Abstract
Background
Animal-derived elicitors can be used by plants to detect herbivory but they function only in specific insect–plant interactions. How can plants generally perceive damage caused by herbivores? Damaged-self recognition occurs when plants perceive molecular signals of damage: degraded plant molecules or molecules localized outside their original compartment.
Methodology/Principal Findings
Flame wounding or applying leaf extract or solutions of sucrose or ATP to slightly wounded lima bean (Phaseolus lunatus) leaves induced the secretion of extrafloral nectar, an indirect defense mechanism. Chemically related molecules that would not be released in high concentrations from damaged plant cells (glucose, fructose, salt, and sorbitol) did not elicit a detectable response, excluding osmotic shock as an alternative explanation. Treatments inducing extrafloral nectar secretion also enhanced endogenous concentrations of the defense hormone jasmonic acid (JA). Endogenous JA was also induced by mechanically damaging leaves of lima bean, Arabidopsis, maize, strawberry, sesame and tomato. In lima bean, tomato and sesame, the application of leaf extract further increased endogenous JA content, indicating that damaged-self recognition is taxonomically widely distributed. Transcriptomic patterns obtained with untargeted 454 pyrosequencing of lima bean in response to flame wounding or the application of leaf extract or JA were highly similar to each other, but differed from the response to mere mechanical damage. We conclude that the amount or concentration of damaged-self signals can quantitatively determine the intensity of the wound response and that the full damaged-self response requires the disruption of many cells.
Conclusions/Significance
Numerous compounds function as JA-inducing elicitors in different plant species. Most of them are, contain, or release, plant-derived molecular motifs. Damaged-self recognition represents a taxonomically widespread mechanism that contributes to the perception of herbivore feeding by plants. This strategy is independent of insect-derived elicitors and, therefore, allows plants to maintain evolutionary control over their interaction with herbivores.
Introduction
How can plants recognize damage that is inflicted by herbivores? The discovery of the ‘wound response’ of tomato [1] initiated a series of studies to determine how local damage is perceived and translated into mobile signals that regulate a systemic resistance response [2], [3], [4]. Fragments of plant-derived molecules were among the first described defense elicitors and include peptides such as systemin [5] and cell wall-derived pectins, oligogalacturonides and oligosaccharides [6], [7], [8]. The early research therefore indicated that the plant itself contains the molecules that are required for defense induction. Concurrent research by several different research groups focused on the feeding insects and discovered specific herbivore-associated molecular patterns (HAMPs) [4], [9], [10], [11], including, for example, volicitin and other fatty acid–amino acid conjugates from the regurgitate of feeding caterpillars, bruchins and caeliferins [12], [13], [14], and plant-derived protein fragments that are formed specifically during insect feeding [15], [16], [17]. HAMPs boost resistance when applied to wounded plant tissues and usually elicit responses that differ from the response to mechanical damage.
However, assuming that plants use exclusively HAMPs to perceive that they are damaged by herbivores causes several conceptual problems. First, and most intriguingly, HAMPs are active only in certain insect–plant interactions [11], [18]. How do plants respond to the feeding damage caused by the majority of herbivores, including mammals? Second, it is unclear whether insect regurgitate is applied to plant tissue during the normal feeding process [19]. Finally, if a resistance induction is based only on the specific recognition of certain HAMPs, the herbivores, in principle, could change the molecular structure of their specific HAMPs and thereby avoid perception, or simply avoid applying their HAMPs to the wounded plant tissue [19]. By contrast, ‘damaged-self recognition’ would allow the plants to mount a general response to herbivory and retain evolutionary control over this vital process [20]. Recognition of the damaged self could be based principally on intact plant molecules localized outside their usual cell compartment or on fragments of plant molecules [20].
We investigated whether lima bean (Phaseolus lunatus L.) responds to chemical motifs that are indicative of the damaged self and whether the recognition of these signals triggers jasmonate signaling. Jasmonic acid (JA) plays a central role in activating the systemic defense after herbivory and in other vital processes, including flower development and senescence [3], and in floral nectar secretion [21]. One JA-dependent defense response is the secretion of extrafloral nectar (EFN), which attracts ants and other predatory insects as a means of indirect defense [22], [23]. We quantified EFN secretion in response to treatments that were likely to elicit damaged-self recognition and in response to chemically similar compounds that are not released from disintegrated plant cells. We also monitored endogenous JA synthesis and used pyrosequencing to compare the transcriptomic patterns induced in response to damaged-self signals with the response to JA. Damaged-self signals induced the synthesis of JA and the overall transcriptomic patterns induced by damaged-self signals in lima bean were similar to those induced by the hormone itself.
Results and Discussion
Induction of an indirect defense
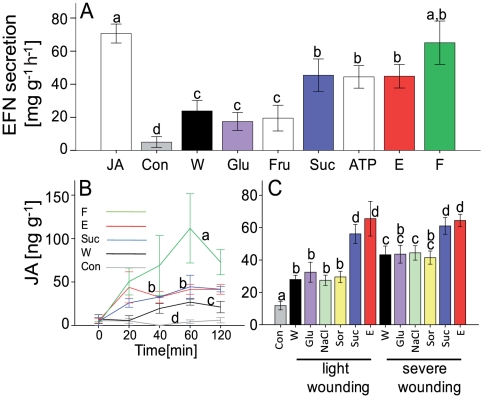
Wild-type plants of P. lunatus were subjected to flame wounding, or to mechanical wounding with subsequent application of either water or 1 mM aqueous solutions of glucose, fructose, sucrose, ATP, or JA (positive control). EFN secretion quantified 2 h after treatment differed significantly among treatments (univariate ANOVA: F = 8.340, P<0.001, n = 7). Wounding alone increased EFN secretion over control levels but the response was significantly stronger after application of ATP, sucrose, leaf extract, or flame wounding. Glucose and fructose caused no further increase as compared to wounding alone (Fig. 1A). Sucrose is the major photosynthetic product and the dominant sugar in the phloem [24] and its extracellular concentration will inevitably increase upon tissue disruption, whereas neither glucose nor fructose is an abundant sugar in the leaf tissue. A sudden increase in the extracellular concentration of sucrose, but less so of the monosaccharides, would therefore appear to be a suitable indicator of severe tissue disruption. ATP is released from cells by secretion or by wounding and it has been suggested that ATP plays a role in plant defense signaling [25]. Flame wounding physically destroys entire cells, releasing their contents into the extracellular space. Flame wounding as applied in our study can induce proteinase inhibitor genes, which are classical markers of wound-induced genes whose expression depends on JA signaling [26], [27], and has been related to Ca2+ signaling [28], which is a well-known early step in the perception of herbivore-feeding by plants [29], [30]. Finally, leaf extract contains all the molecules that are released when cells become disrupted. Patterns in EFN secretion thus support the prediction that common plant molecules or their fragments are monitored in the extracellular space for damaged-self recognition in lima bean.
Figure 1. Damaged-self recognition in lima bean.
(A) EFN secretion [mg soluble solids per gram leaf fresh mass and hour], (B) time course in endogenous jasmonic acid (JA) levels [ng per gram leaf fresh mass], (C) endogenous JA level 60 min after different treatments. Identical colors in different panels indicate identical treatments, values shown are means ± SE (of n = 7 biological replicates in panel A and n = 5 in panels B and C). Bars or lines marked with different letters indicate treatments that were significantly different (P<0.05) according to posthoc analysis with least significant difference tests. Abbreviations: Con = control, E = leaf extract, EFN = extrafloral nectar, F = flame wounding, Fru = fructose, Glu = glucose, Sor = sorbitol, Suc = sucrose, W = mechanical wounding.
Induction of endogenous JA
We applied the above treatments and quantified endogenous JA using gas chromatography coupled with single-ion mass spectrometry (GC-SIM-MS). Wounding induced endogenous JA, but leaf extract, sucrose and flame wounding had significantly greater effects (Fig. 1B, Table 1). The effect of wounding alone was dependent on the severity of the damage, and glucose, sorbitol or salt (NaCl) at 1 mM had no significant effect on the induction of endogenous JA compared with the levels of JA induced by wounding alone (Fig. 1C), which excludes osmotic shock as the reason for the JA response. Similar effects were observed in other plant species. Mechanical wounding of leaves of Arabidopsis thaliana (Brassicaceae), tomato (Solanum lycopersicum, Solanaceae), strawberry (Fragaria×ananassa, Rosaceae), sesame (Sesamum indicum, Pedaliaceae) and maize (Zea mays, Poaceae) caused a significant induction of endogenous JA in all species compared with the level of endogenous JA in intact leaves (Fig. 2, Table 1). The application of sucrose induced the greatest increase in endogenous JA levels in maize, whereas in sesame and tomato the application of leaf extract induced a greater increase in JA concentration than that induced by mechanical damage (Fig. 2), confirming earlier reports of an induction of JA-dependent plant defenses after the application of leaf extract [1], [31], [32], [33]. Therefore, both the amount of damage (Fig. 1C) and the species (Fig. 2) can affect whether any of the damaged-self signals boost the JA response above the levels that are seen after mere mechanical damage. We conclude that all the plants investigated here can respond to mechanical damage to some degree, whereas the specific signals perceived and the degree of the response varies among species. Damaged-self recognition represents a taxonomically common mechanism that is subject to physiological and evolutionary flexibility.
Table 1. Results of ANOVA for levels of endogenous JA.
| Species | Factor | df | F value | P value |
| Lima bean | Treatment | 4 | 13.730 | <0.001 |
| Time | 4 | 7.494 | <0.001 | |
| Treatment×time | 16 | 1.632 | 0.096 | |
| Sesame | Treatment | 3 | 7.289 | <0.001 |
| Time | 4 | 11.087 | <0.001 | |
| Treatment×time | 12 | 1.745 | 0.073 | |
| Tomato | Treatment | 4 | 15.188 | <0.001 |
| Time | 3 | 25.347 | <0.001 | |
| Treatment×time | 12 | 7.296 | <0.001 | |
| Arabidopsis | Treatment | 4 | 21.302 | <0.001 |
| Time | 3 | 18.077 | <0.001 | |
| Treatment×time | 12 | 5.777 | <0.001 | |
| Strawberry | Treatment | 4 | 8.661 | <0.001 |
| Time | 3 | 10.652 | <0.001 | |
| Treatment×time | 12 | 2.822 | 0.003 | |
| Maize | Treatment | 3 | 51.031 | <0.001 |
| Time | 4 | 31.361 | <0.001 | |
| Treatment×time | 12 | 8.418 | <0.001 |
ANOVA was conducted separately for every species on the effects of treatment and time as fixed factors on endogenous levels of JA.
Figure 2. Endogenous levels of jasmonic acid (JA).
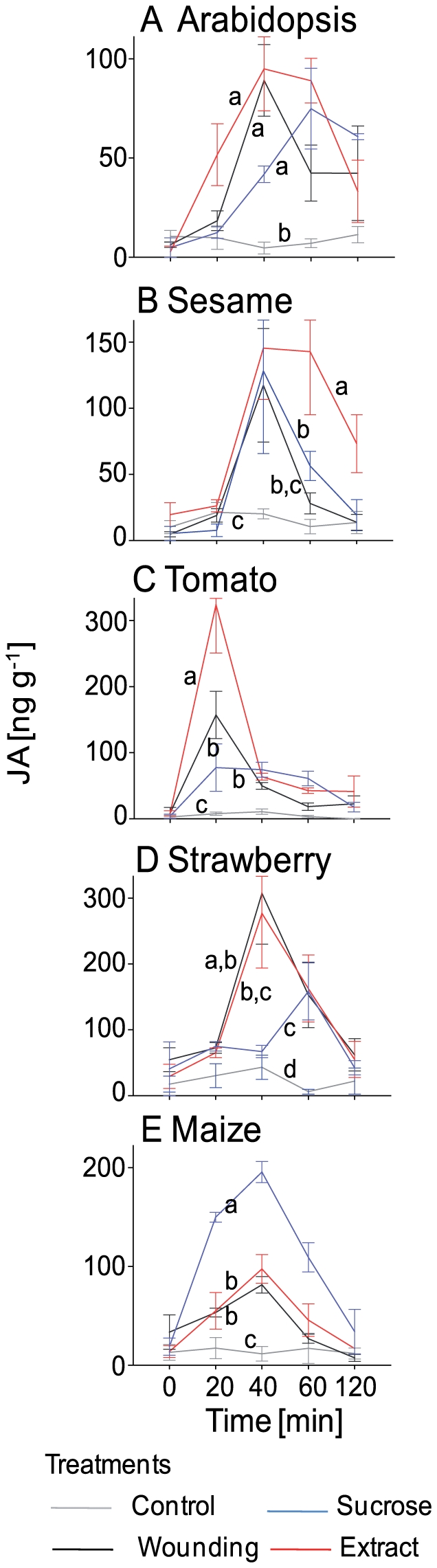
Endogenous JA levels were quantified (ng per gram leaf fresh mass) at different times after the treatment in (A) Arabidopsis, (B) sesame, (C) tomato, (D) strawberry, and (E) maize. Lines marked with different letters indicate statistically significant differences among treatments (least significant difference post hoc tests, n = 5 biological replicates per mean).
Transcriptional changes after damaged-self recognition
The application of leaf extract or sucrose induced a greater increase in endogenous JA than that induced by mere wounding in lima bean, sesame, tomato and maize, and mere wounding increased endogenous JA over control levels in all species investigated here (Figs. 1 and 2). In lima bean, an increase in the severity of the wounding led to an increase in the level of endogenous JA that was dependent on the severity of the damage, whereas the application of leaf extract or sucrose elicited a strong JA response that was independent of the damage level applied before (Fig. 1C). Finally, several different damaged-self signals were able to induce EFN secretion by lima bean (Fig. 1A). All these observations appear contradictory to reports that HAMPs are required for a full resistance response [5], [9], [10], [12], [14], [15], [16], [18], [33], [34], [35]. To resolve this apparent contradiction, we must consider the quantitative aspect: studies aimed at elucidating the particular role of damaged-self recognition in the overall response to herbivore feeding could compare endogenous JA concentrations after different damage levels (with and without the application of leaf extract) to the levels observed after damage caused by different types of herbivores, and they should consider the effects downstream of JA. Our preliminary results indicate that the relative importance of damaged-self recognition is likely to vary among species.
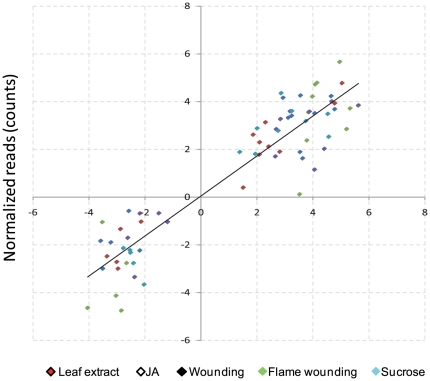
How similar are transcription patterns after damaged-self recognition to the full set of JA-dependent transcriptional responses in lima bean leaves? We used 454 pyrosequencing [36] of cDNA libraries to investigate transcriptomic changes (in relation to the transcriptome of untreated control plants) after JA treatment, flame wounding, mechanical wounding, and mechanical wounding with subsequent application of leaf extract or sucrose. We extracted mRNA, produced cDNA, tagged the resulting libraries and then subjected them to two sequencing runs [37]. During 454 sequencing, the number of sequencing reads representing the same gene can be taken as a measure of transcript abundance (see Text S1).
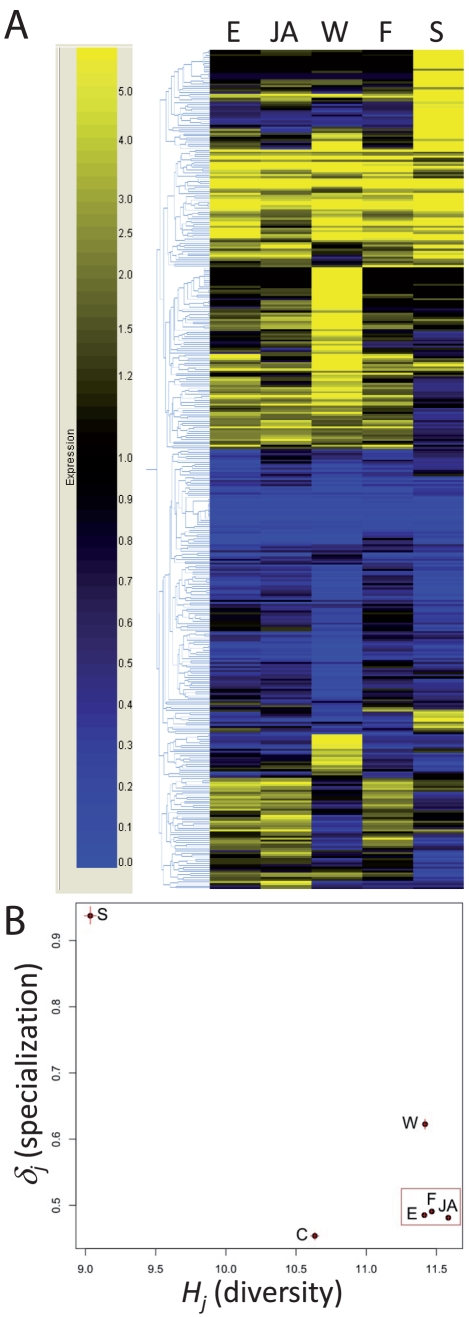
The overall patterns of up- and down-regulated genes (see Table S1 for the list of contigs, accession numbers, annotation results and expression levels of differentially expressed genes) as compared to the control were surprisingly similar among lima bean leaves that had been treated with JA and leaves that had been subjected to fire or to mechanical wounding with subsequent application of leaf extract (Fig. 3). By contrast, mechanical wounding alone and mechanical wounding with subsequent application of sucrose solution caused clearly distinct patterns (Fig. 3).
Figure 3. Transcriptomic response of lima bean leaves to damaged-self signals and jasmonic acid (JA).
(A) Transcriptomic patterns (genes up- and downregulated in comparison to untreated controls) are presented for JA treatment (JA) in comparison to flame wounding (F), mechanical wounding (W), and mechanical wounding with subsequent application of leaf extract (E) or 1 mM solution of sucrose (S). (B) Scatter plot of Hj (diversity) versus δj (specialization) for each treatment tested.
Leaf extract application and particularly flame wounding elicited very similar transcriptomic patterns to those elicited by JA (Fig. 3A). Forty-three genes were induced and 71 were repressed by all three treatments. The transcriptome elicited by JA overlapped with a further 48 genes (29 up, 19 down) in the wounding-induced transcriptome and with 36 genes (33 up, 3 down) in the extract-induced transcriptome (Table 2). According to a Gene Ontology classification using the BioMaps tool from the VirtualPlant webpage (http://virtualplant.bio.nyu.edu/cgi-bin/vpweb/) [38] most of the upregulated genes represent categories such as defense and virulence, interaction with the environment and responses to wounding or stress (Table S2). Several of the highly upregulated genes (by factors >10) are involved in JA synthesis (e.g. LOX, lipoxygenase; AOS, allene oxide synthase; and AOC, allene oxide cyclase) [3] or in the regulation of JA-isoleucine-responsive genes downstream of JA (JAZ1, jasmonate ZIM-domain) [39], [40], [41]. Genes related to photosynthesis or primary metabolism appeared downregulated, which is in line with the repressing effect of JA on photosynthesis and other growth-related processes [42]. Only a few of the induced genes were related to osmotic or salt stress (Table S3), which further supports our view that our treatments caused a specific response rather than general osmotic stress.
Table 2. Differentially expressed genes in response to wounding, JA and leaf extract.
| Genes | Significant response to | |||
| E | JA | W | ||
| Upregulated | 43 | + | + | + |
| 33 | + | + | 0 | |
| 29 | + | 0 | + | |
| 14 | 0 | + | + | |
| 63 | 0 | 0 | + | |
| 7 | + | 0 | 0 | |
| 26 | 0 | + | 0 | |
| Downregulated | 71 | − | − | − |
| 3 | − | − | 0 | |
| 19 | − | 0 | − | |
| 13 | 0 | − | − | |
| 33 | 0 | 0 | − | |
| 5 | − | 0 | 0 | |
| 5 | 0 | − | 0 | |
Numbers of genes are indicated that responded with a significant up (+) or down (−) regulation to at least one of the treatments. Genes were regarded as differentially expressed when their expression was ≥2.0 or ≤0.5 as compared to the untreated control. E = extract, JA = jasmonic acid, W = mechanical wounding.
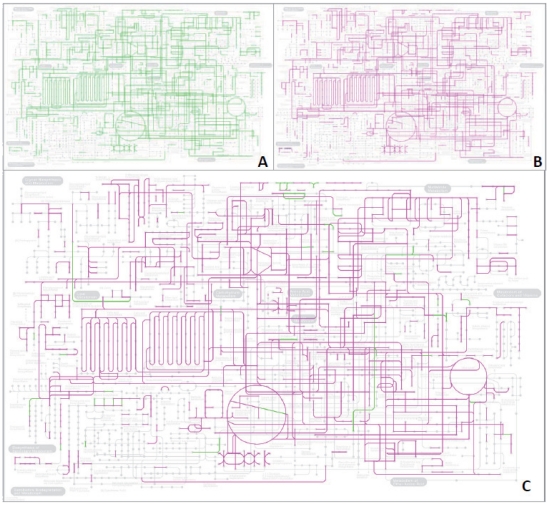
Because the metabolic pathways that we obtained from the P. lunatus unigene set yielded an almost complete coverage of a global metabolic map according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) classification (Fig. 4), we are confident that our transcriptome is sufficiently complete as to allow conclusions concerning global changes in gene expression. To quantify the similarities among these transcriptomes, we compared their diversity (Hj) and specialization (δj), defining Hj as the Shannon entropy of the frequency distribution of a transcriptome and δj as the average specificity of the genes expressed under each condition [43]. Plotting the transcriptomes in a two-dimensional space defined by Hj and δj as well as calculating Euclidean distances among the distributions of the transcriptomes confirmed that the transcriptomes obtained after JA treatment, leaf extract application and flame wounding clustered closely together (Fig. 3B, Table 3). Apparently, the global changes in gene expression after the application of leaf extract after mechanical wounding or after damaging the tissue via flame wounding were mediated via jasmonate signaling.
Figure 4. Metabolic pathways represented in the Phaseolus lunatus unigene set.
Global metabolism map constructed combining existing pathway maps and corresponding genes referenced in the KEGG database for Arabidopsis, Populus, Vitis, Oryza and Sorghum (green lines). (B) Global metabolism map represented by the P. lunatus unigene set (magenta lines). (C) Overlap comparison of the KEGG metabolic global map of flowering plants (Arabidopsis, Populus, Vitis, Oryza and Sorghum) with the metabolic map represented by the P. lunatus unigene set.
Table 3. Euclidean distances between lima bean leaf transcriptomes in a two-dimensional space defined by δj (specialization) and Hj (diversity).
| C | E | JA | W | F | |
| E | 0.0235 | ||||
| JA | 0.0342 | 0.0197 | |||
| W | 0.0524 | 0.0404 | 0.0284 | ||
| F | 0.0224 | 0.0151 | 0.0223 | 0.0452 | |
| S | 0.0954 | 0.0958 | 0.1047 | 0.1113 | 0.10127 |
C: control, E: leaf extract, JA: jasmonic acid, W: mechanical wounding, F: flame wounding, S: sucrose.
Mere mechanical wounding overall elicited similar transcriptomic patterns but was clearly distinguishable from the transcriptomic patterns elicited by JA, flame wounding, or leaf extract application after mechanical wounding. We observed a set of genes which were either induced only after wounding or that were repressed by wounding but induced by JA, or when wounding was caused by fire or complemented by the application of leaf extract (see lower parts in Fig. 3A). Several of the genes that were repressed by mechanical wounding alone, but not repressed by JA or flame wounding or when leaf extract was applied to the wounded leaves, appeared to be related to photosynthesis (Table S1). Genes that were induced at least fourfold by mechanical wounding, but not induced by leaf extract application to wounded leaves, or by JA application or flame wounding, appeared, among others, to have ATPase and other ATP binding functions (Table S1).
Mechanical damage as applied in our study is likely to release some damaged-self signals. However, the transcriptomic patterns elicited by our mechanical wounding treatment differed in some parts from the patterns that resulted when the entire contents of many cells were applied to the wounded tissue. Sucrose caused gene expression alterations that overlapped partly, but in general differed from the gene expression induced by the other treatments (Fig. 3). Although sucrose induced both EFN secretion and endogenous JA-levels in lima bean (Fig. 1), sucrose-signaling is also involved in multiple independent physiological responses [24], [44]. It therefore appears to be likely that an exogenous application of sucrose induced a mixed response whose components can be related to both damaged-self recognition and other physiological processes.
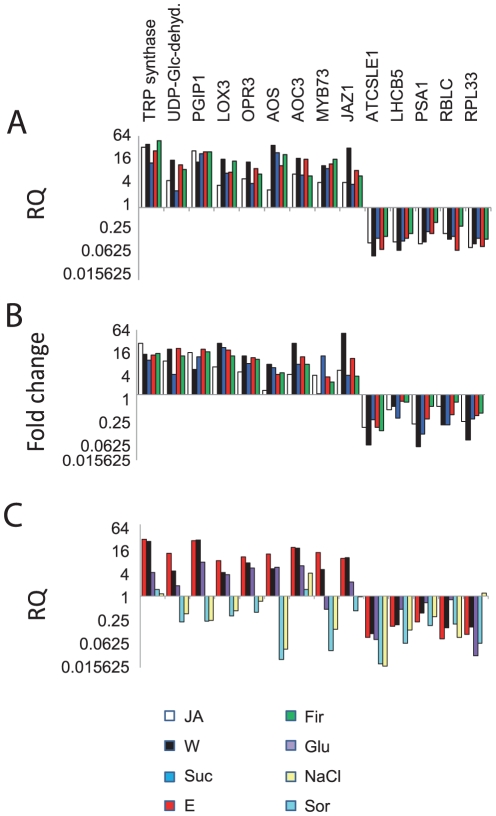
The expression patterns of 14 genes were confirmed with quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR, see Table S4 for primer sequences). We found a good correlation between expression levels obtained by 454 sequencing and those obtained by qRT-PCR (Figs. 5 and 6). Interestingly, leaf extract, sucrose and flame wounding treatments induced the expression of AOS, AOC, LOX, OPR and JAZ1 more strongly than JA itself (Fig. 5). At the phenotypic level, the same treatments induced high levels of endogenous JA in lima bean, with flame wounding eliciting the highest levels, a result that is fully congruent with the strong induction of EFN secretion that occurred following this treatment (Fig. 1). NaCl and sorbitol induced gene expression patterns that were the opposite of those induced by the damaged-self signals. The level of induction of most of the up-regulated genes was much lower following glucose application than for the other treatments, and MYB73, a transcription factor that was induced by all damaged-self signals, including sucrose, was repressed by glucose (Figure 5C). Although the signals that trigger the defense response represent common plant molecules (or changes in their extracellular concentration), the specificity that we observed in the transcriptomic changes appears to be high enough to allow a fine-tuning of the response.
Figure 5. Expression of functionally important genes.
Expression patterns of tryptophan synthase, UDP-glucose-6 dehydrogenase, PGIP1 (polygalacturonase-inhibiting protein 1), LOX3 (Lipoxygenase3), OPR3 (12-oxophytodienoate-reductase 3), AOS (allene oxide synthase), AOC3 (allene oxide cyclase 3), MYB73 (MYB domain protein 73, transcription factor), JAZ1 (jasmonate-ZIM-domain repressor protein 1), ATCSLE1 (cellulose synthase/transferase), LHCB5 (light harvesting complex of photosystem II subunit 5), PSA1 (photosystem I subunit I), RBCL (large subunit of Rubisco) and RPL33 (chloroplast ribosomal protein 33) are presented, obtained with qRT-PCR (A) and 454 sequencing (B). Panel (C) depicts qRT-PCR results in response to control treatments (glucose, NaCl and sorbitol). Bars in panel (A) and (C) present means of three independent PCR runs. See Table S1 for unigene identities, accession numbers, annotation results and expression levels, and Table S4 for primer sequences. Treatments applied were: E = leaf extract, F = flame wounding, Glu = glucose, JA = jasmonic acid, NaCl = sodium chloride, Sor = sorbitol, Suc = sucrose, W = mechanical wounding.
Figure 6. Correlation of expression levels as obtained from 454 sequencing with those obtained with qRT-PCR.
Conclusions
Plants require a general wound-recognition system that is independent of the detailed nature of the attacking herbivore and that cannot be controlled by the animal at the physiological or evolutionary level. Damaged-self recognition is based on the detection of plant-derived molecules and, thus, does fulfill these requirements.
Plants are likely to use both strategies, damaged-self recognition and HAMP-based specific responses, to optimize their response to herbivore feeding [4]. In our study, the application of leaf extract to slightly wounded lima bean leaves and flame wounding elicited almost the same gene transcriptional patterns as those elicited by JA. The similarities among gene expression patterns seen after the application of leaf extract and after flame wounding indicate that the systemic induction of proteinase-inhibitor genes after flame wounding [26], [27], [45], [46] is likely to be an effect of the cellular events that are common to both folivory and strong heat stress: the rapid release of the cellular content into the extracellular space and the disruption of plant macro-molecules. The transcriptomic patterns elicited by mechanical damage alone differed in some parts from the patterns that resulted when the entire contents of many cells were applied to the wounded tissue, indicating that damaged-self recognition allows specific responses without the need for insect-derived elicitors. Common molecules such as ATP and sucrose induced JA synthesis, whereas chemically similar molecules that are not released from damaged plant cells did not. When occurring at high concentrations in the extracellular space, sucrose, ATP and fragments of plant macromolecules can indicate the presence of disrupted cells.
Damaged-self recognition in plants shows surprising similarities to the role played by extracellular ATP in wound perception in the human skin [47]. Monitoring fragments of molecules, or molecules that are localized outside their normal compartment, could thus be a common principle by which organisms detect injuries. For plants, we suggest that damaged-self recognition, which we found in taxonomically unrelated species from both the monocots and the dicots, represents an evolutionarily ancient mechanism that allows a general response to herbivory without depending on animal-derived elicitors. Evolutionarily more derived mechanisms that monitor specific animal-derived molecules might then allow faster and more intensive responses to encounters with specialist enemies.
Materials and Methods
EFN secretion
Plants of P. lunatus growing at their natural site (México, Pacific coast, ∼15°55′N and 97°09′W) were assigned to groups of seven and subjected to the following treatments: control, flame wounding (placing the flame of a lighter at distance of 3 cm below the leaf tip for 2 sec, see refs. [26], [27], [45], [46]), mechanical damage (entire leaves were slightly wounded with a metal brush, causing approximately five punctures with a diameter of 0.1 mm per cm2, and then immediately sprayed with water) and mechanical damage followed immediately by the application of leaf extract (ca. 0.5 g fresh leaf material per ml water homogenized in a mortar, left to sediment and supernatant then used without further preparation) or of a 1 mM aqueous solution of glucose, fructose, sucrose, ATP, or JA. EFN secretion was quantified 2 h later using microcapillaries and a portable refractometer [23].
Endogenous JA levels
Plants of all species were cultivated in a greenhouse for six weeks and then subjected to the following treatments: control, mechanical damage, and mechanical damage followed by the application of leaf extract or sucrose (lima bean was also subjected to the flame wounding treatment). In a second experiment, lima beans leaves were subjected to two different levels of damage (ca. 2 and ca. 8 punctures per cm2) followed by the application of leaf extract or a 1 mM aqueous solution of sucrose, glucose, sorbitol or NaCl. 500 mg of leaf material was collected immediately before treatment and at 20, 40, 60 and 120 min after treatment (second experiment with lima bean: 60 min only), stored in liquid nitrogen and extracted with ethyl acetate [48]. After adding [9,10-H2] dihydrojasmonic acid as an internal standard, JA was derivatized by adding 10 µl pentafluorobenzyl bromide and resuspended in 100 µl methanol [49]. One microliter of the sample was injected in the splitless mode and analyzed by gas chromatography-single ion-mass spectrometry in an Agilent Technologies Gas Chromatograph 7890A using a DB-1MS column (60 m×0.25 mm×0.5 µm Agilent Technologies) coupled to a MSD 5973 detector in SIM mode for 141, 181, 390 and 392 m/z. Other gas chromatography and mass spectrometry conditions were as described previously [50].
Transcriptomic analyses
Leaves were treated (control, JA treatment, flame wounding, mechanical damage, and mechanical damage followed by application of leaf extract or sucrose) and collected 120 min later for mRNA extraction using Trizol® and Qiagen RNAeasy columns (Qiagen, México D.F.). Multiplex IDentifiers were used to tag cDNA libraries. Two FLX pyrosequencing runs were performed for all six conditions. A total of 955,244 reads were generated with an estimated average size of 217 bases representing a total of ≈207.52 Mbp. Sequences were assembled using Newbler (v1.1.03.24) software, producing a total of 44,653 singletons and 25,803 contigs. Every contig comprised on average 35 reads and had 396 bp. A P. lunatus unigene set was generated by combining 25,783 assembled contigs and 18,373 non-assembled reads (singlets), considering sequences with a minimum size of 100 bp. The unigene set was annotated by searching for sequence similarities using BLASTx against Arabidopsis thaliana (TAIR v9.0) gene models. The expression profiles from 454-sequencing were achieved by counting the number of sequencing reads per unigene, in this case represented by the number of BLASTn query 454 reads for each target unigene ID in each of the six samples (control, JA treatment, flame wounding, mechanical damage, and mechanical damage followed by application of leaf extract or sucrose). The frequency at which the sequence of a given gene is read in expressed sequencing tag projects should reflect the relative abundance of the corresponding mRNA [51], [52], [53]. See Text S1 for detailed methods.
Ethics statement
Because lima bean is a wild species and all the authors work in Mexican Institutions, no permits were required to realize the field experiments.
Supporting Information
Differentially expressed genes in the Phaseolus lunatus transcriptome. Complete list of those 406 non-redundant unigenes (contigs, accession numbers, annotation results and expression levels) that exhibited putative differential expression in at least one condition evaluated.
(XLS)
Over-represented functional categories in the Phaseolus lunatus transcriptome. The over-represented functional categories were defined according to the MIPS classification using BioMaps tool from the VirtualPlant webpage (URL: http://virtualplant.bio.nyu.edu/cgi-bin/vpweb/). Hypergeometric method and a Bonferroni correction were used for the analysis with a cutoff of 0.01.
(XLS)
Expression profiles of genes represented in the “osmotic and salt stress response” MIPS categories.
(XLS)
Primer sequences used for qRT-PCR.
(XLS)
Annotation of the Phaseolus lunatus ESTs. A detailed explanation of all bioinformatic steps taken during the analyses of the transcriptomic data presented in this study.
(DOC)
Acknowledgments
We thank Betty Jimenez and Berenice Cuevas-Torres for technical assistance, Jurriaan Ton, Jonathan Gershenzon and Wilhelm Boland for valuable comments on earlier versions of this manuscript and Caroline Woods for careful proofreading.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by grant no. 130656 from CONACyT (Consejo Nacional de Ciencia y Tecnología de México; www.conacyt.mx). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Green TR, Ryan CA. Wound-induced proteinase inhibitor in plant leaves: a possible defense mechanism against insects. Science. 1972;175:776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- 2.Heil M, Ton J. Long-distance signalling in plant defence. Trends in Plant Science. 2008;13:264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Wasternack C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany. 2007;100:681–697. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JQ, Baldwin IT. New insights into plant responses to the attack from insect herbivores. Annual Review of Genetics. 2010;44:1–24. doi: 10.1146/annurev-genet-102209-163500. [DOI] [PubMed] [Google Scholar]
- 5.Ryan CA, Pearce G. Systemins: a functionally defined family of peptide signals that regulate defensive genes in Solanaceae species. Proceedings of the National Academy of Sciences of the USA. 2003;100:14577–14580. doi: 10.1073/pnas.1934788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doares SH, Syrovets T, Weiler EW, Ryan CA. Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proceedings of the National Academy of Sciences of the USA. 1995;92:4095–4098. doi: 10.1073/pnas.92.10.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergey DR, Orozco-Cardenas M, de Moura DS, Ryan CA. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proceedings of the National Academy of Science USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creelman RA, Mullet JE. Oligosaccharins, brassinolides, and jasmonates: Nontraditional regulators of plant growth, development, and gene expression. Plant Cell, The. 1997;9:1211–1223. doi: 10.1105/tpc.9.7.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiology. 2008;146:825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felton GW, Tumlinson JH. Plant–insect dialogs: complex interactions at the plant–insect interface. Current Opinion in Plant Biology. 2008;11:457–462. doi: 10.1016/j.pbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Alborn T, Turlings TCJ, Jones TH, Stenhagen G, Loughrin JH, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 13.Doss RP, Oliver JE, Proebsting WM, Potter SW, Kuy SR, et al. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proceedings of the National Academy of Sciences of the USA. 2000;97:6218–6223. doi: 10.1073/pnas.110054697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alborn HT, Hansen TV, Jones TH, Bennett DC, Tumlinson JH, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proceedings of the National Academy of Sciences of the USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmelz EA, Carroll MJ, LeClere S, Phipps SM, Meredith J, et al. Fragments of ATP synthase mediate plant perception of insect attack. Proceedings of the National Academy of Sciences of the USA. 2006;103:8894–8899. doi: 10.1073/pnas.0602328103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huffaker A, Ryan CA. Endogenous peptide defense signals in Arabidopsis differentially amplify signaling for the innate immune response. Proceedings of the National Academy of Sciences USA. 2007;104:10732–10736. doi: 10.1073/pnas.0703343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce G, Yamaguchi Y, Barona G, Ryan CA. A subtilisin-like protein from soybean contains an embedded, cryptic signal that activates defense-related genes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14921–14925. doi: 10.1073/pnas.1007568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmelz EA, Engelberth J, Alborn HT, Tumlinson JH, III, Teal PEA. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proceedings of the National Academy of Sciences of the USA. 2009;106:653–657. doi: 10.1073/pnas.0811861106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiffer M, Felton GW. Do Caterpillars Secrete “Oral Secretions”? Journal of Chemical Ecology. 2009;35:326–335. doi: 10.1007/s10886-009-9604-x. [DOI] [PubMed] [Google Scholar]
- 20.Heil M. Damaged-self recognition in plant herbivore defence. Trends in Plant Science. 2009;14:356–363. doi: 10.1016/j.tplants.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Radhika V, Kost C, Boland W, Heil M. The role of jasmonate signalling in floral nectar secretion. PLoS ONE. 2010;5:e9265. doi: 10.1371/journal.pone.0009265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heil M. Indirect defence via tritrophic interactions. New Phytologist. 2008;178:41–61. doi: 10.1111/j.1469-8137.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 23.Heil M, Koch T, Hilpert A, Fiala B, Boland W, et al. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proceedings of the National Academy of Sciences of the USA. 2001;98:1083–1088. doi: 10.1073/pnas.031563398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57:675–709. doi: 10.1146/annurev.arplant.57.032905.105441. [DOI] [PubMed] [Google Scholar]
- 25.Roux SJ, Steinebrunner I. Extracellular ATP: an unexpected role as a signaler in plants. Trends In Plant Science. 2007;12:522–527. doi: 10.1016/j.tplants.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Coker JS, Vian A, Davies E. Identification, accumulation, and functional prediction of novel tomato transcripts systemically upregulated after fire damage. Physiologia Plantarum. 2005;124:311–322. [Google Scholar]
- 27.Davies E, Vian A, Vian C, Stankovic B. Rapid systemic up-regulation of genes after heat-wounding and electrical stimulation. Acta Physiologiae Plantarum. 1997;19:571–576. doi: 10.1007/s11738-997-0055-0. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann MR, Felle HH. Dissection of heat-induced systemic signals: superiority of ion fluxes to voltage changes in substomatal cavities. Planta. 2009;229:539–547. doi: 10.1007/s00425-008-0850-x. [DOI] [PubMed] [Google Scholar]
- 31.Ryan CA. Assay and biochemical properties of the proteinase inhibitor inducing factor, a wound hormone. Plant Physiology. 1974;54:328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turlings TCJ, McCall PJ, Alborn HT, Tumlinson JH. An elicitor in caterpillar oral secretions that induces corn seedlings to emit chemical signals attractive to parasitic wasps. Journal of Chemical Ecology. 1993;19:411–425. doi: 10.1007/BF00994314. [DOI] [PubMed] [Google Scholar]
- 33.Mattiacci L, Dicke M, Posthumus MA. Beta-glucosidase — an elicitor of herbivore-induced plant odor that attracts hostsearching parasitic wasps. Proceedings of the National Academy of Sciences of the USA. 1995;92:2036–2040. doi: 10.1073/pnas.92.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiology. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce G, Siems WF, Bhattacharya R, Chen YC, Ryan CA. Three hydroxyproline-rich glycopeptides derived from a single petunia polyprotein precursor activate defensin I, a pathogen defense response gene. Journal of Biological Chemistry. 2007;282:17777–17784. doi: 10.1074/jbc.M701543200. [DOI] [PubMed] [Google Scholar]
- 36.Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–380. doi: 10.1038/nature03959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer M, Stenzel U, Hofreiter M. Parallel tagged sequencing on the 454 platform. Nature Protocols. 2008;3:267–278. doi: 10.1038/nprot.2007.520. [DOI] [PubMed] [Google Scholar]
- 38.Katari MS, Nowicki SD, Aceituno FF, Nero D, Kelfer J, et al. VirtualPlant: a software platform to support systems biology research. Plant Physiology. 2010;152:500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–672. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 40.Chini A, Boter M, Solano R. Plant oxylipins: COI1/JAZs/MYC2 as the core jasmonic acid-signalling module. FEBS Journal. 2009;276:4682–4692. doi: 10.1111/j.1742-4658.2009.07194.x. [DOI] [PubMed] [Google Scholar]
- 41.Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, et al. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–666. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 42.Reinbothe C, Springer A, Samol I, Reinbothe S. Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS Journal. 2009;276:4666–4681. doi: 10.1111/j.1742-4658.2009.07193.x. [DOI] [PubMed] [Google Scholar]
- 43.Martínez O, Reyes-Valdes MH. Defining diversity, specialization, and gene specificity in transcriptomes through information theory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9709–9714. doi: 10.1073/pnas.0803479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–U910. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 45.Stankovic B, Davies E. Both action potentials and variation potentials induce proteinase inhibitor gene expression in tomato. Febs Letters. 1996;390:275–279. doi: 10.1016/0014-5793(96)00672-2. [DOI] [PubMed] [Google Scholar]
- 46.Stankovic B, Davies E. The wound response in tomato involves rapid growth and electrical responses, systemically up-regulated transcription of proteinase inhibitor and calmodulin and down-regulated translation. Plant And Cell Physiology. 1998;39:268–274. [Google Scholar]
- 47.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochimica et Biophysica Acta-Biomembranes. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 48.Pluskota WE, Qu N, Maitrejean M, Boland W, Baldwin IT. Jasmonates and its mimics differentially elicit systemic defence responses in Nicotiana attenuata. Journal of Experimental Botany. 2007;58:4071–4082. doi: 10.1093/jxb/erm263. [DOI] [PubMed] [Google Scholar]
- 49.Mueller MJ, Brodschelm W. Quantification of jasmonic acid by capillary gas chromatography-negative chemical ionization-mass spectrometry. Analytical Biochemistry. 1994;218:425–435. doi: 10.1006/abio.1994.1202. [DOI] [PubMed] [Google Scholar]
- 50.Ramírez-Chávez E, López-Bucio J, Herrera-Estrella L, Molina-Torres J. Alkamides isolated from plants promote growth and alter root development in Arabidopsis. Plant Physiology. 2004;134:1058–1068. doi: 10.1104/pp.103.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okubo K, Hori N, Matoba R, Niiyama T, Fukushima A, et al. Large scale cDNA sequencing for analysis of quantitative and qualitative aspects of gene expression. Nature Genetics. 1992;2:173–179. doi: 10.1038/ng1192-173. [DOI] [PubMed] [Google Scholar]
- 52.Lee NH, Weinstock KG, Kirkness EF, Earle-Hughes JA, Fuldner RA, et al. Comparative expressed-sequence-tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment. Proceedings of the National Academy of Science USA. 1995;92:8303–8307. doi: 10.1073/pnas.92.18.8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franco GR, Rabelo EML, Azevedo V, Pena HB, Ortega JM, et al. Evaluation of cDNA libraries from different developmental stages of Schistosoma mansoni for production of expressed sequence tags (ESTs). DNA Research. 1997;4:231–240. doi: 10.1093/dnares/4.3.231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differentially expressed genes in the Phaseolus lunatus transcriptome. Complete list of those 406 non-redundant unigenes (contigs, accession numbers, annotation results and expression levels) that exhibited putative differential expression in at least one condition evaluated.
(XLS)
Over-represented functional categories in the Phaseolus lunatus transcriptome. The over-represented functional categories were defined according to the MIPS classification using BioMaps tool from the VirtualPlant webpage (URL: http://virtualplant.bio.nyu.edu/cgi-bin/vpweb/). Hypergeometric method and a Bonferroni correction were used for the analysis with a cutoff of 0.01.
(XLS)
Expression profiles of genes represented in the “osmotic and salt stress response” MIPS categories.
(XLS)
Primer sequences used for qRT-PCR.
(XLS)
Annotation of the Phaseolus lunatus ESTs. A detailed explanation of all bioinformatic steps taken during the analyses of the transcriptomic data presented in this study.
(DOC)