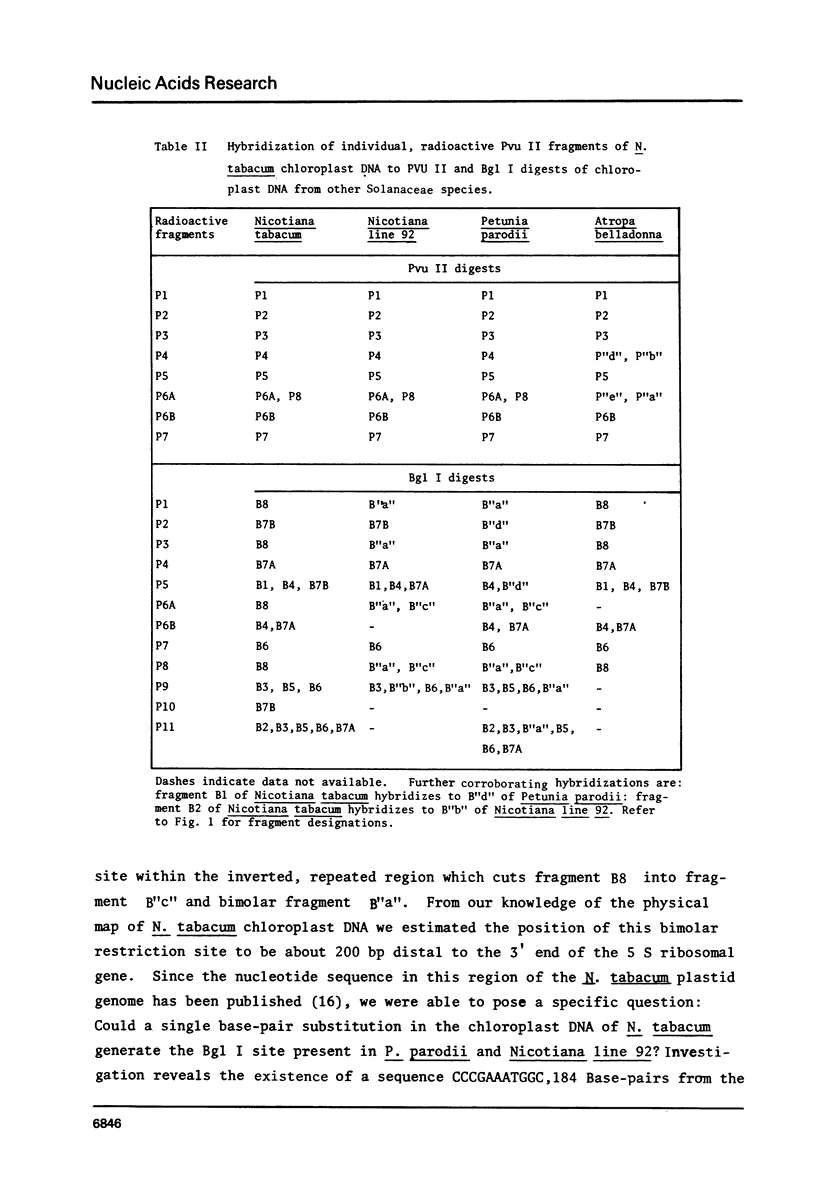
Abstract
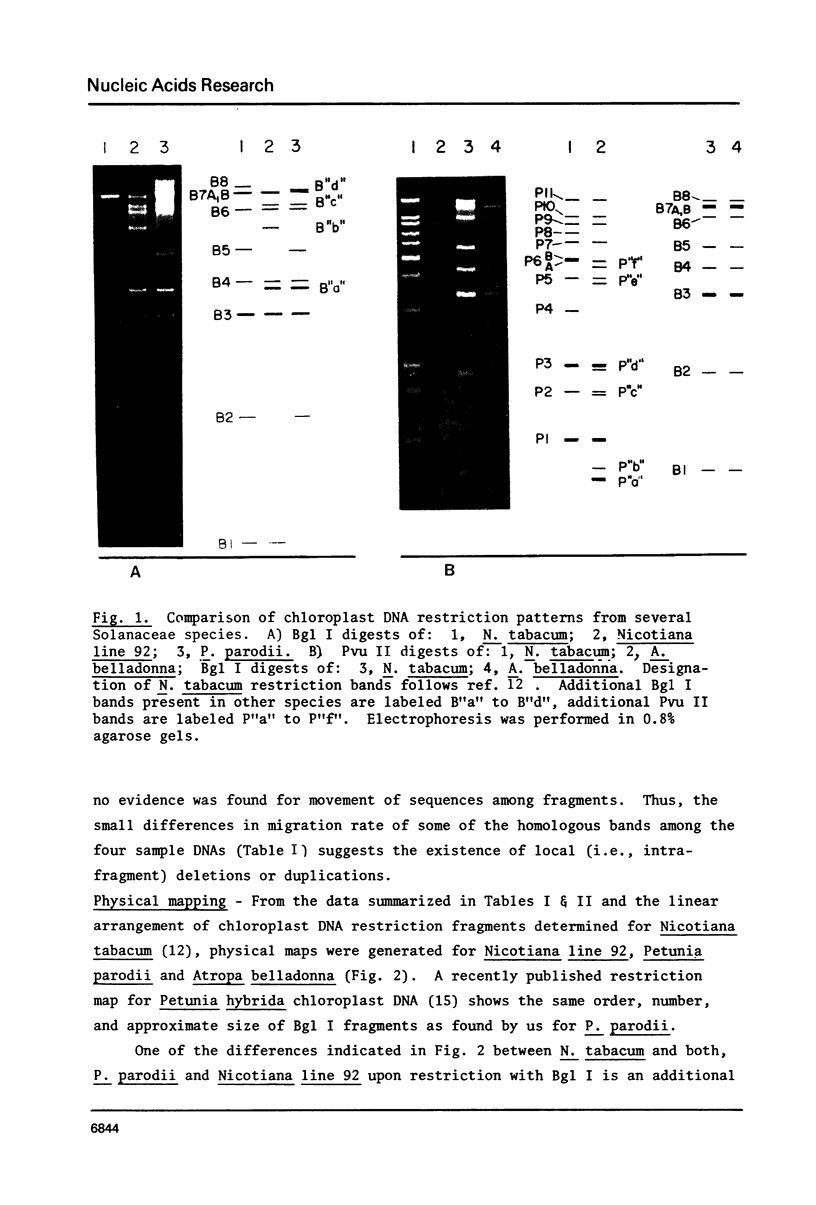
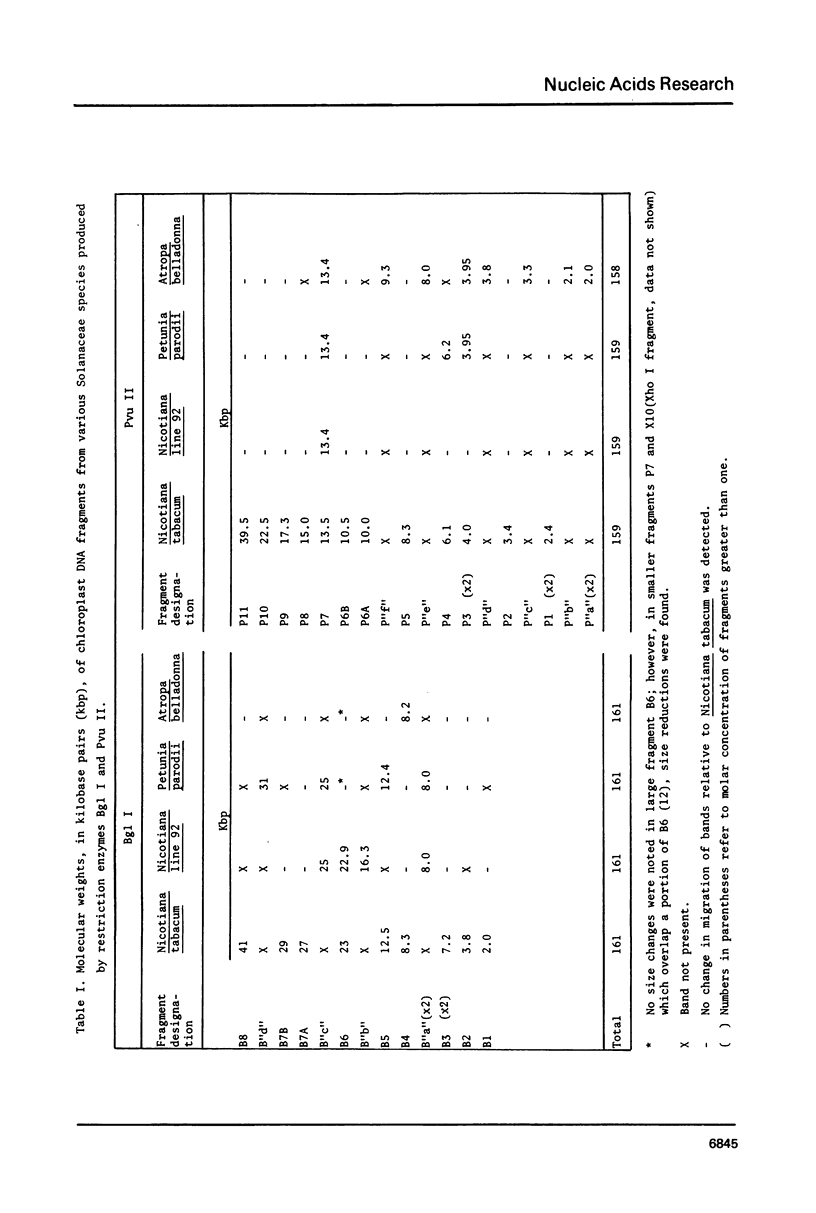
Isolated, nick-translated Pvu II fragments of Nicotiana tabacum chloroplast DNA produce specific intra- and intergeneric hybridization signals with chloroplast DNA digests from several representatives of the Solanaceae. These data, along with similarities in restriction enzyme patterns, permit construction of physical maps for Nicotiana line 92 (a cytoplasmic substitution line), Atropa belladonna and Petunia parodii. Plastid-DNA map differences among the Solanaceae are shown to result from single base-pair substitutions as well as local deletions or insertions. Several of these differences of Nicotiana tabacum chloroplast DNA fragments to a chloroplast DNA digest of Spinacia oleracea defines a sequential arrangement of fragments for spinach DNA which is very similar to its published physical map. This is achieved although chloroplast-DNA restriction enzyme patterns from the two organisms are grossly dissimilar. Alignment differences which have been revealed involve the edges of the inverted repeat region where certain single copy stretches in tobacco have been duplicated in spinach.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T. A., Ineichen K. The DNA sequence recognised by BglI. Gene. 1980 May;9(3-4):205–212. doi: 10.1016/0378-1119(90)90323-j. [DOI] [PubMed] [Google Scholar]
- Bohnert H. J., Driesel A. J., Crouse E. J., Gordon K., Herrmann R. G., Steinmetz A., Mubumbila M., Keller M., Burkard G., Weil J. H. Presence of a transfer RNA gene in the spacer sequence between the 16 S and 23 S rRNA genes of spinach chloroplast DNA. FEBS Lett. 1979 Jul 1;103(1):52–56. doi: 10.1016/0014-5793(79)81248-x. [DOI] [PubMed] [Google Scholar]
- Bovenberg W. A., Kool A. J., Nijkamp H. J. Isolation, characterization and restriction endonuclease mapping of the Petunia hybrida chloroplast DNA. Nucleic Acids Res. 1981 Feb 11;9(3):503–517. doi: 10.1093/nar/9.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R. G., Whitfeld P. R., Bottomley W. Construction of a SalI/PstI restriction map of spinach chloroplast DNA using low-gelling-temperature-agarose electrophoresis. Gene. 1980 Jan;8(2):179–191. doi: 10.1016/0378-1119(80)90036-0. [DOI] [PubMed] [Google Scholar]
- Jurgenson J. E., Bourque D. P. Mapping of rRNA genes in an inverted repeat in Nicotiana tabacum chloroplast DNA. Nucleic Acids Res. 1980 Aug 25;8(16):3505–3516. doi: 10.1093/nar/8.16.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Edwards K., Kössel H. Sequencing of the 16S-23S spacer in a ribosomal RNA operon of Zea mays chloroplast DNA reveals two split tRNA genes. Cell. 1981 Jul;25(1):203–213. doi: 10.1016/0092-8674(81)90245-2. [DOI] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. Inverted repeats in chloroplast DNA from higher plants. Proc Natl Acad Sci U S A. 1979 Jan;76(1):41–45. doi: 10.1073/pnas.76.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubumbila M., Burkard G., Keller M., Steinmetz A., Crouse E., Weil J. H. Hybridization of bean, spinach, maize and Euglena chloroplast transfer RNAs with homologous and heterologous chloroplast DNAs. An approach to the study of homology between chloroplast tRNAs from various species. Biochim Biophys Acta. 1980 Aug 26;609(1):31–39. doi: 10.1016/0005-2787(80)90198-7. [DOI] [PubMed] [Google Scholar]
- Ramirez J. L., Dawid I. B. Mapping of mitochondrial DNA in Xenopus laevis and X. borealis: the positions of ribosomal genes and D-loops. J Mol Biol. 1978 Feb 15;119(1):133–146. doi: 10.1016/0022-2836(78)90273-5. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D. Restriction endonuclease map of the chloroplast DNA of Chlamydomonas reinhardii. J Mol Biol. 1978 Dec 25;126(4):597–617. doi: 10.1016/0022-2836(78)90011-6. [DOI] [PubMed] [Google Scholar]
- Thompson B. J., Escarmis C., Parker B., Slater W. C., Doniger J., Tessman I., Warner R. C. Figure-8 configuration of dimers of S13 and phiX174 replicative form DNA. J Mol Biol. 1975 Feb 5;91(4):409–419. doi: 10.1016/0022-2836(75)90269-7. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakour R. A., Bultmann H. Evoluation of Drosophila mitochondrial DNAs. Analysis of heteroduplex molecules. Biochim Biophys Acta. 1979 Sep 27;564(2):342–351. doi: 10.1016/0005-2787(79)90231-4. [DOI] [PubMed] [Google Scholar]