Abstract
Emerging evidence suggests that the pathogenesis of depressive disorders (DDs) is associated with neuronal abnormalities in brain microtubule function, including changes in α-tubulin isoforms. Currently available antidepressant drugs may act by rescuing these alterations, but only after long-term treatment explaining their delayed therapeutic efficacy. The microtubule associated protein type-2 (MAP-2) modulates neuronal microtubule dynamics. Our hypothesis is that MAP-2 represents an innovative target for the treatment of DDs. The synthetic pregnenolone-derivative MAP4343 (3β-methoxy-pregnenolone) binds MAP-2 in vitro and increases its ability to stimulate tubulin assembly. Here, we show that MAP4343 has antidepressant efficacy in rats and advantages compared with the selective serotonin reuptake inhibitor (SSRI) fluoxetine. A single injection of MAP4343 changes the expression of α-tubulin isoforms indicative of increased microtubule dynamics in the hippocampus of naïve Sprague–Dawley rats, whereas fluoxetine had no effects. MAP4343 has positive efficacy in the rat forced swimming test (FST), the most used assay to screen potential antidepressant drugs by decreasing immobility behavior. In the rat isolation-rearing model of depression, administration of MAP4343 showed more rapid and more persistent efficacy compared with fluoxetine in recovering “depressive-like” behaviors. These effects were accompanied by modifications of α-tubulin isoforms in the hippocampus, amygdala, and prefrontal cortex. Our findings suggest the potential therapeutic use of MAP4343 for the treatment of DDs, based on a unique mechanism of action.
Keywords: neurosteroids, cytoskeleton, mood disorders
Depressive disorders (DDs) are frequent, representing an important burden for contemporary society. The hypothesis of DDs postulates a deregulation of the central nervous system (CNS) monoamine, and available antidepressant drugs target monoamines reuptake transporters or receptors (1). The clinical efficacy of these drugs is reached only after 4–8 wk of administration (2). Compounds such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine (FLX) have disturbing side effects that may cause discontinuation of treatment (1). Importantly, it has been estimated that 50% of severely depressed patients responds only partially or remain refractory to currently used treatment (3).
Recent animal studies suggest a possible role in changes of hippocampal MAP-2 expression and ratio of α-tubulin isoforms in the pathogenesis and pharmacology of DDs (4–6). Microtubule dynamics is involved in the formation and maintenance of axons, dendrites, and dendritic spines (7). The microtubule associated protein type-2 (MAP-2) is mainly expressed in neuronal cell bodies, dendrites, and dendritic spines where it modulates microtubule assembly and dynamics (7). Posttranslational modifications (i.e., isoforms) of α-tubulin include the stable microtubule marker acetylated α-tubulin (Acet-Tub) and products of the cycle of detyrosination/tyrosination such as the dynamic microtubule marker tyrosinated α-tubulin (Tyr-Tub) and the stable microtubule marker detyrosinated α-tubulin (Glu-Tub) (8). Our hypothesis is that MAP-2 can be a unique target for the treatment of DDs because of its role in modulating microtubule dynamics. It has been demonstrated by our group that MAP-2 is a specific intracellular neuronal receptor of neurosteroids such as pregnenolone (9, 10). Pregnenolone specifically binds MAP-2, increases the in vitro tubulin assembly via MAP-2 activity, increases the extension of neurites in PC-12 cell cultures, and protects PC-12 and SH-SY5Y cells from toxic agents (9, 10). The synthetic pregnenolone-derivative MAP4343 (3β-methoxy-pregnenolone) has been selected after screening of a large library of natural and synthetic steroids. MAP4343 has similar in vitro activity as pregnenolone (10); it cannot be converted into metabolites with hormonal activities, and has been recently shown to have in vivo beneficial effects in rat models of spinal cord injury (11). MAP4343 has an interesting pharmacological profile because no in vitro affinity for any CNS neurotransmitter receptor was found.
Here, we show that MAP4343 has antidepressant efficacy in rats and displays advantageous properties compared with FLX. A single injection of MAP4343 rapidly changes the ratio of α-tubulin isoforms indicative of increased microtubule dynamics in the rat hippocampus, whereas FLX has no such effect. MAP4343 has positive efficacy in the rat forced swimming test, the most used assay to screen antidepressant drugs (12, 13), by decreasing immobility behavior (i.e., passive coping behavior). In the rat isolation-rearing model of depression, administration of MAP4343 showed persistent efficacy in recovering recognition memory deficit, stronger and more rapid anxiolytic activity, and more rapid rescue of passive coping behavior compared with FLX. The behavioral effects of MAP4343 correlated with changes in α-tubulin isoforms in the hippocampus, amygdala, and prefrontal cortex (PFC). Our findings suggest the potential use of MAP4343 as an innovative antidepressant drug having a unique mechanism of action.
Results
MAP4343: Pharmacological Profile.
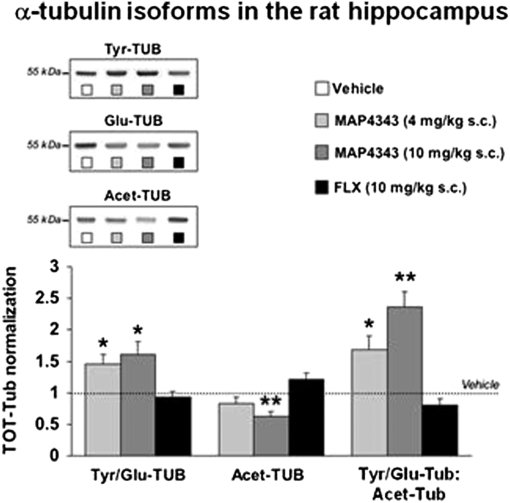
Adult male (250–300 g) Sprague–Dawley rats (SD) received a single s.c. injection of MAP4343 at the doses of 4 (n = 6) and 10 mg/kg (n = 6), FLX (10 mg/kg; n = 6) or the vehicle (n = 6). The hippocampus was dissected 3 h after the injection and prepared for the Western Blot analysis of α-tubulin isoforms (Tyr-Tub, Glu-Tub, and Acet-Tub). The densitometric analysis of Tyr-Tub and Glu-Tub was expressed as a ratio (Tyr-Tub/Glu-Tub). Another ratio was calculated, between Tyr-Tub/Glu-Tub and the densitometric values of Acet-Tub. Compared with vehicle-treated rats, a single injection of MAP4343 induced changes in α-tubulin isoforms indicative of significant dose–response increased microtubule dynamics (Fig. 1). In contrast, a single injection of FLX only induced minor changes, indicating a slight decrease in microtubule dynamics (Fig. 1).
Fig. 1.
A single injection of MAP4343 changes the expression of hippocampal α-tubulin isoforms. Animals received a single injection of MAP4343 at the doses of 4 mg/kg s.c. (n = 6) or 10 mg/kg s.c. (n = 6), FLX (10 mg/kg s.c.; n = 6), or the vehicle (n = 6) and were killed 3 h after treatments. Western blot was used to analyze α-tubulin isoforms in hippocampus homogenates. Representative Western blot bands are shown at top. Data are expressed as mean ± SEM. **P < 0.01; *P < 0.05 vs. vehicle treated rats (one-way ANOVA followed by Fisher's LSD test).
MAP4343: Positive Efficacy in the Forced Swimming Test (FST).
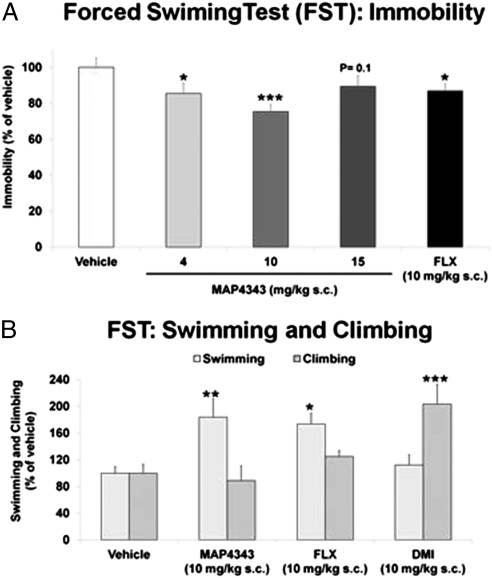
The FST is the most used assay to initially screen the potential in vivo antidepressant activity of drugs (12, 13). Rats are placed in an inescapable cylinder filled with water where they develop an immobile passive coping behavior. The time rats spend immobile is specifically decreased only by drugs exerting antidepressant activity. Naïve SD rats received s.c. administrations of the vehicle (n = 12) or MAP4343 at doses of 4, 10, or 15 mg/kg (n = 8 per dose). FLX (10 mg/kg s.c.; n = 6) was used as the reference antidepressant drug. Drugs were administered after the conventional dosing regimen used in the FST consisting of three injections made 24, 5, and 1h before testing (12). Animals administered with MAP4343 showed significantly decreased immobility, compared with vehicle treated animals, in a dose-dependent fashion at 4 (P < 0.05) and 10 mg/kg (P < 0.001), but significant efficacy was lost at 15 mg/kg (Fig. 2A). As expected, FLX decreased immobility behavior. Active coping behaviors can also be detected in the FST, namely climbing and swimming. It has been postulated that swimming behavior is sensitive to serotonergic drugs such as FLX, whereas climbing is sensitive to noradrenergic ones such as desipramine (DMI) (12). An additional experiment was set up to study the effects of MAP4343 (10 mg/kg s.c.; n = 12), FLX (10 mg/kg s.c.; n = 6), and DMI (10 mg/kg s.c.; n = 6) on active coping behaviors in the FST, compared with vehicle (n = 12) treated rats. MAP4343 confirmed positive efficacy in the FST by significantly (P < 0.01) increasing swimming behavior (Fig. 2B). In agreement with previous studies, FLX increased (P < 0.05) swimming, whereas DMI increased climbing (P < 0.001) (Fig. 2B). These findings showed positive efficacy of MAP4343 in the FST, which was dose-dependent in the 4–10 mg/kg range and characterized by decreased immobility and increased swimming behaviors.
Fig. 2.
MAP4343: positive efficacy in the rat FST and comparison with the conventional antidepressant drugs fluoxetine (FLX) and desipramine (DMI). (A) MAP4343 decreased the time (seconds) rats spent immobile in the FST. Animals were treated with the vehicle (n = 12), MAP4343 at either doses of 4, 10, or 15 mg/kg (n = 8 per dose) or FLX (10 mg/kg s.c.; n = 6). (B) MAP4343 increased the active swimming behavior (counts) in the FST. Rats were treated with the vehicle (n = 12), MAP4343 (10 mg/kg s.c.; n = 12), FLX (10 mg/kg s.c.; n = 6), or DMI (10 mg/kg s.c.; n = 6). Drugs were administered 24, 5, and 1 h before testing. Data are presented as percent of vehicle-treated rats and expressed as mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 vs. vehicle treated rats (one-way ANOVA followed by Fisher's LSD test).
MAP4343: Persistent, Stronger and More Rapid Antidepressant Activity Compared with FLX in the Rat Isolation-Rearing Model of Depression.
Experimental design.
Briefly, male SD rats (postnatal day 21–25) were housed in groups of four per cage (grouped, n = 16) or singly housed (isolated, n = 48) for 40 d. Thereafter, the animals started receiving daily (once a day) injections of treatments as follow: (i) Grouped+Vehicle (control; n = 16); (ii) Isolated+Vehicle (n = 16); (iii) Isolated+MAP4343 (10 mg/kg; n = 16) and (iv) Isolated+Fluoxetine (10 mg/kg; n = 16). To study the effects of the drugs in relation to the duration of treatments, the animals were split in two cohorts (n = 8 per each treatment group), namely the acute phase cohort with behavioral assays performed between days 1 and 4 of treatment (treatment injection received before behavioral testing), and the subchronic phase cohort with behavioral assays performed between days 7 and 10 of treatment (treatment injection received after behavioral testing). Rats were killed 16–18 h after the last injection and the hippocampus, amygdala, and PFC were dissected to be prepared for the analysis on α-tubulin isoforms.
Novel object recognition (NOR) task: Recognition memory.
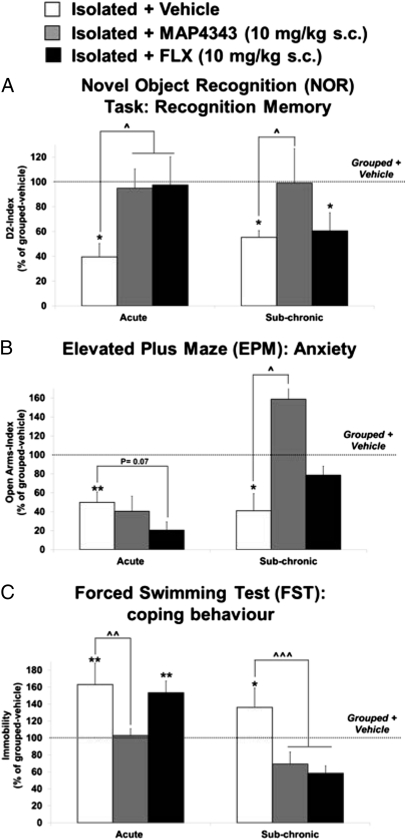
Recognition memory was tested in the NOR task 2 h after a single injection (acute phase) and after 7 d of daily injections (subchronic phase). Rodents naturally explore more a novel rather than a familiar object, which is the basis of the NOR task (14). The ability to discriminate between the familiar and the novel object is calculated as D2 index (Materials and Methods). Our results confirmed that isolation-reared rats had significantly decreased (P < 0.01) D2 index compared with Grouped+Vehicle in both the acute and subchronic phases (Fig. 3A). Significantly increased D2 index was observed in both Isolated+MAP4343 (P < 0.05) and Isolated+FLX rats compared with Isolated+Vehicle (Fig. 3A). In the subchronic phase, MAP4343 showed persistent efficacy in significantly (P < 0.05) recovering D2 index in isolated animals because this effect continued during the 7 d of repeated administration, whereas FLX completely lost its initial beneficial activity (Fig. 3A).
Fig. 3.
MAP4343: persistent, stronger, and more rapid antidepressant activity in the rat isolation-rearing model of depression compared with FLX. (A) MAP4343 has persistent efficacy in rescuing isolation-rearing induced recognition memory deficits in the NOR task compared with FLX. The ability to discriminate between the familiar and the novel object is expressed as D2 index. (B) MAP4343 has stronger and more rapid efficacy in recovering isolation-rearing induced anxiety in the EPM compared with FLX. Anxiety behavior is measured as Open Arms index. (C) MAP4343 has rapid efficacy in recovering isolation-rearing induced increase in passive coping behavior compared with FLX. Passive coping behavior is measured as time spent immobile. Data are presented as percent of Grouped+Vehicle rats. Each bar represents the mean ± SEM of n = 6–8. **P < 0.01, *P < 0.05 vs. Grouped+Vehicle rats; ^^^P < 0.001, ^^P < 0.01, ^P < 0.05 vs. Isolated+Vehicle rats (one-way ANOVA followed by Fisher's LSD test).
Elevated plus maze (EPM) test: Anxiety.
Anxiety was tested in the EPM after 2 d (acute phase) and 8 d of daily injections (subchronic phase). The EPM has four arms (two open and two enclosed), and the assay relies on the spontaneous preference of rodents toward dark and enclosed spaces (closed arms) and an unconditioned fear of open spaces (open arms) (15). Anxiety behavior is measured as the ratio of length of time spent in the open arms and closed arms, respectively (i.e., Open Arms index; Materials and Methods). Our data showed significantly decreased Open Arms index in Isolated+Vehicle rats compared with Grouped+Vehicle rats during both the acute (P < 0.01) and the subchronic (P < 0.05) phase (Fig. 3B). The increased anxiety of the isolated rats was not recovered by MAP4343 in the acute phase, whereas FLX appeared to have additional anxiogenic effects in isolated rats. Importantly, major anxiolytic efficacy of MAP4343 was observed in the subchronic phase because Isolated+MAP4343 animals had significantly (P < 0.05) increased Open Arms-Index compared with Grouped+Vehicle (Fig. 3B). Subchronic FLX treatment only induced a slight increase in Open Arms index, which was not different from that of Isolated+Vehicle rats (Fig. 3B). These data are indicative of a stronger and more rapid anxiolytic activity of subchronic MAP4343 compared with FLX in isolated rats.
Forced swimming test (FST): Coping Behavior.
Coping behavior was measured as immobility in the FST after 4 (acute phase) and 10 d (subchronic phase) of daily injections. Immobility in the FST is considered a valuable marker of depressive-like behavior because it has been found increased in a number of animal models of depression (12). Our results confirmed that Isolated+Vehicle rats have increased immobility in the FST compared with Grouped+Vehicle animals in either the acute phase (P < 0.01) or the subchronic phase (P < 0.05) (Fig. 3C). In the acute phase, MAP4343 significantly decreased FST immobility in isolated rats (Isolated+MAP4343 vs. Isolated+Vehicle; P < 0.01), whereas FLX showed no efficacy (Fig. 3C). Indeed, MAP4343 showed rapid antidepressant efficacy by switching the coping strategy of isolated rats from a passive to an active behavior. In the subchronic phase, both Isolated+MAP4343 and Isolated+FLX significantly (P < 0.001) decreased immobility behavior compared with Isolated+Vehicle (Fig. 3C).
Expression of α-tubulin isoforms in the hippocampus, amygdala, and PFC.
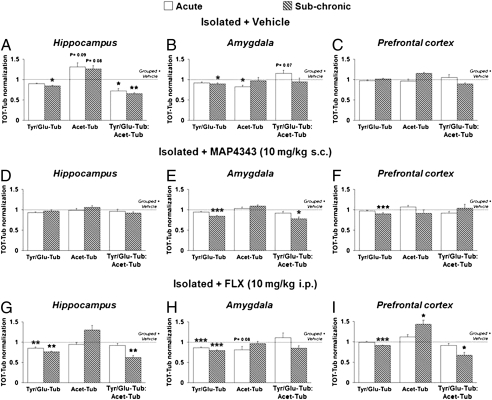
The expression of α-tubulin isoforms was analyzed in the hippocampus, amygdala, and PFC by using the infrared Western blot technique. Isolated rats had changes in α-tubulin isoforms suggestive of decrease hippocampal microtubule dynamics in both the acute and subchronic phases compared with Grouped+Vehicle (Fig. 4A). Moreover, in the amygdala, the changes in α-tubulin isoforms were suggestive of a tendency (P = 0.07) to increase microtubule dynamics in the acute phase and a slight tendency to decrease it in the subchronic one (Fig. 4B). In the PFC, no significant changes induced by isolation rearing were detected in α-tubulin isoforms, although these showed a similar pattern as in the amygdala (Fig. 4C). Treatment with MAP4343 fully recovered changes in α-tubulin isoforms induced by isolation rearing in the hippocampus after both the acute and subchronic phase (Fig. 4D). In the amygdala, MAP4343 also recovered the changes in α-tubulin isoforms and microtubule dynamics index induced by isolation-rearing in the acute phase, but significantly (P < 0.001) decreased Tyr-Tub/Glu-Tub below the Grouped+Vehicle levels in the subchronic phase (Fig. 4E). In the PFC, MAP4343 induced α-tubulin isoform changes that appeared to reverse the effects of isolation-rearing in both the acute and subchronic phases (Fig. 4F). Administration of FLX changed hippocampal α-tubulin isoforms, indicative of rescue of the isolation-induced decrease in microtubule dynamics in the acute phase, but failed to do so in the subchronic one (Fig. 4G). In the amygdala, FLX did not show any rescue of the isolation induced changes in α-tubulin isoforms after either the acute or the subchronic phase (Fig. 4H). In the PFC, FLX induced changes in α-tubulin isoforms in the acute phase similar to those of MAP4343, whereas major changes suggesting decreased microtubule dynamics compared with Grouped+Vehicle rats were evident in the subchronic phase. These sets of data indicate that the antidepressant behavioral effects of MAP4343 are accompanied by normalization of changes in α-tubulin isoforms in the three brain regions examined.
Fig. 4.
Expression of α-tubulin isoforms in the hippocampus, amygdala, and PFC. Effects of isolation-rearing (Isolated+Vehicle) in the hippocampus (A), the amygdala (B), and the PFC (C). Effects of MAP4343 treatment in isolated rats (Isolated+MAP4343) in the hippocampus (D), the amygdala (E), and the PFC (F). Effects of FLX treatment in isolated rats (Isolated+FLX) in the hippocampus (G), the amygdala (H), and the PFC (I). Each bar represents the mean ± SEM of n = 6–7. ***P < 0.001, **P < 0.01, *P < 0.05 vs. Grouped+Vehicle rats (four-way ANOVA followed by Fisher's LSD test).
Discussion
Currently available antidepressant drugs have similar mechanisms of action, and most of them target monoamines transporters or receptors (1). Despite being blockbusters in the drug market, antidepressants have well-known limitations including late onset of efficacy (from 4 to 8 wk), disturbing side effects leading to treatment discontinuation, and 50% of severely depressed individuals are partial or nonresponders to treatment (1–3). Because of the economic and social burden represented by DDs, the urgency for faster, more efficacious, and safer treatments becomes evident.
Growing evidence suggest a possible role in changes of hippocampal MAP-2 expression and ratio of α-tubulin isoforms in the pathogenesis and pharmacology of DDs (4–6). Our hypothesis is that MAP-2 represents a unique target for the treatment of DDs because of its role in modulating microtubule dynamics. However, the possibility of targeting microtubules for the treatment of DDs was only hypothesized but not considered feasible because of the lack of molecules having neuronal microtubule specificity and safer pharmacological profile (4). The recent discovery that MAP-2 is a neurosteroid receptor and that pregnenolone acts as agonist by stimulating the function of MAP-2 (9, 10) opens the path to new treatments for CNS disorders including DDs.
This study shows that the synthetic pregnenolone-derivative MAP4343 has positive efficacy in the rat FST by decreasing immobility (passive coping behavior) and increasing swimming (active coping behavior), which is considered suggestive of potential antidepressant activity (12, 13). MAP4343 showed a U-shape dose–response curve for which we have no explanation, but this U-shape curve is frequently observed in some behavioral paradigms after treatment with psychotropic drugs.
The antidepressant efficacy of MAP4343 was investigated in the isolation-rearing model that has been shown to induce “depressive-like” behaviors including recognition memory deficits (5), increased anxiety (16), and increased passive coping behavior (17). In the isolation-rearing model of depression, MAP4343 demonstrated clear antidepressant activity and important advantages compared with FLX. MAP4343 had persistent efficacy in recovering recognition memory deficits in isolated rats after either acute or subchronic treatment. In contrast, FLX was effective only after acute treatment, which is in agreement with studies reporting beneficial effects of a single injection of FLX on memory (18), whereas no effects were observed after repeated administrations (19). Moreover, MAP4343 rescued anxiety in isolated rats after subchronic treatment more efficaciously and more rapidly than FLX. Acute FLX treatment appeared to deteriorate anxiety in isolated rats consistently with reports of increased anxiety observed in depressed patients during the first days of FLX treatment (20). Passive coping behavior was rapidly recovered in isolated rats by MAP4343 after acute treatment and such activity was persistent after subchronic administration. In contrast, FLX rescued passive coping behavior only after subchronic treatment. This finding is in full agreement with previous papers showing activity of FLX on passive coping behavior after at least 7 d of daily administrations (21). Noteworthy, our behavioral studies are of particular relevance because they have been performed on isolated (i.e., “depressed-like”) and not naïve rats, and the isolation-rearing condition is likely to further delay the efficacy of FLX treatment. Internal data clearly show that single or repeated administrations of MAP4343 and FLX do not alter locomotor activity in isolated rat. Thus, the possibility that the observed responses in the behavioral tests are due to changes in locomotor activity can be excluded.
Isolation-rearing induced hippocampal changes in α-tubulin isoforms suggestive of decreased microtubule dynamics, as reported (5). Other authors reported similar changes in hippocampal α-tubulin isoforms in the chronic unpredictable mild stress model of depression in the rat (22). Here, we show that isolation-rearing induced different pattern of α-tubulin isoforms changes in the amygdala and possibly to a much minor extent in the PFC. The behavioral effects of MAP4343 correlated with rescue of the alterations induced by isolation-rearing in α-tubulin isoforms and microtubule dynamics in the hippocampus, amygdala, and PFC. The three brain areas examined are important in all of the behavioral tests used (23–25) and have been shown to differently respond to stress in terms of alterations in dendritic structure (26). It is possible to speculate that the different effects on microtubule dynamics induced by MAP4343 in the three brain regions are associated with restoration of neuronal integrity and physiological behavioral patterns. In vitro study showed that MAP4343 increases neurite outgrowth via a MAP-2–dependent mechanism, suggesting that MAP4343 may induce neuronal remodeling of dendritic structure (10). However, further studies are required to elucidate the impact of MAP4343 on neuronal function and structure in vivo, even we report here that MAP4343 appears to exert more rapid and persistent efficacy compared with currently used antidepressant drugs. Additionally, it has been recently suggested that a functional link may exist between microtubule dynamics and central serotonin neurotransmission (27). Interestingly, MAP4343 was found to increase swimming (serotonergic dependent) but not climbing (noradrenergic dependent) behavior in the FST. Therefore, the possibility that MAP4343 can indirectly affect the serotonergic system cannot be excluded.
The behavioral effects of FLX were also accompanied by changes in α-tubulin isoforms of different onset, pattern, and brain region specificity compared with those of MAP4343. Repeated daily administration (3–4 wk) of different classes of antidepressant drugs have been reported to affect microtubules in the rat hippocampus by changing the ratio of hippocampal α-tubulin isoforms suggestive of increased microtubule dynamics (6, 28) and by increasing MAP-2 phosphorylation (22, 28). The modulation of microtubule dynamics induced by repeated administrations of antidepressant drugs is a slow process associated to postsynaptic activation of intracellular protein kinases pathways (4) and translocation of Gsa from plasma membrane (29) to cytosol, where it increases microtubule dynamics (30). Therefore, the present data are in favor of additional postsynaptic mechanisms, which may explain the delayed therapeutic efficacy of FLX and other antidepressant drugs.
The lack of biomarkers and the spectrum of symptoms characterizing depression underline the difficulties in reproducing the disorder in the laboratory in terms of translational meaning for human clinical practice (13). Therefore, our findings need to be confirmed in more animal models.
In this report, we present results of experiments indicating activities of the neurosteroid derivative MAP4343, specifically dealing with its remarkable positive effects for treating experimental depressive disorders. The biochemical and subcellular background of MAP4343 activities in the nervous system is related to its interaction with the MAP-2 protein acting as neurosteroid receptor (10); thus, stimulating microtubule dynamics and modifying α-tubulin isoforms.
We have demonstrated therapeutic activity of MAP4343 on spinal cord injuries (11). Considering the important generalized role of microtubule pathophysiology in the CNS, this recently unveiled mechanism may demonstrate useful applications in the treatment of other neuronal alterations. In the specific case of depressive syndromes, we will soon submit for publication other results including detailed pharmacological properties of the compound, and effects in other animal species. In this paper, we publish the primary results to attract attention to the possible use of a neurosteroid derivative in one of the most wild-spread neuropathology, because the potential therapeutic use of the proposed mechanism is still unknown so far. We are willing to share our views with interested colleagues.
Materials and Methods
Animals.
Adult male (250–300 g) SD rats were obtained from Janvier. Animals were group-housed (n = 4 per cage) and maintained under controlled conditions (21 ± 1 °C, 12 h/12 h light/dark cycles lights on at 0700) with food and water available ad libitum. The experiments started after 1 wk of animal acclimatisation to the facilities environment. The experimental procedures were in accordance with the European Communities Council Directive (86/609/EEC) and approved by the internal scientific committees of MAPREG and Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche 788.
Drug Preparation and Administration.
MAP4343 (US Patent 2006/0199790 A1) was dissolved in sesame oil, whereas FLX (Biotrend) was dissolved in water. The drug formulations were prepared freshly each day of treatment.
Western Blot of α-Tubulin Isoforms.
The hippocampi, amygdalae, and PFC were dissected and prepared for Western Blot analyses as described (28).
Colorimetric Western Blot Detection of α-Tubulin Isoforms.
Analyses of α-tubulin isoforms (Tyr-, Glu-, and Acet-Tub) and of total α-tubulin (TOT-Tub) using colorometric Western Blot detection were performed as reported (28).
Infrared Western Blot Detection of α-Tubulin Isoforms.
Tyr-, Glu-, Acet-, and TOT-Tub were analyzed by using the Odyssey infrared imaging system (Li-Cor). After electrophoresis, proteins were transferred onto PVDF membranes (Millipore-Immobilon) and the membranes were blocked with Odyssey blocking buffer (Li-Cor). The membranes were then incubated with primary antibodies, washed, and incubated with secondary antibodies coupled with infrared dyes (Li-Cor). The intensities of the protein bands were quantified by using the Odyssey V3.0 software. α-tubulin isoforms data were normalized to those of total α-tubulin expression. The (Tyr-Tub/Glu-Tub)/Acet-Tub ratio was used as microtubule dynamics index.
Isolation-Rearing Protocol.
Male SD rats (Janvier) were obtained immediately after weaning (postnatal day 21–25) from eight different mothers. They were housed either individually (isolated, n = 48) or four per cage (grouped, n = 16), such that each of the eight litters provided both isolated and grouped animals. All rats were housed in opaque plastic cages lined with sawdust and fitted with metal grid lids for 52 d. Isolated rats were housed in cages measuring 38 × 24 × 18 cm, whereas group housed rats were maintained in cages measuring 35 × 35 × 18 cm. All animals were housed in the same room under controlled conditions (21 ± 1 °C, 12 h/12 h light/dark cycles lights on at 0700, with food and water available ad libitum). The experiments were in accordance with the European Communities Council Directive (86/609/EEC) and approved by the internal scientific committees of MAPREG and Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche 788.
NOR Task.
The NOR task was performed as described (5). An inter-trial interval (ITI) of 2 h was used between the familiarization and the choice trial. The exploration of the objects was defined as the time (seconds) spent sniffing, touching, and having moving vibrissae on each object. The D2 index, which represents the ability to discriminate the novel from familiar object, was calculated {[novel object (sec) − familiar object (sec)]/[novel object (sec) + familiar object (sec)]} according to the method of Ennaceur and Delacour (14).
Elevated Plus-Maze.
The applied procedure has been described in details (15). Briefly, rats were initially placed onto the center of the maze, and the time spent in each arm was recorded for 5 min by using a computerized system (VideoTrack V2.5; ViewPoint). The open arms-index was calculated as a measure of anxiety as follows: time in the open arms (sec)/[time in open arms (sec) + time in closed arms (sec)].
FST.
The animals were submitted to FST as described in a previous study (28).
Statistical Analysis.
Appropriate multifactorial analysis of variance (ANOVA) was used to detect statistical significance of dependent variables. The Fisher least difference (LSD) test was used for post hoc analyses. Probability values of P < 0.05 were considered as statistically significant. Statistical analyses were performed by using InVivoStat V1.2 (31).
Acknowledgments
We thank Dr. N. Ladurelle for her continued and high quality contribution; Dr. S. Bate for performing the accurate and elegant statistical analysis of the data; and Dr. A. Viggiano, C. Perier, C. Potard, and L. Paresys for assistance with the behavioral experiments. The present work was partially supported by the European Union Eureka/Eurostars Depression and Steroids Grant E!5291.
Footnotes
The authors declare no conflict of interest.
References
- 1.O'Donnell JM, Shelton RC. Drug therapy of depression and anxiety disorders. In: Brunton L, Chabner B, Chabner B, Knollman B, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 12th Ed. New York: McGraw–Hill; 2011. pp. 397–415. [Google Scholar]
- 2.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi M, Hagan JJ, Heidbreder CA. Neuronal plasticity, stress and depression: Involvement of the cytoskeletal microtubular system? Curr Drug Targets CNS Neurol Disord. 2005;4:597–611. doi: 10.2174/156800705774322012. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M, et al. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–2902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi M, et al. Fluoxetine administration modulates the cytoskeletal microtubular system in the rat hippocampus. Synapse. 2009;63:359–364. doi: 10.1002/syn.20614. [DOI] [PubMed] [Google Scholar]
- 7.Conde C, Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 8.Janke C, Kneussel M. Tubulin post-translational modifications: Encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33:362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Murakami K, Fellous A, Baulieu EE, Robel P. Pregnenolone binds to microtubule-associated protein 2 and stimulates microtubule assembly. Proc Natl Acad Sci USA. 2000;97:3579–3584. doi: 10.1073/pnas.97.7.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine-Lenoir V, et al. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci USA. 2006;103:4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duchossoy Y, David S, Baulieu EE, Robel P. Treatment of experimental spinal cord injury with 3β-methoxy-pregnenolone. Brain Res. 2011;1403:57–66. doi: 10.1016/j.brainres.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 12.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Fernando AB, Robbins TW. Animal models of neuropsychiatric disorders. Annu Rev Clin Psychol. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 15.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellemans KG, Benge LC, Olmstead MC. Adolescent enrichment partially reverses the social isolation syndrome. Brain Res Dev Brain Res. 2004;150:103–115. doi: 10.1016/j.devbrainres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Brenes JC, Fornaguera J. The effect of chronic fluoxetine on social isolation-induced changes on sucrose consumption, immobility behavior, and on serotonin and dopamine function in hippocampus and ventral striatum. Behav Brain Res. 2009;198:199–205. doi: 10.1016/j.bbr.2008.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Flood JF, Cherkin A. Fluoxetine enhances memory processing in mice. Psychopharmacology (Berl) 1987;93:36–43. doi: 10.1007/BF02439584. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Palacios G, Bonilla-Jaime H, Velazquez-Moctezuma J. Antidepressant-like effects of the acute and chronic administration of nicotine in the rat forced swimming test and its interaction with flouxetine. Pharmacol Biochem Behav. 2004;78:165–169. doi: 10.1016/j.pbb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Gordon JA, Hen R. The serotonergic system and anxiety. Neuromolecular Med. 2004;5:27–40. doi: 10.1385/NMM:5:1:027. [DOI] [PubMed] [Google Scholar]
- 21.Vázquez-Palacios G, Bonilla-Jaime H, Velázquez-Moctezuma J. Antidepressant effects of nicotine and fluoxetine in an animal model of depression induced by neonatal treatment with clomipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:39–46. doi: 10.1016/j.pnpbp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Yang C, Wang G, Wang H, Liu Z, Wang X. Cytoskeletal alterations in rat hippocampus following chronic unpredictable mild stress and re-exposure to acute and chronic unpredictable mild stress. Behav Brain Res. 2009;205:518–524. doi: 10.1016/j.bbr.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Moreira CM, Masson S, Carvalho MC, Brandão ML. Exploratory behaviour of rats in the elevated plus-maze is differentially sensitive to inactivation of the basolateral and central amygdaloid nuclei. Brain Res Bull. 2007;71:466–474. doi: 10.1016/j.brainresbull.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Bessa JM, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–773, 739. doi: 10.1038/mp.2008.119. [DOI] [PubMed] [Google Scholar]
- 25.Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31:10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- 27.Fournet V, et al. The deletion of the microtubule-associated STOP protein affects the serotonergic mouse brain network. J Neurochem. 2010;115:1579–1594. doi: 10.1111/j.1471-4159.2010.07064.x. [DOI] [PubMed] [Google Scholar]
- 28.Ladurelle N, et al. Agomelatine (S20098) modulates the expression of cytoskeletal microtubular proteins, synaptic markers and BDNF in the rat hippocampus, amygdala and PFC. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2597-5. [DOI] [PubMed] [Google Scholar]
- 29.Donati RJ, Rasenick MM. Chronic antidepressant treatment prevents accumulation of gsalpha in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (lipid rafts) Neuropsychopharmacology. 2005;30:1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- 30.Davé RH, et al. A molecular and structural mechanism for G protein-mediated microtubule destabilization. J Biol Chem. 2011;286:4319–4328. doi: 10.1074/jbc.M110.196436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark RA, Shoaib M, Hewitt KN, Stanford SC, Bate ST. A comparison of InVivoStat with other statistical software packages for analysis of data generated from animal experiments. J Psychopharmacol. November 8, 2011 doi: 10.1177/0269881111420313. [DOI] [PubMed] [Google Scholar]