Abstract
The attrition of telomeres, the ends of eukaryote chromosomes, is thought to play an important role in cell deterioration with advancing age. The observed variation in telomere length among individuals of the same age is therefore thought to be related to variation in potential longevity. Studies of this relationship are hampered by the time scale over which individuals need to be followed, particularly in long-lived species where lifespan variation is greatest. So far, data are based either on simple comparisons of telomere length among different age classes or on individuals whose telomere length is measured at most twice and whose subsequent survival is monitored for only a short proportion of the typical lifespan. Both approaches are subject to bias. Key studies, in which telomere length is tracked from early in life, and actual lifespan recorded, have been lacking. We measured telomere length in zebra finches (n = 99) from the nestling stage and at various points thereafter, and recorded their natural lifespan (which varied from less than 1 to almost 9 y). We found telomere length at 25 d to be a very strong predictor of realized lifespan (P < 0.001); those individuals living longest had relatively long telomeres at all points at which they were measured. Reproduction increased adult telomere loss, but this effect appeared transient and did not influence survival. Our results provide the strongest evidence available of the relationship between telomere length and lifespan and emphasize the importance of understanding factors that determine early life telomere length.
Keywords: aging, Taeniopygia guttata, senescence, bird
Understanding the physiological processes underlying variation in lifespan is of central importance to evolutionary ecology, biomedical research, and conservation science (1). One cellular mechanism thought to be particularly important in this regard is the attrition of telomeres (2). Telomeres are highly conserved, noncoding, repetitive sequences of DNA that, together with a number of shelterin proteins, form caps at the ends of eukaryotic chromosomes, enabling chromosome ends to be distinguished from double-stranded breaks (3). In the absence of restoration, telomeres shorten during each round of normal somatic cell division because RNA polymerase cannot completely replicate the lagging strand (3, 4). The loss of DNA from the telomeric cap protects the coding sequences from attrition and also limits cell replicative potential; once telomeres reach a critically shortened length, cells stop dividing and enter a state of replicative senescence (3, 5). Telomere loss can be prevented by the enzyme telomerase, a ribonucleoprotein that adds telomeric sequences to telomere ends (6). However, in long-lived mammals, after embryogenesis, telomerase is down-regulated in most somatic tissues, which is thought to have evolved as a tumor-suppressing mechanism (7). The pattern in birds is less well understood, but in some species, like the zebra finch Taeniopygia guttata, the pattern is similar to that in long-lived mammals (8–10). Progressive telomere shortening has been linked to both normal aging and to various degenerative diseases in a wide range of studies mainly in humans and mice (2), and telomere lengthening has been shown to have a restorative effect (11). Thus, intraspecific variation in age-specific telomere length is expected to be a good indicator of biological age.
Average telomere length in cell samples has been found to decrease with donor age in a number of cell types in diverse taxa (12, 13). Individuals with longer telomeres have been reported to have a longer subsequent lifespan in some studies of vertebrate species, with the predictive power of age per se being lower (12, 13). Other studies have not found such a relationship, and the results in humans in particular have been mixed (14–16). However, because most of these studies have been cross-sectional (i.e., repeat samples were not collected from the same individuals throughout life) it is not possible to separate within-individual effects from population-level processes such as cohort effects or prior selection events producing sample biases. The few longitudinal studies that do exist suggest that telomere attrition is greatest during early life when growth and development are still occurring (17–20). Those studies that have examined the relationship between telomere dynamics and survival have measured telomere length at a single life-stage (early or late in life) and/or monitored survival over a short period of the lifespan. In the case of human studies, telomere measurement has often been made when the individuals involved were already old or very old (13). Accordingly, we do not know whether those individuals with relatively long telomeres early in life are those whose total life expectancy is greatest nor have we yet been able to assess whether biases are created when only relatively old individuals are sampled. Furthermore, because variation in the rate at which telomere loss occurs may be indicative of variation in exposure to agents that cause more widespread damage to macromolecules, longitudinal studies are also needed to assess whether telomere length or loss rate is a better predictor of longevity (12, 13). The key studies that need to be done to address these questions require telomere length to be monitored from early life in groups of normally aging individuals whose natural lifespans are then recorded.
Individuals of the same age generally vary greatly in telomere length (13). Some of this variation is due to genetic factors (21), but the environmental factors that influence telomere loss are also likely to be very important (22–25). Evidence is accumulating that some of these environmental effects are likely to be mediated by exposure to oxidative stress, which might accelerate telomere loss (26, 27) or increase cell turnover rates (28). Metabolically expensive activities such as reproduction might increase telomere loss if individuals are unable to up-regulate antioxidant defenses and/or activate telomere restoration processes to the required extent. Because individuals might adjust reproductive effort to their physiological capability, thereby minimizing adverse effects, experimental manipulations of effort are essential if we are to understand the mechanisms causing telomere attrition. Such data are still very limited. In mice, females that were allowed to breed then had shorter telomeres than females that were not (29); on the other hand, in Adelie penguins (Pygoscelis adeliae), an experimentally increased reproductive workload was not found to influence telomere length, but this could be because the manipulation was not sufficient to increase oxidative stress levels in this relatively long-lived species (30).
In this study, we examined telomere length in red blood cell samples from early in life (at 25 d) and at various points thereafter in a group of zebra finches (n = 99) that were allowed to live out their natural lifespan (ranging from 210 d to 8.7 y). We also examined the effect of reproduction on adult telomere length, by experimentally manipulating whether, and how often, individuals were allowed to breed. These data enabled us to uniquely examine the relationship between telomere dynamics from early in life and total lifespan and reveal that telomere length in early life is a highly significant predictor of the age of death.
Results
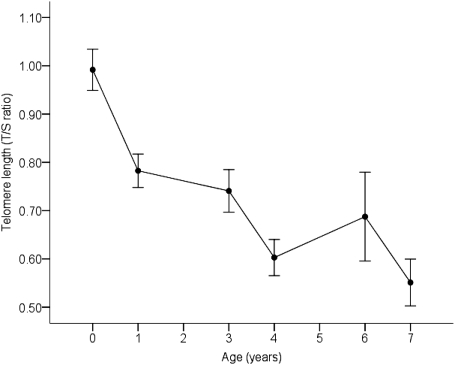
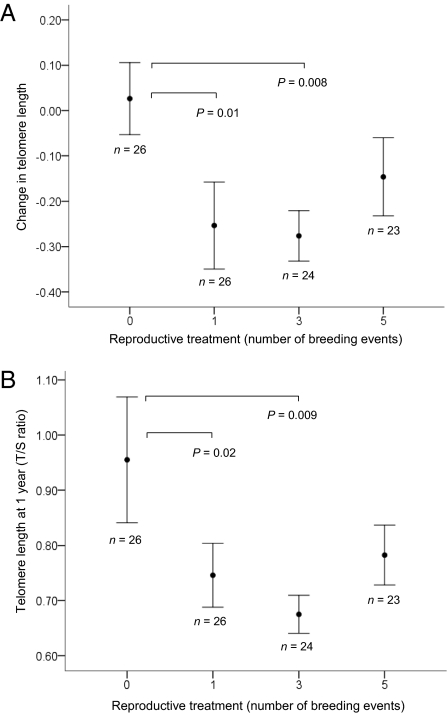
Telomere length declined with age (F5, 158.92 = 20.92, P < 0.001), with loss being most marked during the first year (Fig. 1). The pattern did not differ significantly between the sexes (F1, 81.22 = 1.571, P = 0.214). Data plotted at the individual level are shown in Fig. S1. We found that engaging in reproduction accelerated telomere shortening. Those birds that bred had a greater average telomere attrition between 25 d and 1 y than those that were prevented from reproducing during this period (F3, 73.852 = 3.55, P = 0.018, Fig. 2A); the number of times they were allowed to breed during that year had no effect. Consequently, the birds in the reproductive treatments had significantly shorter telomeres at 1 y than those in the nonreproductive treatment (F3, 72.29 = 2.739, P = 0.050, Fig. 2B). However, this effect of reproduction on telomere length did not persist; when telomere length was measured at the next sampling point (3 y, by which time the reproductive treatment had ceased), there was no measurable effect of reproduction on telomere length (F3, 20.10 = 0.762, P = 0.528), but the number of birds measured at this point was reduced.
Fig. 1.
Relationship between mean telomere length (± SEM, measured by T/S ratio using qPCR) and age at measurement in 99 zebra finches. The first sample (shown as year = 0) was collected at 25 d. The data are plotted at the individual level in Fig. S1.
Fig. 2.
(A) Mean change in telomere length (measured using qPCR) between 25 d and 1 y (± SEM) for zebra finches in each reproductive treatment group. Birds were randomly assigned to reproductive treatment groups (allowed to breed zero, one, three, or five times) at 100 d of age. P values are based on least significant difference (LSD) post hoc tests and are only shown where significant at P < 0.05. (B) Mean telomere length (T/S ratio from qPCR) at 1 y (± SEM) for zebra finches in each reproductive treatment group. P values are based on least significant difference (LSD) post hoc tests and are only shown where significant at P < 0.05.
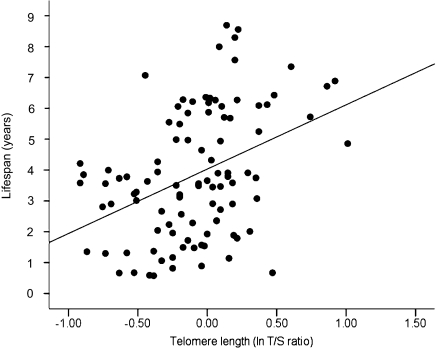
There was a highly significant relationship between early life telomere length and longevity: individuals that had longer telomeres at 25 d had a significantly longer lifespan (F1, 86.11 = 16.75, P < 0.001, Fig. 3). There was substantial variation in the magnitude of telomere loss over the first year of life (mean change in telomere repeat copy number/single control gene copy number, T/S ratio = −0.179, SD = 0.374, CV 48%). For those birds sampled at 1 y of age (n = 79), subsequent lifespan was not related to the change in telomere length between 25 d and 1 y or to reproductive treatment or sex (all P > 0.17). Telomere length at 1 y was a significant predictor of subsequent lifespan (F1, 74.51 = 5.16, P = 0.026), but the relationship was weaker than at 25 d and ceased to be significant when the telomere length of the birds at 25 d was included in the model (telomere length at 25 d: F1, 72.97 = 14.65, P = 0.003; telomere length at 1 y: F1, 69.64 = 0.614, P = 0.436; interaction between telomere length at 25 d and 1 y: P = 0.869). There was no significant relationship between telomere length at any of the other time points (i.e., 3, 4, 6, and 7 y) and subsequent lifespan (all P > 0.17), although the sample size at these points is reduced.
Fig. 3.
Relationship between natural log-transformed relative telomere length (T/S ratio from qPCR) at 25 d and lifespan in zebra finches (n = 99).
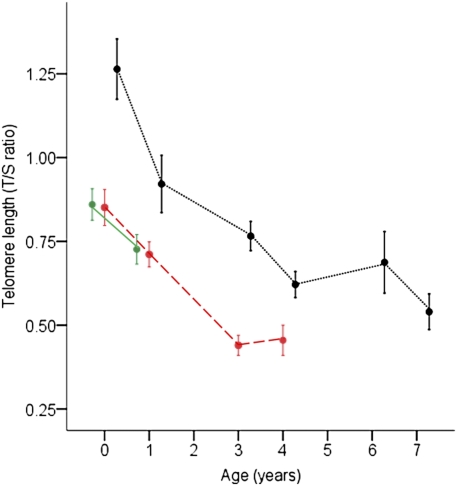
Fig. 4 clearly shows that individuals that go on to live to older ages are those with initially longer telomere lengths, whereas those with initially shorter telomeres are more likely to disappear first from the population. Birds in the longest lifespan category had consistently longer telomere lengths at the points at which they were measured than the birds in the other two lifespan categories (Fig. 4). Fig. 4 also illustrates the sampling bias that occurs when only old individuals are measured, because these are a subset of individuals that have relatively long telomeres from early in life.
Fig. 4.
Relationship between mean (± SEM) telomere length (T/S ratio from qPCR) and age at measurement (first sample, shown as year = 0, was collected at 25 d) in zebra finches in three lifespan categories. Lifespan categories were created by dividing the 99 zebra finches into three equal groups on the basis of their age at death. Birds in the shortest lifespan group (n = 33) lived for an average of 1.6 y and are represented by the solid, green line; birds in the middle lifespan group (n = 33) lived for an average of 3.6 y and are represented by the dashed, red line; and birds in the longest lifespan group (n = 33) lived for an average of 6.3 y and are represented by the dotted, black line. Birds in the shortest and middle lifespan groups did not differ in telomere length at the first two sampling points (at 25 d, P = 0.99 and at 1 y, P = 0.85). Birds in the longest-lived group had consistently longer telomeres than those in the other two groups where sample sizes permitted comparisons (at 25 d, P < 0.001; at 1 y, P = 0.02; and at 3 y, P = 0.05). P values are based on Fisher's least significant difference (LSD) tests and significant differences, *P < 0.05.
Discussion
Although reduced telomere length has been associated with a number of degenerative diseases in humans, there has been increasing interest in their role in the aging process in otherwise normal individuals (31). The results of this study clearly show that telomere length early in life is predictive of longevity. Previous studies of the relationship between telomere length and lifespan have only tracked survival for a relatively short period after sampling and have been based on individuals sampled either relatively early or late in life (13). In this study, we were able to examine the relationship between telomere length measured early in life (i.e., at 25 d, toward the end of the main growth period), and at various time points thereafter, and subsequent lifespan. Those individuals with longer telomeres at 25 d went on to have longer lives. As is evident from Fig. 3, we had substantial variation in lifespan among the birds in our sample, even though this sample involved a captive population, with some birds dying within their first year of life and others living to almost 9 y of age. As is evident from Fig. 3, telomere length, although predictive of lifespan, does not explain all of the variation observed. In addition to other, unrelated factors, there is likely to be a stochastic element to which body systems fail first when telomere lengths start to become relatively short, and so the actual cause of death may differ among individuals. Predation, starvation, and obvious signs of infection were not involved, so the birds appear to have died from intrinsic causes. We do not know the cause of death in these birds, and it is of course possible that those individuals that died relatively early in life died from internal disorders less likely to be related to telomere length. However, even if we exclude the birds from the shortest lifespan category (i.e., the third of the population with the shortest lifespans) from this analysis, telomere length at 25 d remains a very strong predictor of lifespan (P < 0.001). The effect of early life telomere length on longevity may be a consequence of inherited variation in telomere length or variation in telomere loss during the early growth period itself, and both are likely to be involved. There is a substantial inherited component to telomere length (21), and environmental conditions are also known to affect the rate of telomere loss (22, 23, 32). The pattern of early growth, and exposure to stress in early life, have been associated with reduced telomere length in early adulthood (25, 33). Because these early life effects are known to induce changes in oxidative stress pathways (34), which are known to affect telomere loss (2), this seems the most likely route whereby conditions before 25 d would influence telomere length. Variation in the degree of exposure and response to such early life effects may be a consequence of gene-by-environment interactions or stochastic processes (35, 36). Detailed studies are required to tease apart direct genetic, maternal, and direct environmental effects on early life telomere length.
This positive relationship between telomere length and lifespan became weaker at later sampling ages. The power to detect the relationship between telomere length and lifespan in our analyses was most certainly reduced at later ages because of decreasing sample sizes and the loss of variation on both axes—telomere length and lifespan. However, in the 79 birds whose telomere length was measured at both 25 d and at 1 y of age, the 25-d telomere length remained the best predictor of lifespan, and telomere length at 1 y did not have any additional explanatory power. Following the method used by Entringer et al. for humans (25), we can calculate a rough estimate of telomere loss in kilobases over the first year of life, on the basis of the regression equation relating T/S ratio to telomere length in base pairs given by Criscuolo et al. (37). On average, telomere length at 25 d varied between around 18 and 29 kb and telomere length decreased by 1,300 bp during the first year and around 500 bp per year across the remaining 6 y, which is consistent with other studies of telomere loss rates in this species (38). Telomere loss could be related to life expectancy independently of its effect on telomere length, because loss rate might be indicative of more general exposure to stressful physiological conditions, which could accelerate telomere loss and also cause more extensive oxidative damage to cells and tissues. In our study, however, telomere loss, measured during the first year of life, was not predictive of subsequent survival, and loss rate did not explain any additional variation in observed lifespan over that explained by telomere length.
Our data clearly show that when telomere length is measured at later life stages, selective loss of individuals with shorter telomere lengths has already occurred. When individuals were divided into thirds on the basis of their longevity, it is readily apparent that those in the longest-lived third represent a skewed subset with respect to their telomere length. Those individuals still alive at later ages are those that initially had longer telomere lengths, and these long-lived birds retained relatively long telomeres at each point at which they were measured. These results highlight the considerable bias that can be introduced by restricting sampling to a single life-stage, particularly individuals that live to be quite old. Our data show that it is likely to be more difficult to find a relationship with survival the later in life that telomere length is measured and therefore offer a possible explanation for why studies do not always find a positive relationship between telomere length and subsequent survival (39–41).
This study is primarily concerned with organismal level outcomes of changes in telomere length and provides a definitive “yes” to the question of whether telomere length is predictive of lifespan in otherwise healthy individuals; our data clearly show that it is length in early life that is most predictive. Whether telomere length change itself plays a directly causative role in determining the pace of decline and age of death is an active area of research. Several mechanistic routes have been identified, mainly from in vitro studies, whereby shortened telomeres can accelerate aging and reduce longevity, primarily involving activation of cellular checkpoints of apoptosis, cell cycle arrest, and impaired stem cell and tissue function (2). A clear association between telomere dysfunction and the presence of significant numbers of senescent cells in older individuals has been demonstrated in vivo (42). Our study shows that the changes associated with reduced telomere length do have the predicted relationship with organism life expectancy.
We also found that engaging in reproduction accelerated telomere loss. In zebra finches, females have the extra burden of producing eggs, but both sexes incubate them (43), and incubation is known to be a relatively costly component of parental care (44, 45). Birds that were allowed to produce and incubate eggs, irrespective of sex or of the number of clutches produced, had greater telomere loss between 25 d and 1 y, and hence shorter telomeres at 1 y, than birds that did not breed. This is consistent with a previous study in mice in which females that bred experienced greater telomere loss and consequently had shorter telomeres than females that did not (29). Reproduction can increase exposure to oxidative stress (34), which could accelerate telomere loss. It is possible that it is being in a reproductive state (with the concomitant substantial hormonal and body composition changes that occur, refs. 46–48) that causes an increase in telomere loss, rather than the level of reproductive effort. In support of this possible effect of being in a reproductive state, we found that greater breeding effort did not increase telomere loss, which is similar to the result reported in Adelie penguins (30). The effect of reproductive rate on telomere length did not, however, appear to be long lasting and reproductive rate was not predictive of lifespan.
The results of this study clearly show that telomere length early in life (at the end of the main growth period) is strongly predictive of lifespan. That those individuals living relatively long lives have demonstrably long telomeres from early in life highlights the biases introduced when telomere length is not measured until late in adult life. It also reveals why it will be more difficult to demonstrate links to life expectancy in such studies, because those individuals with the shortest telomeres will have already died. Further, telomere length early in life rather than in adulthood might be more important in predicting lifespan because it has more of an effect on subsequent tissue function. The extent to which variation in early-life telomere length is a consequence of genetic inheritance, maternal effects, and/or conditions during growth are important areas for future research.
Materials and Methods
Study Subjects.
Experimental birds were bred in 2001–2002 from randomly paired, adult stock birds taken from an out-bred captive population at the University of Glasgow. They were reared and subsequently maintained under standardized conditions as part of a study on the consequences of early diet on later performance (49–51). A brief summary of the early diet manipulation is as follows: after hatching, whole families were randomly assigned to receive either a standard-quality or lower-quality rearing diet for 15 d from the hatching of the first egg, after which they all received the same standard-quality diet. Food was always provided ad libitum, and the primary difference between the two diets was that the standard-quality diet had higher levels of protein, carotenoids, vitamin E, and vitamin A (ref. 49 provides a detailed description of nutrient composition).
Experimental Manipulation of Reproductive Effort.
When the birds were 100 d old, they were randomly assigned a mate from the same early diet treatment group and allocated to one of four reproductive categories: nonbreeding, or breeding one, three, or five times per year. The reproductive regimes lasted for 2.5 y. Breeding birds were always paired with the same mate if possible; if a pair member died during the 2.5-y treatment period, the remaining bird was assigned a new partner. During each breeding round, pairs in the breeding treatments were placed in their own breeding cage and allowed to build nests and lay eggs. Because the eggs were required for other purposes, they were collected shortly after laying and replaced with dummy eggs; accordingly, the birds did not rear any chicks. Once they had completed the normal period of incubation, pairs were separated and returned to single-sex holding cages; birds in the nonbreeding treatment group remained in single-sex holding cages (see ref. 50 for a detailed description). After the 2.5-y period of reproduction, all birds were kept in single-sex holding cages (five to eight birds per cage) until they died; birds were randomly allocated to these holding cages irrespective of diet or reproductive treatments. Cages were checked daily and the condition of all birds examined for any signs of injury or infection. The date of death of each bird was recorded and the body carefully examined for any signs of external injuries. Cause of death was generally unknown, but was not due to obvious signs of infection or injury. Interspecific aggression was minimal, and in the few cases where it did occur, the affected birds were transferred to other groups and the aggression ceased. As would be expected, because individuals were randomly allocated to the reproductive treatments, there were no differences among the reproductive treatment groups in their average telomere length at the measurement point before reproduction (25 d; F3, 85.17 = 0.367, P = 0.777).
Telomere Measurement.
Blood samples had been collected for other purposes from subsets of the birds by brachial venipuncture at various time points throughout their lives (25 d, 1, 3, 4, 6, and 7 y). These blood samples therefore gave us the opportunity to examine the relationship between telomere length in the nestling stage, and at various adult ages, and individual lifespan. The blood was collected into heparinized capillary tubes, centrifuged, separated, and the red cell samples snap frozen in liquid N2 before being stored at −80 °C until telomere analysis. We analyzed the telomere lengths in the red blood cells for all individuals (n = 99; 42 males and 57 females) that were first sampled at 25 d and had died of natural causes. Red cells are nucleated in birds; blood cells represent a proliferative tissue, and a number of studies in humans and mice have reported good correspondence in telomere length among proliferative somatic tissue types (52–58). Levels of telomerase are relatively low in red cells and in bone marrow in hatchling zebra finches, and almost undetectable thereafter (8); thus, avian red cells represent a good tissue in which to examine variation in telomere length. The sample size for measurement of telomere lengths later in life was gradually reduced because of natural mortality and because samples were not available for all of the birds at each of the later time points because in some cases, a particular sample for an individual had been lost or used for other purposes (n = 79 at 1 y, n = 26 at 3 y, n = 18 at 4 y, n = 12 at 6 y, and n = 9 at 7 y old).
We used the DNeasy Blood and Tissue kit (Qiagen) to extract genomic DNA from the red blood cells following the manufacturer's protocol. The quantity and purity of the DNA was measured using a Nanodrop 1000 spectrophotometer (Thermo Scientific) and we used the quantitative PCR method, as described in ref. 59 for measuring relative telomere length, as adapted for use in zebra finches in ref. 37. This method, which gives a relative value for telomere length, is widely used for interspecific comparisons; double blind studies have recently shown that it correlates well (r = 0.85, n = 50, P < 0.001) with measurements made on the same samples in different laboratories using the telomere restriction fragment (TRF) method (60). We found a highly significant correlation (r = 0.87, n = 26, P < 0.001) between telomere measurements of the same zebra finch samples by the TRF and quantitative PCR methods (37). Briefly, the relative telomere length of each sample was measured by determining the ratio (T/S) of telomere repeat copy number (T) to single control gene copy number (S), relative to a reference sample. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the single control gene. The following forward and reverse primers were used to amplify the telomere: Tel1b (5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′), Tel2b (5′-GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT-3′) and zebra finch-specific GAPDH sequences: GAPDH-F (5′-AACCAGCCAAGTACGATGACAT-3′), GAPDH-R (5′-CCATCAGCAGCAGCCTTCA-3′). The telomere and GAPDH reactions were carried out on separate plates, and in both reactions the number of PCR cycles (Ct) required for the products to accumulate enough fluorescent signal to cross a threshold was determined. Individuals with relatively short telomeres are characterized by high Ct values, whereas those with relatively long telomere are characterized by low Ct values (59). For detailed descriptions of the PCR conditions see ref. 37. To measure the efficiencies of the reactions, we included a standard curve, produced by serially diluting the same reference sample on every plate. The reference sample was of pooled red blood cells from six zebra finches that were ∼100 d old at the time of collection. In all cases, efficiencies were within an acceptable range (i.e., 100 ± 15%) and samples fell within the bounds of the standard curve. This same reference sample was used to set the Ct thresholds for each reaction and to calculate both the within- and among-plate variation. All samples, including the standard curve, were run in triplicate, and average values were used to calculate the relative T/S ratios for each sample relative to the reference sample according to the following formula: 2ΔΔCt, where ΔΔCt = (Cttelomere – CtGAPDH) reference − (Cttelomere – CtGAPDH) focal (introduction to qPCR: methods and application guide by Stratagene, 2007). The average intraplate variation of the Ct values was 1.46% for the telomere assays and 0.48% for the GAPDH assays, respectively, and the average interplate variation for the ΔCt values was 12.04%. To give an approximate value of the T/S ratio in base pairs (bp), we used the regression equation relating the two (37) (bp = 23.28 + 6.28 ⋅ T/S ratio).
Statistics.
Linear mixed effect models (LMEs) were used for all analyses. Variance components were estimated using a restricted maximum likelihood (REML) function and all models had normal error structures. Family was included as a random effect to control for siblings in the analyses. We used a backward elimination process to exclude variables with P > 0.05, starting with interactions, to produce minimum adequate models. Telomere lengths were log transformed to achieve a normal distribution and all other variables met the assumptions of the LME. We found no significant differences between the two early diet treatments and no interactions with reproductive treatment in either telomere lengths at any time point or in survival (P > 0.34 in all cases); hence, the early diet treatment was dropped from all models.
To examine whether telomere length changed with age and whether this varied between the sexes, we included bird identity as a random factor to control for repeated measurements from the same individuals, and age at sampling, and sex as fixed factors. To examine the potential influence of reproduction on telomere length at 1 and 3 y, and the change in telomere length between 25 d and 1 y, we included reproductive treatment, sex, and an interaction between sex and reproductive treatment as fixed factors. To identify where the differences among the four reproductive treatment groups occurred, we used Fisher's least significant difference (LSD) test.
In a separate analysis, we examined whether lifespan was influenced by the following fixed factors: telomere length (at 25 d and 1, 3, 4, 6, and 7 y), the change in telomere length between 25 d and 1 y, reproductive treatment, and sex. The period over which within-individual change in telomere length was measured was restricted to the first time period, as investigation of the effect of later time periods would, by definition, have excluded all birds with short lifespans, and also sample size was substantially reduced. (Fig. 3 shows distribution of ages of death). We ran a separate model for each measurement point because sample sizes were much smaller at later time points. Family had to be excluded from the models for telomere length at 3, 4, 6, and 7 y to get the models to resolve; however, if anything, this is a less conservative test and we report that there were no significant effects of telomere length at later ages on lifespan (Results).
To show the pattern of telomere change during their lives for individuals with differing lifespans, and to illustrate more clearly the bias introduced when sampling only individuals that live to be old, we divided the total sample of 99 birds into three equal-sized groups on the basis of their longevities (i.e., the shortest-lived third of the population, the middle third, and longest-lived third) and examined the differences in telomere length at the different measurement points for these three lifespan categories. All statistical analyses were performed in PASW Statistics 18 for Windows.
Supplementary Material
Acknowledgments
We are extremely grateful to G. Adams, D. Armstrong, A. Kirk, J. Laurie, G. Law, and H. Lepitak for assistance with animal husbandry. This work was funded by the Natural Environment Research Council and the European Research Council. B.J.H. and W.B. were also supported by Glasgow University Wellcome Trust Value in People Awards, B.J.H. by a National Science Foundation Postdoctoral Fellowship IRFP 0852962, and J.D.B. by a Royal Society University Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113306109/-/DCSupplemental
References
- 1.Ricklefs RE, Wikelski M. The physiology/life-history nexus. Trends Ecol Evol. 2002;17:462–468. [Google Scholar]
- 2.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blackburn EH. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 4.Vaziri H, et al. Evidence for a mitotic clock in human hematopoietic stem cells: Loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornsby PJ. Cellular senescence and tissue aging in vivo. J Gerontol A Biol Sci Med Sci. 2002;57:B251–B256. doi: 10.1093/gerona/57.7.b251. [DOI] [PubMed] [Google Scholar]
- 6.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 7.Gomes NMV, et al. Comparative biology of mammalian telomeres: Hypotheses on ancestral states and the roles of telomeres in longevity determination. Aging Cell. 2011;10:761–768. doi: 10.1111/j.1474-9726.2011.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haussmann MF, Winkler DW, Huntington CE, Nisbet ICT, Vleck CM. Telomerase expression is differentially regulated in birds of differing life span. Ann N Y Acad Sci. 2004;1019:186–190. doi: 10.1196/annals.1297.029. [DOI] [PubMed] [Google Scholar]
- 9.Holmes D, Martin K. A bird's-eye view of aging: What's in it for ornithologists? Auk. 2009;126:1–23. [Google Scholar]
- 10.Swanberg SE, et al. Telomere biology of the chicken: A model for aging research. Exp Gerontol. 2010;45:647–654. doi: 10.1016/j.exger.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Jaskelioff M, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Monaghan P. Telomeres and life histories: The long and the short of it. Ann N Y Acad Sci. 2010;1206:130–142. doi: 10.1111/j.1749-6632.2010.05705.x. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 15.Njajou OT, et al. Health ABC study Association between telomere length, specific causes of death, and years of healthy life in health, aging, and body composition, a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2009;64:860–864. doi: 10.1093/gerona/glp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atzmon G, et al. Evolution in health and medicine Sackler colloquium: Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc Natl Acad Sci USA. 2010;107(Suppl 1):1710–1717. doi: 10.1073/pnas.0906191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeichner SL, et al. Rapid telomere shortening in children. Blood. 1999;93:2824–2830. [PubMed] [Google Scholar]
- 18.Brümmendorf TH, et al. Longitudinal studies of telomere length in feline blood cells: Implications for hematopoietic stem cell turnover in vivo. Exp Hematol. 2002;30:1147–1152. doi: 10.1016/s0301-472x(02)00888-3. [DOI] [PubMed] [Google Scholar]
- 19.Hall ME, et al. Telomere loss in relation to age and early environment in long-lived birds. Proc Biol Sci. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baird DM. Telomeres II. Exp Gerontol. 2008;43:15–19. doi: 10.1016/j.exger.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Njajou OT, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci USA. 2007;104:12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 23.Haussmann MF, Marchetto NM. Telomeres: Linking stress and survival, ecology and evolution. Current Zoology. 2010;56:714–727. [Google Scholar]
- 24.Alder JK, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Entringer S, et al. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci USA. 2011;108:E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattan V, et al. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic Biol Med. 2008;44:1592–1598. doi: 10.1016/j.freeradbiomed.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Shlush LI, et al. Telomere elongation followed by telomere length reduction, in leukocytes from divers exposed to intense oxidative stress—implications for tissue and organismal aging. Mech Ageing Dev. 2011;132:123–130. doi: 10.1016/j.mad.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kotrschal A, Ilmonen P, Penn DJ. Stress impacts telomere dynamics. Biol Lett. 2007;3:128–130. doi: 10.1098/rsbl.2006.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaulieu M, Reichert S, Le Maho Y, Ancel A, Criscuolo F. Oxidative status and telomere length in a long-lived bird facing a costly reproductive event. Funct Ecol. 2011;25:577–585. [Google Scholar]
- 31.Gadalla SM, et al. Correlation of telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Blood. 2009;114:446. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakaysa SL, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 33.Tarry-Adkins JL, et al. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–1528. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 34.Metcalfe NB, Alonso-Alvarez C. Oxidative stress as a life-history constraint: The role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol. 2010;24:984–996. [Google Scholar]
- 35.Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: Role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 36.Herndon LA, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 37.Criscuolo F, et al. Real-time quantitative PCR assay for measurement of avian telomeres. J Avian Biol. 2009;40:342–347. [Google Scholar]
- 38.Haussmann MF, et al. Telomeres shorten more slowly in long-lived birds and mammals than in short-lived ones. Proc Biol Sci. 2003;270:1387–1392. doi: 10.1098/rspb.2003.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin-Ruiz CM, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp RGJ. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: A population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff C, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- 41.Harris SE, et al. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 42.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 43.Zann RA. Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford: Oxford Univ Press; 1996. [Google Scholar]
- 44.Thomson DL, Monaghan P, Furness RW. The demands of incubation and avian clutch size. Biol Rev Camb Philos Soc. 1998;73:293–304. [Google Scholar]
- 45.Reid JM, Monaghan P, Ruxton GD. Resource allocation between reproductive phases: The importance of thermal conditions in determining the cost of incubation. Proc Biol Sci. 2000;267:37–41. doi: 10.1098/rspb.2000.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Tienhoven A. Reproductive Physiology of Vertebrates. NY: Cornell Univ Press, Ithaca; 1983. [Google Scholar]
- 47.Wallen K, Schneider J, editors. Reproduction in Context: Social and Environmental Influences on Reproduction. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- 48.Nelson RJ. Introduction to Behavioral Endocrinology. Sunderland, MA: Sinauer; 2005. [Google Scholar]
- 49.Blount JD, et al. Neonatal nutrition, adult antioxidant defences and sexual attractiveness in the zebra finch. Proc Biol Sci. 2003;270:1691–1696. doi: 10.1098/rspb.2003.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blount JD, Metcalfe NB, Arnold KE, Surai PF, Monaghan P. Effects of neonatal nutrition on adult reproduction in a passerine bird. Ibis. 2006;148:509–514. [Google Scholar]
- 51.Arnold KE, et al. Sex-specific differences in compensation for poor neonatal nutrition in the zebra finch Taeniopygia guttata. J Avian Biol. 2007;38:356–366. [Google Scholar]
- 52.Butler MG, et al. Comparison of chromosome telomere integrity in multiple tissues from subjects at different ages. Cancer Genet Cytogenet. 1998;105:138–144. doi: 10.1016/s0165-4608(98)00029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youngren K, et al. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–643. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- 54.Friedrich U, et al. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 55.Takubo K, et al. Telomere lengths are characteristic in each human individual. Exp Gerontol. 2002;37:523–531. doi: 10.1016/s0531-5565(01)00218-2. [DOI] [PubMed] [Google Scholar]
- 56.Okuda K, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Flores I, et al. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatakeyama H, et al. The teleost Oryzias latipes shows telomere shortening with age despite considerable telomerase activity throughout life. Mech Ageing Dev. 2008;129:550–557. doi: 10.1016/j.mad.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aviv A, et al. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.