Abstract
This systematic review assesses the effectiveness of interventions in community and workplace settings to reduce sickness absence and job loss in workers with musculoskeletal disorders (MSDs). Relevant studies (randomised controlled trials (RCTs) and cohort studies published since 1990) were identified by screening citations in 35 earlier systematic reviews and from searches of Medline and Embase to April 2010. Among 42 studies (54 reports) including 34 RCTs, 27 assessed return to work, 21 duration of sickness absence, and five job loss. Interventions included exercise therapy, behavioural change techniques, workplace adaptations and provision of additional services. Studies were typically small (median sample size 107 (inter-quartile range (IQR) 77 to 148) and limited in quality. Most interventions were reported as beneficial: the median relative risk (RR) for return to work was 1.21 (IQR 1.00 – 1.60) and that for avoiding MSD-related job loss, 1.25 (IQR 1.06-1.71); the median reduction in sickness absence was 1.11 (IQR 0.32 to 3.20) days/month. However, effects were smaller in the larger and better quality studies, suggesting publication bias. No intervention was clearly superior to others, although effort-intensive interventions were less effective than simple ones. No cost-benefit analyses established statistically significant net economic benefits. Given that benefits are small and of doubtful cost-effectiveness, employers’ practice should be guided by their value judgements about the uncertainties. Expensive interventions should be implemented only with rigorous cost-benefit evaluation planned from the outset. Future research should focus on the cost-effectiveness of simple low cost interventions, and further explore impacts on job retention.
Keywords: Occupational Disease, Epidemiology, Rehabilitation, Systematic review, Psychological techniques, Physiotherapy
Introduction
Musculoskeletal disorders (MSDs) cause substantial suffering and disability among adults of working age, and are costly in demands for healthcare and the lost productivity that arises from impaired work performance, sickness absence and job loss. In the UK Labour Force Survey, 30% of those taking sick leave in the previous week cited MSDs as the main reason [1], while the Health and Safety Executive estimates that over nine million working days are lost annually from work-related MSDs [2]. Given the large burden of disability, high associated costs, and a growing evidence base on the adverse health consequences of job loss and unemployment [3], there is a need for effective, evidence-based strategies to guide the employment and care of patients with MSDs.
Against this background, the UK’s National Institute for Health and Clinical Excellence (NICE) has reviewed the effectiveness of community and workplace-based interventions to limit sickness absence [4], and published public health advice [5], much relating to workers with MSDs. However, the evidence base on which NICE relied contained few effect estimates, and in its evaluation, NICE did not derive quantitative summaries of benefit, either overall or according to anatomical site or type of intervention. Nor was an assessment made of possible publication bias.
To address these outstanding questions, and to assess the effectiveness of different types of non-pharmacological intervention in workplace or community settings in reducing sickness absence and job loss, and promoting return to work among workers with established MSDs, we re-evaluated the MSD-related reports considered by NICE. Additionally, we updated NICE’s search in two bibliographic databases, extended it to encompass MSD-related job loss, evaluated 43 other potentially relevant systematic reviews to check for relevant studies that might have been missed, and sought evidence on the cost-effectiveness of the interventions we identified.
Methods
Data sources
The NICE reviewers screened >18,000 articles (identified from 22 databases and 11 websites) that were published between 1990 and 2007, retrieved 800 papers, and summarised 49 reports on sickness absence, of which 42 concerned MSDs [4]. We retrieved all 42 of these reports. We also checked exclusions from the NICE review, from which we identified three further reports relevant to our inquiry. We re-ran NICE’s search strategy in Medline and Embase for 1st Jan 2007 to 23rd April 2010, to check for later publications; and also for 1990 to 23rd April 2010 using terms for health-related job loss, retirement and disability pensioning in lieu of sickness absence. Our searches identified two 2010 Cochrane reviews, the citations from which were also considered [6,7].
Additionally, we checked a best evidence synthesis by Waddell et al on vocational rehabilitation [8]. This considered several thousand citations identified from five electronic databases, hand searching of 18 journals and the publications of 13 organisations or Government departments, internet searches, citation tracking, and the authors’ personal databases. We retrieved all cited systematic reviews, meta-analyses, and Cochrane reports covering interventions on sickness absence and job loss for MSDs (n=41), excluded nine that fell outside the scope of our inquiry, and checked the primary research reports cited in the remaining 32.
After removal of duplicates, these various sources yielded a total of 2,156 reports which were screened for eligibility.
Inclusion criteria
We retained only peer-reviewed randomised controlled trials (RCTs) and cohort studies published from 1990 onwards, in which subjects were workers who had an MSD and/or were on sick leave with an MSD at entry, or had taken sick leave for an MSD in the past 12 months. We limited inclusion further to studies in which vocational outcomes of interest (sickness absence, MSD-related job loss, return to work during follow-up, or prevalence of work attendance at follow-up) could be quantified for defined worker populations. Qualifying interventions were those delivered in a primary care or workplace setting or conducted in collaboration with primary care providers or employers, excluding drug trials and surgery, but including physical therapies delivered by physiotherapists or chiropractors. Reports relating to external traumatic injury were excluded.
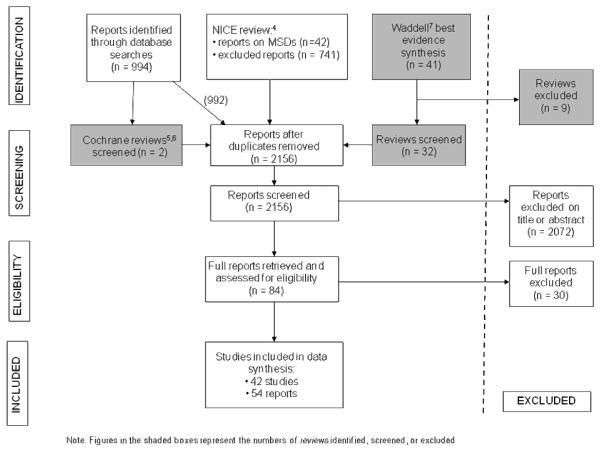
Figure 1 details the selection process in PRISMA [9] format. From the 2,156 reports screened, we excluded 2,072 on title or abstract, and a further 30 that we retrieved and assessed in full. This left 54 reports from 42 separate studies that formed the review base.
Figure 1.
Flow chart of the review’s identification, screening, and selection stages
Data abstraction and quality assessment
One investigator (KTP) systematically abstracted data from each study on: study populations (type of MSD, case definition, exclusion criteria, source of recruitment, setting, participant flow, numbers, baseline characteristics); outcomes; interventions (e.g. nature, timing, intensity, frequency); numbers analysed, analytical methods and treatment of losses to follow-up; effect estimates and confidence intervals or data permitting estimates of precision; and where relevant, procedures for randomisation and blinding. Data abstraction was piloted in ten reports, and then independently checked for all reports by a second investigator (CHL).
Studies were evaluated for their susceptibility to bias using, for RCTs, the Cochrane Back Review Group’s criteria [10], and for cohort studies, a checklist adapted from those criteria. A quality score was derived for each study by assigning one point for each quality measure. For sensitivity analysis, we classified studies in thirds of the distribution of quality score.
Classification of interventions
We identified various physical, psychological, social and environmental interventions directed at (i) the individual, (ii) his/her work or workplace, or (iii) healthcare and other services to which he/she had access.
At the individual level, interventions included exercise therapy, work hardening, or physical therapy (often called a ‘functional restoration programme’); psychological therapy aimed at behavioural or attitudinal change, and general in nature (e.g. CBT, coping, relaxation) or vocationally focussed (at overcoming psychosocial barriers to working, or attitudes to and perceptions of work), often accompanied by education aimed at influencing perceptions and behaviour (e.g. ‘Back School’ education); rehearsal of safer working techniques; and use of lumbar supports.
At the workplace level, approaches included: ergonomic and/or psychosocial risk assessments – aimed at the individual (e.g. how well an exercise programme matched job demands) or at identifying and controlling workplace risks (‘participatory’ ergonomic assessments, involving the individual, his/her manager, and union); ergonomic changes to the physical environment; job modifications (e.g. lighter duties, reduced hours); and interventions directed at managers (education and advice).
At the service level, approaches included: assessment and a co-ordinated action plan, evolved by a multidisciplinary case management team or a case manager; consultation with an occupational physician; education of primary care doctors and/or occupational physicians, and/or formalised agreements between them, to improve liaison; and access to extra external support and referral services.
Some categories were capable of finer delineation e.g. exercise therapy, work hardening, or physical therapy could be subdivided into: exercises to build aerobic capacity, stamina, and endurance; exercises to build anaerobic capacity, and strength and size of muscles – e.g. strength training, weight training; resistance, static, isometric, or isotonic exercises; exercises to improve balance and co-ordination – e.g. stabilising exercises; flexibility exercises such as stretching; exercises which rehearsed work activities (to build endurance and flexibility for everyday work tasks, and mitigate fear-avoidance psychological responses); physical therapy applied by a health care professional to increase mobility or reduce pain – e.g. traction, manipulation, massage, TENS, pulsed electromagnetic therapy, ultrasound, heat/cold. Where accounts were sufficiently detailed, we used the framework of Abraham and Michie [11] to sub-classify behavioural change interventions into component techniques such as: providing information on behaviour health-links, prompting practice, providing feedback on performance, setting graded tasks, prompting identification of barriers, providing contingent rewards, help in specific goal-setting, agreed behavioural contracts, and stress management.
Analysis
Data were abstracted on three main outcomes: return to work, avoidance of health-related job loss, and mean days of sick leave/month over follow-up. The corresponding measures of effect (relative risk (RR) of return to work or job survival and mean days of sick leave/month avoided in treatment vs. comparison group for a given follow-up) were seldom reported, and had to be derived from summary data in reports – e.g. the proportions by group who had returned to work/lost a job and numbers at risk; or the mean sickness absence, standard deviation and numbers studied by group. When proportions were given but not subject numbers, we imputed numbers so that confidence intervals of RRs could be estimated. In the absence of direct author information, we made the simplifying assumption that, for skewed quantitative data on sickness absence, the sampling distribution of the difference in mean days of absence between groups was roughly normal or that violations of this assumption were trivial. Estimates of precision were made using immediate commands in the STATA package.
Preliminary tabulations highlighted substantial heterogeneity of case definitions and interventions, so we decided against combining data in meta-analyses. Instead, findings were summarised descriptively across studies as the median and interquartile range (IQR) of effect estimates, overall and by specified subject characteristics (e.g. pain site, duration of sick leave at baseline), study features (e.g. follow-up time, study size, study quality), and type of intervention. Few investigations analysed or provided raw data on cost-effectiveness. However, we estimated for each comparison the time involved in delivering the intervention, as a proxy for invested resources, and compared outcomes by invested effort. Finally, a check was made for publication bias by constructing funnel plots of the natural logs of treatment effects vs. their standard errors.
Results
Study characteristics
The 42 studies (Table 1) comprised 34 RCTs and 8 cohort studies, and were described in 54 reports [12-65], mostly from northern Europe and North America. Half of studies focussed on the ‘low-back’, three on the ‘back’, five on axial (back and neck, or back, neck and shoulder) pain, one on the neck, two on the upper limbs, and nine on unspecified musculoskeletal pain. Studies varied in case definition, although most required subjects to be on sick leave at entry; durations of absence ranged from a few days to >1 year. Seven studies (17%) tested more than one intervention and 15 studies (36%) reported outcomes at more than one follow-up. Follow-up times ranged from one week to five years, but reached >3 years in only three studies. Return to work was most often studied (64% of studies), followed by sickness absence (55%). Only five reports were found on job loss.
Table 1.
Characteristics of the included trials and cohort studies
| Materials | Interventions | Follow-up | Outcome(s) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (date) | Country | Site(s) of pain |
Weeks of sick leave (*weeks of symptoms) at entry |
Maximum numbers Analysed I/C |
Exercise therapy | Behavioural intervention |
Physical therapy | Workplace intervention |
Service provision | Time point or interval (months) |
Return to work | MSD-related job loss | Sickness Absence during follow-up (days) |
High sickness absence in follow- up (%)b |
At work at time of follow-up |
| Anderson (2007)12 | Norway | Mixed | >12 | 14/20 | X | X | 6, 18 | X | |||||||
| Arnetz (2003)13 | Sweden | Mixed | Not stated | 65/72 | X | X | 6, 12 | X | X | ||||||
| Aure (2003)14 | Norway | LBP | >8 to <26 | 27/22 | X | X | X | 1, 6, 12 | X | ||||||
| Bültmann (2009)15 | Denmark | Mixed | 4 to 12 | 66/47 | X | X | 3, 6, 12 | X | X | ||||||
| Cheng (2007)16 | Hong Kong | UL | >12 | 46/48 | X | X | 0.25 | X | |||||||
| Faber (2005)17 | Netherlands | LBP | 3 to 12 | 56/56 | X | 6 | X | ||||||||
| Fleten (2006)18 | Norway | Mixed | >2 | 495/495 | X | 12 | X | X | |||||||
| Godges (2008)19 | USA | LBP | Not stated | 16/18 | X | X | X | 1, 3 | X | ||||||
| Haldorsen (1998)20-23 | Norway | Mixed | 8 | 312/157 | X | X | X | 12 | X | ||||||
| Haugli (2001)24 | Norway | Mixed | Unclear | 63/33 | X | 6-12 | X | ||||||||
| Heymans (2006)25 | Netherlands | LBP | 3 to 6 | 98/103 | X | X | 12 | X | (X)a | ||||||
| Hlobil (2005)26-29 | Netherlands | LBP | ≥4 | 67/67 | X | X | 12 | X | X | ||||||
| Jensen (1997)30 | Sweden | Axial | >12 to 52 | 33/30 | X | X | 6, 12-18 | X | |||||||
| Jensen (2001/5)31,32 | Sweden | Axial | 4 to 26 | 37/28 | X | X | X | 36 | X | X | X | ||||
| Kääpä (2006)33 | Finland | LBP | (52w LBP*) | 53/54 | X | X | X | 0-12, 12-24 | X | ||||||
| Lambeek (2010)34 | Netherlands | LBP | >12 | 66/68 | X | X | X | X | 12 | X | (X)a | ||||
| Lindh (1997)35 | Sweden | Axial | Not stated1 | 151/134 | X | X | X | X | 6, 12, 18, 24, 60 | X | X | ||||
| Lindström (1992)36-38 | Sweden | LBP | 8 | 51/52 | X | X | X | 1.5, 3, 12-24 | X | X | |||||
| Loisel (1997)39,40 | Canada | Back | >4 to 12 | 56/26 | X | X | X | X | 12 | X | (X)a | ||||
| Marhold (2001)41 | Sweden | Axial | 8-26; >52 | 18/18 | X | 0-2, 2-4, 4-6 | X | ||||||||
| Meijer (2006)42 | Netherlands | UL | Unclear | 20/14 | X | X | X | 2, 6, 12 | X | ||||||
| Mitchell (1994)43 | Canada | Mixed | >12 | 271/271 | X | X | 0-24 | X | X | ||||||
| Molde Hagen (2003)44 |
Norway | LBP | 8-12 | 237/220 | X | X | 12, 24, 36 | X | X | ||||||
| Niemisto (2005)45 | Finland | LBP | (Chronic LBP*) | 81/78 | X | X | X | 0-24 | X | ||||||
| Nystuen (2006)46 | Norway | Mixed | >7 | 46/37 | X | 6 | X | X | |||||||
| Roelofs (2007)47 | Netherlands | LBP | Not stated2 | 183/177 | X | 0-12 | X | ||||||||
| Scheel (2002)48 | Norway | LBP | >2 | 2232/1902 | X | 12 | X | X | |||||||
| Schultz (2008)49 | Canada | Back | 4 to 10 | 35/37 | X | X | X | 3, 6 | X | ||||||
| Soukup (1999)50 | Norway | LBP | Not stated | 34/35 | X | X | 0-12 | X | |||||||
| Stankovic (1990)51 | Sweden | LBP | (4w LBP*) | 50/50 | X | X | 0-3 | X | |||||||
| Steenstra (2006)52-54 | Netherlands | LBP | 2 to 6 | 96/100 | X | X | X | X | 12 | X | |||||
| Torstensen (1998)55 | Norway | LBP | 8 to 52 | 71/70 | X | X | X | 12 | X | X | X | ||||
| Van den Hout (2003)56 |
Netherlands | LBP | Unclear | 44/39 | X | X | X | 6, 12 | X | X | |||||
| Verbeek (2002)57 | Netherlands | LBP | >9 days | 61/59 | X | X | 12, 0-12 | X | X | ||||||
| Cohort | |||||||||||||||
| Brown (1992)58 | USA | LBP | Not stated | 70/70 | X | X | 0-6 | X | |||||||
| Durand (2000)59 | Canada | Back | >12 | 28/49 | X | X | 24 | X | |||||||
| Feuerstein (1993)60 | USA | UL | >12 | 19/15 | X | X | X | 17-18 | X | ||||||
| Gard (1999)61 | Sweden | LBP | Mean = 8 | 40/46 | X | X | 1 | X | X | ||||||
| Jensen (1998)62 | Sweden | Axial | ≤26 | 67/28 | X | X | 0-18 | X | |||||||
| Klemetti (1997)63 | Finland | Neck | (Current NP*) | 35/44 | X | X | 0-6 | X | |||||||
| Leino (1994)64 | Finland | LBP | Not stated | 87/61 | X | X | X | 0-12 | X | ||||||
| Sinclair (1997)65 | Canada | Mixed | ≤3 | ? | X | X | X | 12 | X | ||||||
| All studies (n=42) | 30 | 37 | 5 | 17 | 10 | 27 | 5 | 24 a | 2 | 4 | |||||
I = intervention; C = control; a – 3 studies reported only median days of sick leave by treatment group (results for a and b are restricted to the supplementary tables)
Sample sizes were generally small, the median number of subjects analysed being 107 (inter-quartile range (IQR) 77 to 148). In only six studies (14%) did a comparison involve >300 subjects and in only three was it >500. Table 2 summarises other aspects of methodological quality. The median quality score for RCTs was 45% (IQR 27% to 64%) and that for cohort studies 40% (IQR 15% to 45%). Given the nature of most interventions (below), it proved difficult to blind patients and impossible to blind care-givers. In half of studies, however, outcomes were assessed blinded or outcome data collected from an independent registry. A third of studies had high drop-out rates, and in a third there was doubt about whether intervention and control groups were assessed with comparable follow-up timing. Half of the RCTs lacked a clear protocol for blinded allocation to groups, and half failed to analyse by intention to treat.
Table 2.
Methodological strengths and weaknesses of studies in the review
| (a) RCTs | blinding of allocation | blinding of patient | blinding of assessor, or electronic register of outcome |
blinding of care-giver | co-interventions equal | drop-out rate <20% for main outcome |
compliance acceptablec |
comparable timing | intention to treat | adequately randomized | Similar at baselined | % score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson (2007) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 45% |
| Arnetz (2003) | ? | 0 | 1 | 0 | ? | ? | ? | 1 | ? | 0 | ? | 18% |
| Aure (2003) | 1 | 0 | 0 | 0 | ? | 1 | 1 | 1 | 1 | ? | 1 | 55% |
| Bultmann (2009) | ? | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 64% |
| Cheng (2007) | ? | 0 | 0 | 0 | ? | 1 | ? | 1 | 0 | ? | ? | 18% |
| Faber (2005) | n/a | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 70% |
| Fleten (2006) | ? | 0 | 1 | n/a | ? | 1 | ? | 1 | 1 | ? | ? | 40% |
| Godges (2008) | 0 | 1 | 1 | n/a | ? | 1 | ? | ? | 0 | 0 | ? | 30% |
| Haldorsen (1998) | 1 | 0 | 1 | 0 | ? | 1 | ? | ? | 1 | ? | ? | 36% |
| Haugli (2001) | ? | 0 | 0 | 0 | ? | 0 | 0 | ? | 0 | ? | ? | 0% |
| Heymans (2006) | 1 | 0 | 1 | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 73% |
| Hlobil (2005) | ? | 0 | 1 | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 64% |
| Jensen (1997) | 1 | 0 | 1 | 0 | ? | 1 | ? | ? | 1 | 1 | 1 | 55% |
| Jensen (2005) | ? | 0 | 1 | 0 | 1 | 1 | 1 | ? | 1 | ? | 1 | 55% |
| Kääpä (2006) | 1 | 0 | 0 | 0 | ? | 0 | ? | 1 | 0 | 1 | 1 | 36% |
| Lambeek (2010) | 1 | 0 | 0 | 0 | ? | 1 | 1 | 1 | 1 | 1 | 1 | 64% |
| Lindh (1997) | ? | 0 | 1 | 0 | ? | 1 | ? | 1 | 1 | ? | ? | 36% |
| Lindström (1992) | ? | 0 | 1 | 0 | ? | 1 | 1 | 1 | 1 | ? | ? | 45% |
| Loisel (1997) | 1a | 0 | ? | 0 | ? | 1 | ? | 1 | 0 | 1 | 1 | 45% |
| Marhold (2001) | ? | 0 | 1 | 0 | ? | 1 | ? | ? | ? | ? | ? | 18% |
| Meijer (2006) | 1 | 0 | ? | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 64% |
| Mitchell (1994) | 1 | 0 | 0 | 0 | ? | ? | ? | 1 | ? | 1 | ? | 27% |
| Molde Hagen (2003) | 1 | 0 | 1 | 0 | ? | 1 | ? | 1 | 1 | 1 | ? | 55% |
| Niemisto (2005) | ? | 0 | 0 | 0 | 1 | 0 | ? | 1 | 0 | ? | 1 | 27% |
| Nystuen (2006) | 1 | 0 | 1 | 0 | ? | 0 | ? | ? | 0 | 1 | ? | 27% |
| Roelofs (2007) | 1 | 0 | 1b | n/a | ? | 1 | 1 | 1 | 1 | 1 | ? | 70% |
| Scheel (2002) | 1 | n/a | 1 | 0 | ? | 1 | 0 | 1 | 1 | 1 | 1 | 70% |
| Schultz (2008) | 0 | 0 | ? | 0 | ? | 0 | 0 | ? | 0 | 0 | 1 | 9% |
| Soukup (1999) | ? | 0 | ? | 0 | ? | 1 | 1 | 1 | 0 | 1 | 1 | 45% |
| Stankovic (1990) | ? | 0 | 0 | 0 | ? | ? | ? | ? | ? | 1 | ? | 9% |
| Steenstra (2006) | 1a | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 82% |
| Torstensen (1998) | 1 | 0 | 1 | 0 | ? | 1 | 1 | 1 | 1 | 1 | ? | 64% |
| Van den Hout (2003) | 1 | 0 | 1 | 0 | ? | 0 | ? | 1 | 0 | 1 | 1 | 45% |
| Verbeek (2002) | ? | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 73% |
| All | 17 | 3 | 21 | 0 | 7 | 24 | 14 | 25 | 17 | 20 | 18 |
| (b) Cohort Studies | Outcome assessed independent of exposure |
comparable timing of outcome assessment |
Other major treatments equal |
Adequate control of other major confounders* |
drop-out rate <20% | % score |
|---|---|---|---|---|---|---|
| Brown (1992) | 1 | ? | ? | 0 | 1 | 40% |
| Durand (2000) | 0 | ? | ? | 0 | 1 | 20% |
| Feuerstein (1993) | 0 | 0 | ? | 1 | 1 | 40% |
| Gard (1999) | 0 | ? | ? | ? | ? | 0% |
| Jensen (1998) | 1 | ? | ? | 1 | 0 | 40% |
| Klemetti (1997) | 0 | ? | ? | ? | 0 | 0% |
| Leino (1994) | 1 | 1 | ? | 1 | 1 | 80% |
| Sinclair (1997) | 1 | 1 | ? | 1 | 0 | 60% |
| All | 4 | 2 | 0 | 4 | 4 |
A two-stage factorial design was used with cluster randomisation of one intervention and individual randomisation of a second - concealment could not be applied for the first;
Both computer generated outcomes and self-reported were reported;
Intervention delivered and received;
Adequate control of confounding for any 4 of 5 major outcome determinants – (i) severity/duration, (ii) sickness absence history, (iii) site(s) of pain, (iv) mental health status, (v) health belief status.
As in d above.
Interventions and control comparisons
Among the interventions tested in the 42 studies, 30 prescribed exercises, 37 promoted behavioural change, 17 were at the patient’s workplace, and 10 provided additional services (Table 1). Interventions were often applied in combination – frequently an exercise (functional restoration) regimen combined with behavioural measures to improve compliance (e.g. prompts, encouragement). In 12 studies (29%), interventions were aimed both at personal exercise and behavioural change, and also included workplace adaptations or assessments.
Supplementary tables S1 and S2 (for web-only publication) provide further detail. Common behavioural change approaches involved: providing instruction (75% of all studies), prompting practice (55%), providing feedback on performance (45%), prompting barrier identification (41%), stress reduction (41%), setting graded tasks (39%), prompting intention formation (32%), prompting review of behavioural goals (30%), providing general information on behaviour-health links (30%), and CBT (30%). Physical fitness regimens frequently involved aerobic exercises (60% of studies), strength and resistance training (43%), and stretching and flexibility training (41%). Although most studies employed a combination of these techniques, a few differed substantially from the generality – e.g. a one-off postal information leaflet to participants [18], a brief active vocational interview, assessment, or intervention [44,51,57], workshops for GPs and occupational physicians [17], or fitting of lumbar supports [47].
The control arms in most studies received care as usual (n=33, 79%), or simple advice in lieu of a supervised intervention [55,61]. Occasionally, however, controls received a less intense version of the full intervention [14,19,30,56]; one study contrasted settings (workplace-based vs. clinic-based exercise)[16]; and in two there were contrasting active interventions (exercises vs. back school education [51], and multidisciplinary rehabilitation vs. physiotherapy [33]). (Further details appear in web-supplementary tables S3 to S7.)
Findings
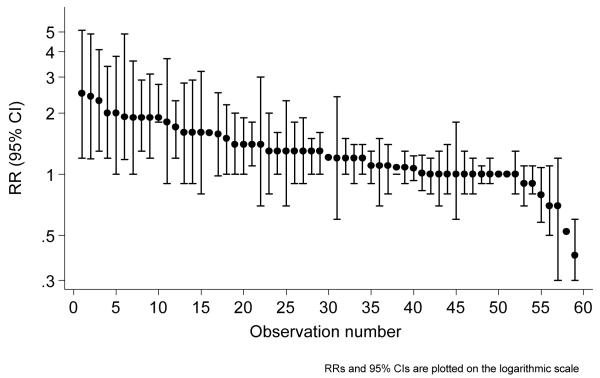
Figure 2 charts, for return to work (the most studied outcome) ranked RRs, with associated 95% confidence intervals where calculable, across all 27 studies and 59 comparisons reporting the outcome. (Not all observations were independent, as reports sometimes included comparisons for the same intervention at multiple follow-ups.) Interventions were mostly beneficial (RR>1 for intervention vs. control group), but seldom strongly beneficial (RR>2), and logarithmic confidence intervals tended to be wider for estimates at extremes of the range. Patterns were similar for other outcomes, and effects were similarly modest.
Figure 2.
Return to work – all comparisons, intervention vs. control
Table 3 sets out in more detail the RRs for return to work and avoidance of health-related job loss, and the estimated differences in mean days of sickness absence/month, overall and by study population and study characteristics. (A tabulation of findings across all studies and interventions appears in web-only supplementary tables S3 to S7.) The median RR for return to work was 1.21 (IQR 1.00 – 1.60) and that for avoidance of health related job loss, across 15 comparisons in five studies, was 1.25 (IQR 1.06-1.71). A total of 21 studies (41 comparisons) provided data on sickness absence, the median effect (mean reduction in sickness absence in intervention vs. control group) across these comparisons being 1.11 (IQR 0.32 to 3.20) days/month.
Table 3.
Effect of interventions on return to work, avoidance of job loss, and sickness absence, overall and by characteristics of studies and study populations
| Return to work | Avoidance of job loss | Mean reduction in sick leave days/month |
|||||
|---|---|---|---|---|---|---|---|
| N studiesa/ comparisons |
Median RR (IQR) |
N studiesa/ comparisons |
Median RR (IQR) |
N studiesa/ comparisons |
Median (IQR) | ||
| All | 27/59 | 1.21 (1.00 to 1.60) |
5/15 | 1.25 (1.06 to 1.71) |
All | 21/41 | 1.11 (0.32 to 3.20) |
| Site: Low-back | 13/25 | 1.20 (1.00 to 1.60) |
4/6 | 2.29 (0.73 to 4.58) |
Site: Low-back | 11/15 | 1.11 (0.15 to 2.74) |
| Back | 3/8 | 1.30 (1.28 to 1.67) |
- | - | Back | - | - |
| Axial | 1/5 | 1.00 (1.00 to 1.10) |
1/9 | 1.25 (1.11 to 1.43) |
Axial | 4/13 | 1.48 (0.80 to 3.30) |
| Upper limb | 2/2 | 1.65 (-) | - | - | Upper limb | - | - |
| Not specified |
8/18 | 1.14 (1.00 to 1.53) |
- | - | Not specified |
6/11 | 2.85 (0.50 to 3.79) |
| Prior sick leave: | Prior sick leave: | ||||||
| <12 weeks | 12/30 | 1.30 (1.02 to 1.60) |
2/2 | 4.17 (-) | <12 weeks | 4/5 | 2.85 (1.67 to 3.13) |
| >12 weeks | 10/17 | 1.20 (1.00 to 1.40) |
2/11 | 1.25 (1.00 to 1.34) |
>12 weeks | 5/15 | 1.25 (0.58 to 2.73) |
| Not specified | 5/12 | 1.14 (1.00 to 1.65) |
- | - | Not specified | 5/12 | 0.69 (0.07 to 3.37) |
|
Follow-up
(months): |
Follow-up (months): |
||||||
| ≤ 3 | 9/9 | 1.40 (1.30 to 1.60) |
1/1 | 5.00 (-) | |||
| 6 | 8/9 | 1.40 (1.00 to 1.80) |
1/1 | 1.30 (-) | 0 to 6 | 6/11 | 2.85 (1.03 to 3.41) |
| 12 | 17/32 | 1.09 (1.00 to 1.60) | 1/3 | 0.56 (-) | 0 to 12 | 12/16 | 0.54 (0.27 to 3.24) |
| 18 | 3/3 | 1.20 (-) | - | - | 0 to 24 | 3/3 | 0.32 (0.11 to 0.78) |
| ≥24 | 6/6 | 1.05 (1.00 to 1.25) |
2/10 | 1.25 (1.15 to 1.43) |
0 to 36 | 2/7 | 1.21 (0.73 to 1.64) |
|
Subjects
analysed: |
Subjects
analysed: |
||||||
| 31-99 | 9/21 | 1.60 (1.30 t 1.90) |
2/11 | 1.34 (1.08 to 1.57) |
31-99 | 5/13 | 2.68 (0.91 to 5.09) |
| 100-150 | 8/16 | 1.30 (1.00 to 1.60) |
3/5 | 1.25 (0.56 to 1.25) |
100-150 | 8/12 | 3.18 (1.96 to 3.48) |
| 151-300 | 4/11 | 1.00 (0.95 to 1.15) |
- | - | 151-500 | 4/6 | 0.36 (0.05 to 0.41) |
| >300 | 5/9 | 1.00 (1.00 to 1.01) |
- | - | >500 | 3/4 | 0.50 (0.25 to 0.83) |
|
Quality score
(%): |
Quality score
(%): |
||||||
| 0-30 | 8/12 | 1.30 (1.30 to 1.90) |
1/1 | 5.0 (-) | 0-30 | 7/13 | 3.20 (1.25 to 3.52) |
| 31-63 | 10/30 | 1.20 (1.00 to 1.59) |
3/12 | 1.25 (1.22 to 1.57) |
31-63 | 8/11 | 0.33 (0.09 to 0.95) |
| 64-82 | 9/17 | 1.10 (1.00 to 1.40) |
1/2 | 0.54 (-) | 64-82 | 6/11 | 1.01 (0.35 to 2.26) |
| >63 + N ≥148b |
3/6 | 1.00 (0.85 to 1.20) |
- | - | >63 + N ≥148b | 3/5 | 0.30 0.20 to 0.40) |
Numbers of studies and comparisons do not always arrive at common totals as: some studies had several intervals of follow-up or compared more than one intervention, and a few studies defined cases by duration of symptoms rather than by duration of sick leave.
Where relevant, the number of subjects given is for the largest comparison.
And number of subjects analysed in the top quartile for all comparisons. RR = relative risk, IQR = interquartile range
Few differences were found by anatomical site; but consistently across all categories of outcome, the benefits of intervention were somewhat greater in workers with <12 weeks of sickness absence at baseline as compared with workers off work for longer. They were also greater in the earlier stages of follow-up. For example, the median RR for return to work was 1.40 at 3 and 6 months vs. 1.09 at 12 months and 1.05 at ≥24 months; and for sickness absence, the median reduction was 2.85 days/month in the first 6 months but 0.32 days/month over 24 months.
There were differences in treatment effect by study size, small studies (<150 subjects) reporting greater benefits than larger ones (>150) (Table 3). In the latter, RRs for return to work tended towards the null value, while the median sick leave avoided amounted to only 0.36 to 0.50 days/month. Similarly, benefits of intervention tended to be lower in high quality than in low quality reports, categorised by third of quality score; and when attention was further restricted to studies in the top third by quality (>63%) and the top quarter by size (≥148 subjects in a comparison), effects were even smaller: a median RR of 1.00 for return to work and a median of 0.30 days/month of sick leave avoided. A plot of log RRs for return to work against their standard errors showed an asymmetric inverted funnel, consistent with publication bias (Figure – web supplement).
Table 4 summarises the magnitudes of effect by type of intervention. No interventions were clearly superior to others, although studies that involved setting graded tasks were slightly more positive (median RR for return to work 1.40 vs. 1.21 overall; median reduction in days lost/month 1.67 vs. 1.1 overall). Interventions involving workplace adaptations/assessments or extra services were somewhat more beneficial in reducing days lost.
Table 4.
Effect of interventions on return to work, avoidance of job loss, and sickness absence, by type and intensity of intervention
| Return to work | Avoidance of job loss | Mean reduction in sick leave days/month |
||||
|---|---|---|---|---|---|---|
| No of studies/ comparisons |
Median RR (IQR) |
No of studies/ comparison s |
Median RR (IQR) |
No of studies/ comparisons |
Median (IQR) | |
| All | 27/59 | 1.21 (1.00 to 1.60) |
5/15 | 1.25 (1.06 to 1.71) |
21/41 | 1.11 (0.32 to 3.20) |
| Workplace intervention(s) | 11/29 | 1.30 (1.00 to 1.60) |
3/12 | 1.25 (1.22 to 1.57) |
6/15 | 1.64 (0.40 to 3.42) |
| Job modification(s) | 4/17 | 1.21 (1.00 to 1.60) |
1/9 | 2.29 (0.73 to 4.58) |
1/6 | 1.29 (0.54 to 1.73) |
| Extra services | 6/36 | 1.25 (1.00 to 1.60) |
- | - | 3/5 | 1.67 (0.31 to 2.85) |
| Behavioural/cognitive intervention(s): | ||||||
| Provide instruction | 18/37 | 1.20 (1.00 to 1.60) |
5/15 | 1.25 (1.06 to 1.71) |
14/27 | 1.11 (0.33 to 2.64) |
| Prompt practice | 13/31 | 1.10 (1.00 to 1.35) |
3/12 | 1.25 (1.00 to 1.43) |
9/18 | 1.23 (0.35 to 2.39) |
| Provide feedback on performance | 10/23 | 1.20 (1.00 to 1.45) |
3/12 | 1.25 (1.22 to 1.57) |
8/17 | 1.25 (0.33 to 2.64) |
| Set graded tasks | 11/23 | 1.40 (1.16 to 1.90) |
5/15 | 1.25 (1.06 to 1.71) |
5/16 | 1.67 (1.11 to 3.30) |
| Prompt barrier identification | 10/20 | 1.15 (1.00 to 1.40) |
2/11 | 1.25 (1.18 to 1.43) |
6/17 | 1.25 (0.33 to 2.15) |
| Prompt review of behavioural goals | 5/15 | 1.00 (1.00 to 1.30) |
1/10 | 1.25 (1.15 to 1.43) |
5/16 | 1.25 (0.35 to 2.44) |
| Provide information on behaviour- health link |
5/10 | 1.20 (1.03 to 1.38) |
3/5 | 1.25 (0.56 to 3.33) |
7/13 | 1.25 (0.66 to 3.23) |
| CBT | 6/17 | 1.10 (1.00 to 1.40) |
1/9 | 1.25 (1.11 to 1.43) |
5/16 | 1.25 (0.35 to 2.44) |
| Stress management | 7/16 | 1.15 (1.00 to 1.30) |
1/9 | 1.25 (1.11 to 1.43) |
9/20 | 1.18 (0.33 to 2.41) |
| Exercise therapy: | ||||||
| Aerobic, anaerobic, core, stretcha | 18/41 | 1.20 (1.00 to 1.60) |
5/15 | 1.25 (1.06 to 1.71) |
11/19 | 1.01 (0.24 to 1.37) |
| Aerobic ± others | 16/37 | 1.20 (1.00 to 1.70) |
5/15 | 1.25 (1.06 to 1.71) |
11/17 | 1.11 (0.33 to 1.48) |
| Work simulation | 11/31 | 1.20 (1.00 to 1.60) |
1/2 | 0.54 (-) | 4/4 | 0.69 (0.10 to 1.22) |
| Physical therapy | 4/8 | 1.25 (1.10 to 1.70) |
1/2 | 0.54 (-) | 1/1 | 0.32 (-) |
| Estimated total hours of intervention: | ||||||
| ≤6 | 6/11 | 1.30 (1.20 to 1.35) |
- | - | 7/14 | 1.44 (0.40 to 3.45) |
| >6-12 | 2/6 | 1.80 (1.33 to 2.08) |
- | - | - | - |
| 12-32 | 8/14 | 1.45 (1.10 to 1.85) |
2/2 | 4.17 (-) | 6/13 | 1.25 (0.80 to 3.30) |
| 33-70 | 3/6 | 1.09 (1.08 to 1.18) |
3/4 | 0.90 (0.55 to 2.19) |
6/11 | 0.73 (0.10 to 1.56) |
| >70 | 2/5 | 1.00 (1.00 to 1.00) |
1/9 | 1.25 (1.11 to 1.43) |
- | - |
| Missing | 4/13 | 1.21 (1.10 to 1.30) |
- | - | - | - |
Numbers of studies and comparisons do not always arrive at common totals as some studies compared more than one intervention.
Any or all of these
In a sensitivity analysis which excluded studies in the lowest third by quality score (0-30%), most interventions appeared less effective. However, median RRs for return to work were somewhat larger for setting graded tasks (20 comparisons, median RR 1.45, IQR 1.05 to 1.90), CBT (16 comparisons, median RR 1.30, IQR 1.06 to 1.68), and physical therapy (6 comparisons, median RR 1.35, IQR 1.09 to 1.90).
Although interventions did not differ clearly in effectiveness, there were substantial differences in the time invested in their delivery (Table 4). Those that involved brief interventions (no more than 12 hours in total), appeared more effective than those that took longer, and above 32 hours there was little evidence of benefit. The pattern of decreasing benefit by increasing effort of intervention was confirmed in a sensitivity analysis restricted to studies in the top third by quality score: for interventions taking >32 hours, the median RR for return to work was 1.05 and the mean reduction in sick leave was 0.17 days/month (albeit based on a much reduced sample of comparisons).
More formal cost-effectiveness analyses were undertaken by the authors of eight of the 54 reports [15,26,32,40,42,52,55,65], but no study clearly proved or disproved a positive return on investment. Typically, estimated benefits in productivity (e.g. averted costs of sickness absence) were weighed against the costs of the intervention. Four studies also assessed benefits from reductions in healthcare expenditure [15,32,40,52], and one focussed only on healthcare costs. Most analyses suggested net savings from the interventions evaluated, but in the two reports which gave 95% confidence limits [28,54], findings were also compatible with substantial net losses.
Discussion
Despite the large peer-reviewed literature on community and workplace interventions to improve employment outcomes from musculoskeletal illness, we found gaps in the evidence on upper limb disorders and MSD-related job loss, few large studies, and few reports with extended follow-up or economic evaluation. Many studies had methodological limitations which may have biased estimates of effect (e.g. unblinded outcome assessment, failure to analyse by intention to treat, poor randomisation protocols) and which could be remedied with better design and execution.
Within these limits, most interventions appeared effective, although better quality and larger studies showed less benefit, suggesting publication bias. In the better conducted studies, the median benefit amounted to a 10% improved chance of returning to work or avoidance on average of 0.3 to 0.5 days/month of sickness absence. At these levels of benefit, there is substantial uncertainty about cost-effectiveness. No type of intervention could clearly be identified as superior to others.
Our review was limited to the care of established illness (i.e. excluding primary prevention), and to vocational outcomes rather than symptoms, function or quality of life. Nevertheless, it covered a broad range of interventions for variously defined case-groups. Because of this heterogeneity, we judged that formal meta-analysis was inappropriate, and instead summarised study results descriptively. An alternative approach would have been to specify our inquiry more tightly in terms of case definition and type of intervention. However, our findings indicate that such restriction would have identified few studies sufficiently similar for useful combined statistical analysis. Moreover, even if such analysis were possible, estimates of benefit would have been small, since magnitudes of effect were small across all of the available studies (e.g. Figure 2).
Other methodological limitations relate to the challenges in identifying all relevant publications and in assessing study quality. However, we screened citations from 35 systematic reviews as well as conducting our own electronic search, and quality assessment followed a well accepted system employed by the Cochrane collaboration. Some studies provided (non-independent) estimates of effect for the same intervention at multiple follow-up times, but findings were stratified by length of follow-up (Table 3), and sensitivity analysis restricting observations to the earliest (or latest) follow-up interval had little impact on estimates of the magnitude of effect (data available on request).
Implications for practice and research
According to the GRADE framework, “if clinicians believe that benefits and risks and burdens are finely balanced, or appreciable uncertainty exists...they must offer a ‘weak’ recommendation” [66]. Ahead of this, GRADE categorises the overall quality of evidence from ‘high’ through to ‘very low’, with judgement as to the likelihood of further research eroding confidence in estimated effects. Without the need for a full formal GRADE review process it is evident that at best only ‘weak’ recommendations can be framed on the basis of our review. The current evidence, comprising largely RCTs, might be assessed provisionally as ‘high’ in quality, but penalty scale downgrades for methodological weaknesses, imprecise data and potential for publication bias would result in a low quality of evidence as defined by GRADE. In addition, the limited and inconclusive cost-benefit data, the modest estimates of effect, and their statistical compatibility with no effect in the strongest studies, mandate weak recommendations.
In framing its recommendations for practice, NICE suggested various possible actions by employers and stakeholders – consideration of, for example: referral to a physiotherapist, rehabilitation specialist, occupational physician or GP; appointing a case manager; employing intensive multidisciplinary treatment over several weeks; small group CBT; or back school education programmes with advice on managing stress and coping [4]. The action to ‘consider’ such measures probably constitutes a ‘weak’ recommendation by the GRADE definition. However, recommendations were not framed in the context of known limitations or with quantitative estimates of uncertainty. Our findings suggest application of this recommendation would at most produce only small benefits in earlier return to work and reduction in sick leave.
We prefer more circumspect conclusions. Resource intensive approaches were ineffective in the studies reviewed. Brief treatments (<12 weeks) were offered more frequently to short-term (<12 weeks) than to long-term absentees (P=0.04). Thus, the finding may reflect in part an effect of case severity (more resource and lengthier treatments being deployed in difficult chronic cases); even so, it is difficult to advocate such interventions for acute cases with a generally more favourable prognosis. Beyond this, the benefits suggested are small and of uncertain cost-effectiveness; many interventions may work, but perhaps only modestly. Practice should be consistent with the balance of available scientific evidence on benefits, costs and adverse effects. However, in the face of doubtful cost-effectiveness, employers must make choices according to their value judgements about the uncertainties. Simple options will appear attractive to some if they are feasible, inexpensive, unlikely to do harm and with potential to be beneficial. If expensive interventions are contemplated, they should be evaluated rigorously, with assessment of costs as well as benefits planned from the outset.
Recommendations for further research can be firmer. As well as strengthening the methodological quality of reports, some gaps in evidence are important to address. A report by Dame Carol Black to the UK Secretaries of State for Health and for Work and Pensions emphasised the large costs of unemployment and sickness absence, and the adverse social and health consequences for unemployed individuals, families and local communities [67]. Further research on measures to promote job retention is urgently needed. Also, there is a need to focus more on the cost-effectiveness of interventions to improve vocational outcomes; pro-active measures are most likely to come from employers, and are most easily justified where there is convincing evidence of a return on investment. In choosing which interventions to evaluate, there are advantages in focussing on, and some evidence in support of, simple low cost measures with a workplace and/or a primary care element. These are unlikely to do harm and may have a better chance of proving cost-effective. By contrast, exercise therapy and interventions aimed at the individual without recourse to changes in work organisation and the working environment have been well studied and are likely at best to have only small benefits, while labour intensive measures are of low priority, given their cost and limited benefit.
Supplementary Material
Key messages.
Most reports of community- and workplace-based Interventions in workers with musculoskeletal disorders indicate reduced sickness absence and job loss.
However, the benefits are small and their cost-effectiveness is uncertain.
Acknowledgements
A full technical report on this work, of which this is an abridged summary, was commented on by expert and lay consultees in correspondence and at a dedicated workshop. We thank the following for their comments: Kim Burton, Peter Buckle, Peter Croft, Gary Macfarlane, Chris Main, Ira Madan, Eira Viikari-Juntura, Alex Burdorf, Sigurd Mikkelsen, Jos Verbeek, Bart Koes, Karen Walker-Bone (research active consultees); John Osman and David Lewis (HSE); Hugh Robertson (TUC); Cynthia Atwell (RCN); Margaret Barrett (NHS Confederation); Siobbain McCurrach (Arthritis Care); Olivia Carlton (Transport for London); Su Wang (independent practitioner); and Alan Silman (Arthritis Research UK). We also wish to acknowledge Janet Cushnaghan’s support in checking the coding of exercise interventions and Miranda Kim for her statistical advice.
Funding: This study was funded by the research charity Arthritis Research UK, with the aim of informing its commissioning strategy.
Footnotes
Conflicts of interest: None.
Contributions: KTP, DC and CC designed the study. KTP executed it, with advice from DC and CC, and support from CH and CL in searches and in data abstraction, data coding and data checking. MB and WL classified the psychological and behavioural interventions. KTP wrote the first draft of the manuscript and all the authors contributed to its revision. KTP acts as a guarantor for the work.
References
- 1.Economic and Social Data Service Nesstar Catalogue http://nesstar.esds.ac.uk/webview/(accessed 9/08/10)
- 2.Health and Safety Executive Self-reported work-related illness (SWI) and workplace injuries: Results from the Labour Force Survey (LFS) - Index of tables. http://www.hse.gov.uk/statistics/lfs/index.htm(accessed 9/08/10)
- 3.Waddell G, Burton AK. Is work good for your health and well-being? The Stationery Office; London: 2006. ISBN 0 11 7036943. [Google Scholar]
- 4.Hillage J, Rick J, Pilgrim H, Jagger N, Carroll C, Booth A. Evidence review 1: Review of the Effectiveness and Cost Effectiveness of Interventions, Strategies, Programmes and Policies to reduce the number of employees who move from short-term to long-term sickness absence and to help employees on long-term sickness absence return to work (May 2008) http://www.nice.org.uk/nicemedia/pdf/PH19EvidenceReview1.pdf(accessed 9/08/10)
- 5.National Institute for Health and Clinical Excellence . Managing Long-Term Sickness Absence and Incapacity for Work. NICE publications; London: 2009. NICE public health guidance 19. http://www.nice.org.uk/nicemedia/pdf/PH19Guidance.pdf(accessed 9/08/10) [Google Scholar]
- 6.Choi BKL, Verbeek JH, Tam WWS, Jiang JY. Cochrane Collaboration Review. Wiley & Sons Ltd; 2010. Exercises for prevention of recurrences of low-back pain. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD006555/pdf_fs.html(accessed 25/05/10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaafsma F, Schonstein E, Whelan KM, Ulvestad E, Kenny DT, Verbeek JH. Cochrane Collaboration Review. Wiley & Sons Ltd; 2010. Physical conditioning programs for improving work outcomes in workers with back pain. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001822/pdf_fs.html(accessed 25/05/10) [DOI] [PubMed] [Google Scholar]
- 8.Waddell G, Burton AK, Kendall NAS. Vocational Rehabilitation: What Works, for Whom and When? The Stationery Office; 2008. ISBN 9780117038615. http://www.workingforhealth.gov.uk/documents/vocational-rehabilitation.pdf(accessed 9/08/10) [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, for the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group for Spinal Disorders. Spine. 2003;28:1290–9. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008;27:379–87. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 12.Anderson B, Strand L, Raheim M. The effect of long-term awareness training succeeding a multimodal cognitive behaviour program for patients with widespread pain. J Musculoskeletal Pain. 2007;15(3):19–29. [Google Scholar]
- 13.Arnetz BB, Sjögren B, Rydéhn B, Meisel R. Early workplace intervention for employees with musculoskeletal-related absenteeism: a prospective controlled intervention study. J Occup Environ Med. 2003;45(5):499–506. doi: 10.1097/01.jom.0000063628.37065.45. [DOI] [PubMed] [Google Scholar]
- 14.Aure OF, Nilsen JH, Vasseljen O. Manual therapy and exercise therapy in patients with chronic low back pain: a randomized, controlled trial with 1-year follow-up. Spine. 2003;28(6):525–31. doi: 10.1097/01.BRS.0000049921.04200.A6. [DOI] [PubMed] [Google Scholar]
- 15.Bültmann U, Sherson D, Olsen J, Hansen CL, Lund T, Kilsgaard J. Coordinated and tailored work rehabilitation: a randomized controlled trial with economic evaluation undertaken with workers on sick leave due to musculoskeletal disorders. J Occup Rehabil. 2009;19:81–93. doi: 10.1007/s10926-009-9162-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AS-K, Hung LK. Randomized controlled trial of workplace-based rehabilitation for work-related rotator cuff disorder. J Occup Rehabil. 2007;17:487–503. doi: 10.1007/s10926-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 17.Faber E, Bierma-Zeinstra SM, Burdorf A, et al. In a controlled trial training general practitioners and occupational physicians to collaborate did not influence sick leave of patients with low back pain. J Clin Epidemiol. 2005;58(1):75–82. doi: 10.1016/j.jclinepi.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Fleten N, Johnsen R. Reducing sick leave by minimal postal intervention: a randomised, controlled intervention study. Occupational and Environmental Medicine. 2006;63(10):676–682. doi: 10.1136/oem.2005.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godges JJ, Anger MA, Zimmerman G, Delitto A. Effects of education on return-to-work status for people with fear-avoidance beliefs and acute low back pain. Phys Ther. 2008;88(17):231–9. doi: 10.2522/ptj.20050121. [DOI] [PubMed] [Google Scholar]
- 20.Haldorsen EMH, Kronholm K, Skouen JS, Ursin H. Predictors for outcome of a multi-modal cognitive behavioural treatment program for low back pain patients - a 12-month follow-up study. Eur J Pain. 1998;2(4):293–307. doi: 10.1016/s1090-3801(98)90028-3. [DOI] [PubMed] [Google Scholar]
- 21.Haldorsen EMH, Grasdal AL, Skouen JS, Risa AE, Kronholm K, Ursin H. Is there a right treatment for a particular patient group? Comparison of ordinary treatment, light multidisciplinary treatment, and extensive multidisciplinary treatment for long-term sick-listed employees with musculoskeletal pain. Pain. 2002;95:49–63. doi: 10.1016/s0304-3959(01)00374-8. [DOI] [PubMed] [Google Scholar]
- 22.Skouen JS, Grasdal AS, Haldorsen EMH, Ursin H. Relative cost-effectiveness of extensive and light multidisciplinary treatment programs versus treatment as usual for patients with chronic low back pain on long-term sick leave. Spine. 2002;27(9):901–10. doi: 10.1097/00007632-200205010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Skouen JS, Kvale A. Different outcomes in subgroups of patients with long-term musculoskeletal pain. Norsk Epidemiologi. 2006;16(2):127–135. [Google Scholar]
- 24.Haugli L, Steen E, Laerum E, Nygard R, Finset A. Learning to have less pain - is it possible? A one-year follow-up study of the effects of a personal construct group learning programme on patients with chronic musculoskeletal pain. Patient Educ Couns. 2001;45(2):111–8. doi: 10.1016/s0738-3991(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 25.Heymans MW, de Vet HCW, Bongers PM, Knol DL, Koes BW, van Mechelen W. The effectiveness of high-intensity versus low-intensity back schools in an occupational setting. Spine. 2006;31(10):1075–82. doi: 10.1097/01.brs.0000216443.46783.4d. [DOI] [PubMed] [Google Scholar]
- 26.Hlobil H, Staal JB, Twisk J, et al. The effects of a graded activity intervention for low back pain in occupational health on sick leave, functional status and pain: 12-Month results of a randomized controlled trial. J Occupl Rehab. 2005;15(4):569–80. doi: 10.1007/s10926-005-8035-y. [DOI] [PubMed] [Google Scholar]
- 27.Staal JB, Hlobil H, Twisk JWR, Smid T, Köke AJA, van Mechelen W. Graded activity for low back pain in occupational health care. Ann of Intern Med. 2004;140:77–84. doi: 10.7326/0003-4819-140-2-200401200-00007. [DOI] [PubMed] [Google Scholar]
- 28.Hlobil H, Uegaki K, Staal JB, de Bruyne M, Smid T, van Mechelen W. Substantial sick-leave cost savings due to graded activity intervention for workers with non-specific sub-acute low back pain. Eur Spine J. 2007;16:919–24. doi: 10.1007/s00586-006-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staal JB, Hlobil J, Köke AJA, Twisk JWR, Smid T, van Mechelen W. Graded activity for workers with low back pain: who benefits most and how does it work? Arthritis Rheum. 2008;59(5):642–9. doi: 10.1002/art.23570. [DOI] [PubMed] [Google Scholar]
- 30.Jensen I, Dahlquist C, Nygren A, Royen E, Stenberg M. Treatment for ‘helpless’ women suffering from chronic spinal pain: A randomised controlled 18 month follow up study. J Occup Rehabil. 1997;7(4):225–38. [Google Scholar]
- 31.Jensen IB, Bergström G, Ljungquist T, Bodin L, Nygren AL. A randomized controlled component analysis of a behavioural medicine rehabilitation program for chronic spinal pain: are the effects dependent on gender? Pain. 2001;91:65–78. doi: 10.1016/s0304-3959(00)00420-6. [DOI] [PubMed] [Google Scholar]
- 32.Jensen IB, Bergstrom G, Ljungquist T, Bodin L. A 3-year follow-up of a multidisciplinary rehabilitation programme for back and neck pain. Pain. 2005;115(3):273–83. doi: 10.1016/j.pain.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Kääpä EH, Frantsi K, Sarna S, Malmivaara A. Multidisciplinary group rehabilitation versus individual physiotherapy for chronic nonspecific low back pain. Spine. 2006;31(4):371–6. doi: 10.1097/01.brs.0000200104.90759.8c. [DOI] [PubMed] [Google Scholar]
- 34.Lambeek LC, van Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ. 2010;340:1–7. doi: 10.1136/bmj.c1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindh M, Lurie M, Sanne H. A randomized prospective study of vocational outcome in rehabilitation of patients with non-specific musculoskeletal pain: a multidisciplinary approach to patients identified after 90 days of sick-leave. Scand J Rehabil Med. 1997;29(2):103–12. [PubMed] [Google Scholar]
- 36.Lindström I, Öhlund C, Eek C, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant-conditioning behavioural approach. Physical therapy. 1992;72(4):279–93. doi: 10.1093/ptj/72.4.279. [DOI] [PubMed] [Google Scholar]
- 37.Lindström I, Öhlund C, Eek C, Wallin L, Petersen L-E, Nachemson A. Mobility, strength, and fitness after a graded activity program for patients with subacute low back pain. Spine. 1992b;17(6):641–9. doi: 10.1097/00007632-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lindström I, Öhlund C, Nachemson A. Physical performance, pain, pain behaviour and subjective disability in patients with subacute low back pain. Scand J Rehabil Med. 1995;27:153–60. [PubMed] [Google Scholar]
- 39.Loisel P, Abenhaim L, Durand P, et al. A population-based, randomized clinical trial on back pain management. Spine. 1997;22(24):2911–2918. doi: 10.1097/00007632-199712150-00014. [DOI] [PubMed] [Google Scholar]
- 40.Loisel P, Lemaire J, Poitras S, et al. Cost-benefit and cost-effectiveness analysis of a disability prevention model for back pain management: a six year follow up study. Occupational Environmental Medicine. 2002;59:807–15. doi: 10.1136/oem.59.12.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marhold C, Linton SJ, Melin L. A cognitive-behavioural return-to-work program: effects on pain patients with a history of long-term versus short-term sick leave. Pain. 2001;91(1-2):155–63. doi: 10.1016/s0304-3959(00)00431-0. [DOI] [PubMed] [Google Scholar]
- 42.Meijer EM, Sluiter JK, Heyma A, Sadiraj K, Frings-Dresen MH. Cost-effectiveness of multidisciplinary treatment in sick-listed patients with upper extremity musculoskeletal disorders: a randomized, controlled trial with one-year follow-up. Int Arch Occup Environ Health. 2006;79(8):654–64. doi: 10.1007/s00420-006-0098-3. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell RI, Carmen GM. The functional restoration approach to the treatment of chronic pain in patients with soft tissue and back injuries. Spine. 1994;19(6):633–642. doi: 10.1097/00007632-199403001-00001. [DOI] [PubMed] [Google Scholar]
- 44.Hagen E Molde, Grasdal A, Eriksen HR. Does early intervention with a light mobilization program reduce long-term sick leave for low back pain: a 3-year follow-up study. Spine. 2003;28(20):2309–15. doi: 10.1097/01.BRS.0000085817.33211.3F. [DOI] [PubMed] [Google Scholar]
- 45.Niemisto L, Rissanen P, Sarna S, Lahtinen-Suopanki T, Lindgren KA, Hurri H. Cost-effectiveness of combined manipulation, stabilizing exercises, and physician consultation compared to physician consultation alone for chronic low back pain: a prospective randomized trial with 2-year follow-up. Spine. 2005;30(10):1109–15. doi: 10.1097/01.brs.0000162569.00685.7b. [DOI] [PubMed] [Google Scholar]
- 46.Nystuen P, Hagen KB. Solution-focused intervention for sick listed employees with psychological problems or muscle skeletal pain: a randomised controlled trial [ISRCTN39140363] BMC Public Health. 2006;6:69. doi: 10.1186/1471-2458-6-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roelofs PDDM, Bierma-Zeinstra SMA, van Poppel MNM, et al. Lumbar supports to prevent recurrent low back pain among home care workers. Ann Intern Med. 2007;147(10):685–92. doi: 10.7326/0003-4819-147-10-200711200-00004. [DOI] [PubMed] [Google Scholar]
- 48.Scheel IB, Hagen K Birger, Herrin J, Carling C, Oxman AD. Blind faith? The effects of promoting active sick leave for back pain patients: A cluster-randomized controlled trial. Spine. 2002;27(23):2734–40. doi: 10.1097/00007632-200212010-00014. [DOI] [PubMed] [Google Scholar]
- 49.Schultz IZ, Crook J, Berkowitz J, Milner R, Meloche GR, Lewis ML. A prospective study of the effectiveness of early intervention with high-risk back-injured workers – a pilot study. J Occup Rehabil. 2008;18:140–51. doi: 10.1007/s10926-008-9130-7. [DOI] [PubMed] [Google Scholar]
- 50.Soukup MG, Glomsrod B, Lonn JH, Bo K, Larsen S. The effect of a Mensendieck exercise program as secondary prophylaxis for recurrent low back pain: a randomised, controlled trial with 12 month follow-up. Spine. 1999;24(15):1585–92. doi: 10.1097/00007632-199908010-00013. [DOI] [PubMed] [Google Scholar]
- 51.Stankovic R, Johnell O. Conservative treatment of acute low-back pain. A prospective randomized trial: McKenzie method of treatment versus patient education in ‘Mini back school’. Spine. 1990;15(2):120–3. [PubMed] [Google Scholar]
- 52.Steenstra IA, Anema JR, van Tulder MW, Bongers PM, de Vet HCW, van Mechelen W. Economic evaluation of a multi-stage return to work program for workers on sick-leave due to low back pain. J Occup Rehabil. 2006;16(4):557–78. doi: 10.1007/s10926-006-9053-0. [DOI] [PubMed] [Google Scholar]
- 53.Anema JR, Steenstra IA, Bongers PM, et al. Multidisciplinary rehabilitation for subacute low back pain: graded activity or workplace intervention or both? Spine. 2007;32(3):291–8. doi: 10.1097/01.brs.0000253604.90039.ad. [DOI] [PubMed] [Google Scholar]
- 54.Steenstra IA, Knol DL, Bongers PM, Anema JR, van Mechelen W, de Vet HCW. What works best for whom? Spine. 2009;34(12):1243–9. doi: 10.1097/BRS.0b013e3181a09631. [DOI] [PubMed] [Google Scholar]
- 55.Torstensen TA, Ljunggren AE, Meen HD, Odland E, Mowinckel P, Geijerstam S. Efficiency and costs of medical exercise therapy, conventional physiotherapy, and self-exercise in patients with chronic low back pain: A pragmatic, randomized, single-blinded, controlled trial with 1-year follow-up. Spine. 1998;23(23):2616–24. doi: 10.1097/00007632-199812010-00017. [DOI] [PubMed] [Google Scholar]
- 56.van den Hout JH, Vlaeyen JW, Heuts PH, Zijlema JH, Wijnen JA. Secondary prevention of work-related disability in nonspecific low back pain: Does problem-solving therapy help? A randomized clinical trial. Clin J Pain. 2003;19(2):87–96. doi: 10.1097/00002508-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Verbeek JH, van der Weide WE, van Dijk FJ. Early occupational health management of patients with back pain: a randomized controlled trial. Spine. 2002;27(17):1844–51. doi: 10.1097/00007632-200209010-00006. [DOI] [PubMed] [Google Scholar]
- 58.Brown KC, Sirles AT, Hilyer JC, Thomas MJ. Cost-effectiveness of a back school intervention for municipal employees. Spine. 1992;17(10):1224–8. doi: 10.1097/00007632-199210000-00016. [DOI] [PubMed] [Google Scholar]
- 59.Durand MJ, Loisel P. Therapeutic Return to Work: Rehabilitation in the workplace. Work. 2000;17(1):57–63. [PubMed] [Google Scholar]
- 60.Feuerstein M, Callan-Harris S, Hickey P, Dyer D, Armbruster W, Carosella AM. Multidisciplinary rehabilitation of chronic work-related upper extremity disorders: Long-term effects. J Occup Med. 1993;35(4):396–403. [PubMed] [Google Scholar]
- 61.Gard F, Gille K, Grahn B. Functional activities and psychosocial factors in the rehabilitation of patients with low back pain. Scandinavian Journal of Caring Science. 1999;14:75–81. [PubMed] [Google Scholar]
- 62.Jensen I, Bodin L. Multimodal cognitive-behavioural treatment for workers with chronic spinal pain: A matched cohort study with an 18-month follow-up. Pain. 1998;76(1-2):35–44. doi: 10.1016/s0304-3959(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 63.Klemetti M, Santavirta N, Sarvimäki A, Björvell H. Tension neck and evaluation of a physical training course among workers in a bank corporation. J Adv Nurs. 1997;26:962–7. doi: 10.1046/j.1365-2648.1997.00390.x. [DOI] [PubMed] [Google Scholar]
- 64.Leino P, Kivekäs J, Hänninen K. Effects of work-oriented fitness courses in lumberjacks with low back pain. J Occup Rehabil. 1994;4(2):67–76. doi: 10.1007/BF02110046. [DOI] [PubMed] [Google Scholar]
- 65.Sinclair SJ, Hogg-Johnson SH, Mondloch MV, Shields SA. The effectiveness of an early active intervention program for workers with soft-tissue injuries. The Early Claimant Cohort Study. Spine. 1997;22(24):2919–31. doi: 10.1097/00007632-199712150-00015. [DOI] [PubMed] [Google Scholar]
- 66.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Black C. Working for a healthier tomorrow. The Stationery Office; London: 2008. Dame Carol Black’s Review of the health of Britain’s working age population. ISBN 978011702513 4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.